Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
- -
- Serum M-component (the absolute increase must be >0.5 g/dL), and/or
- -
- Urine M-component (the absolute increase must be >200 mg/24 h), and/or
- -
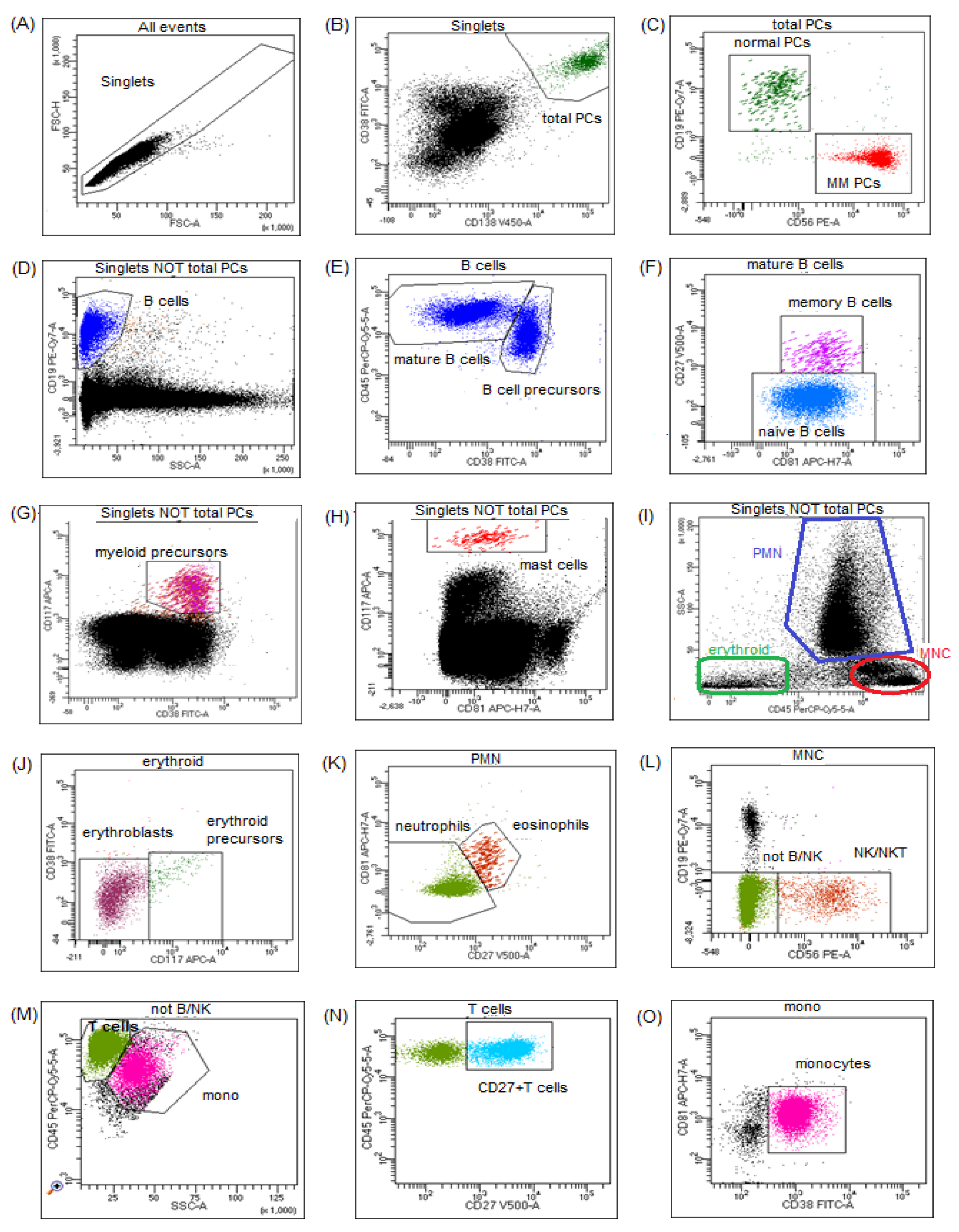
2.2. Flow Cytometry Assessment of Plasma Cells and BM Microenvironment
2.3. Statistical Analysis
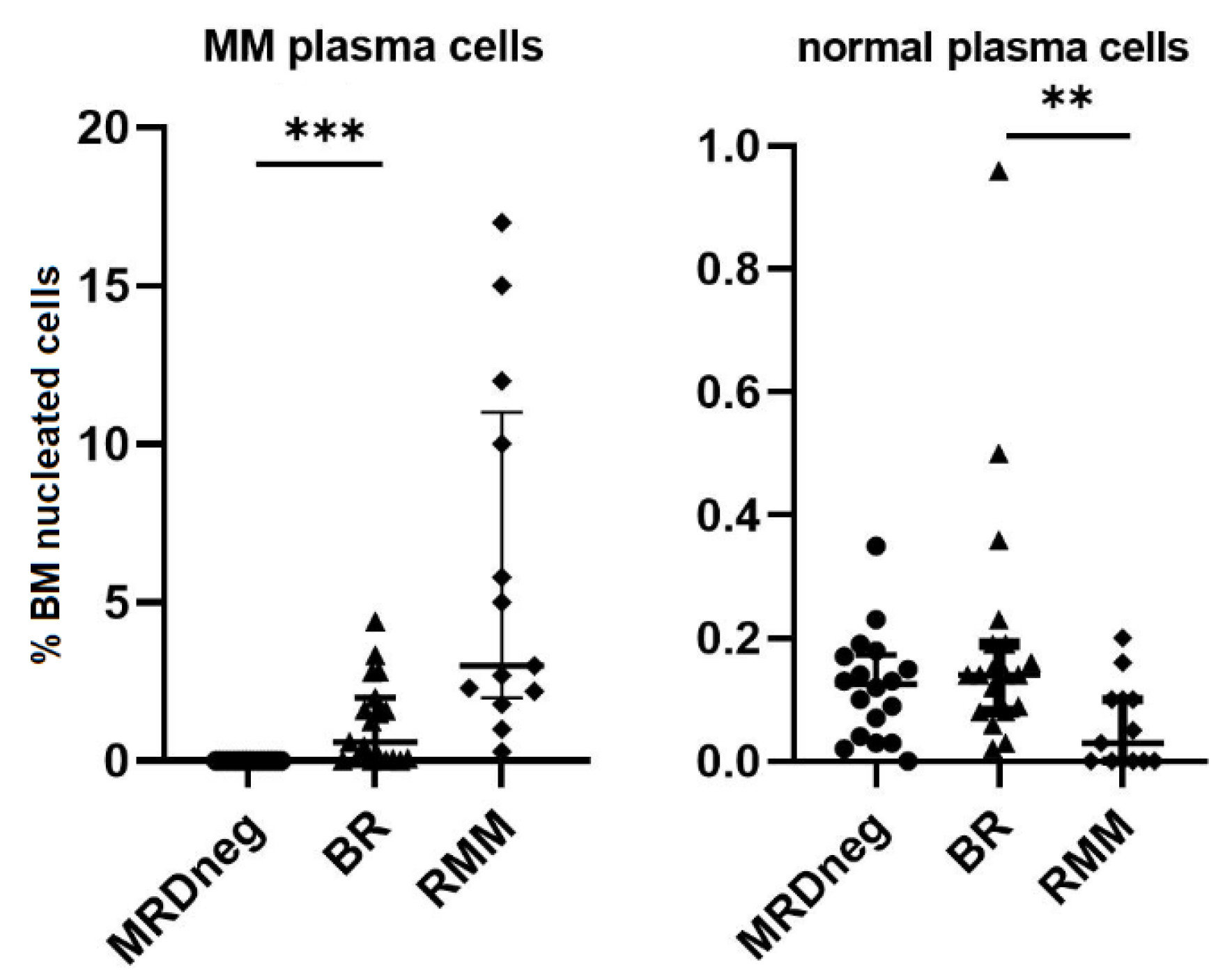
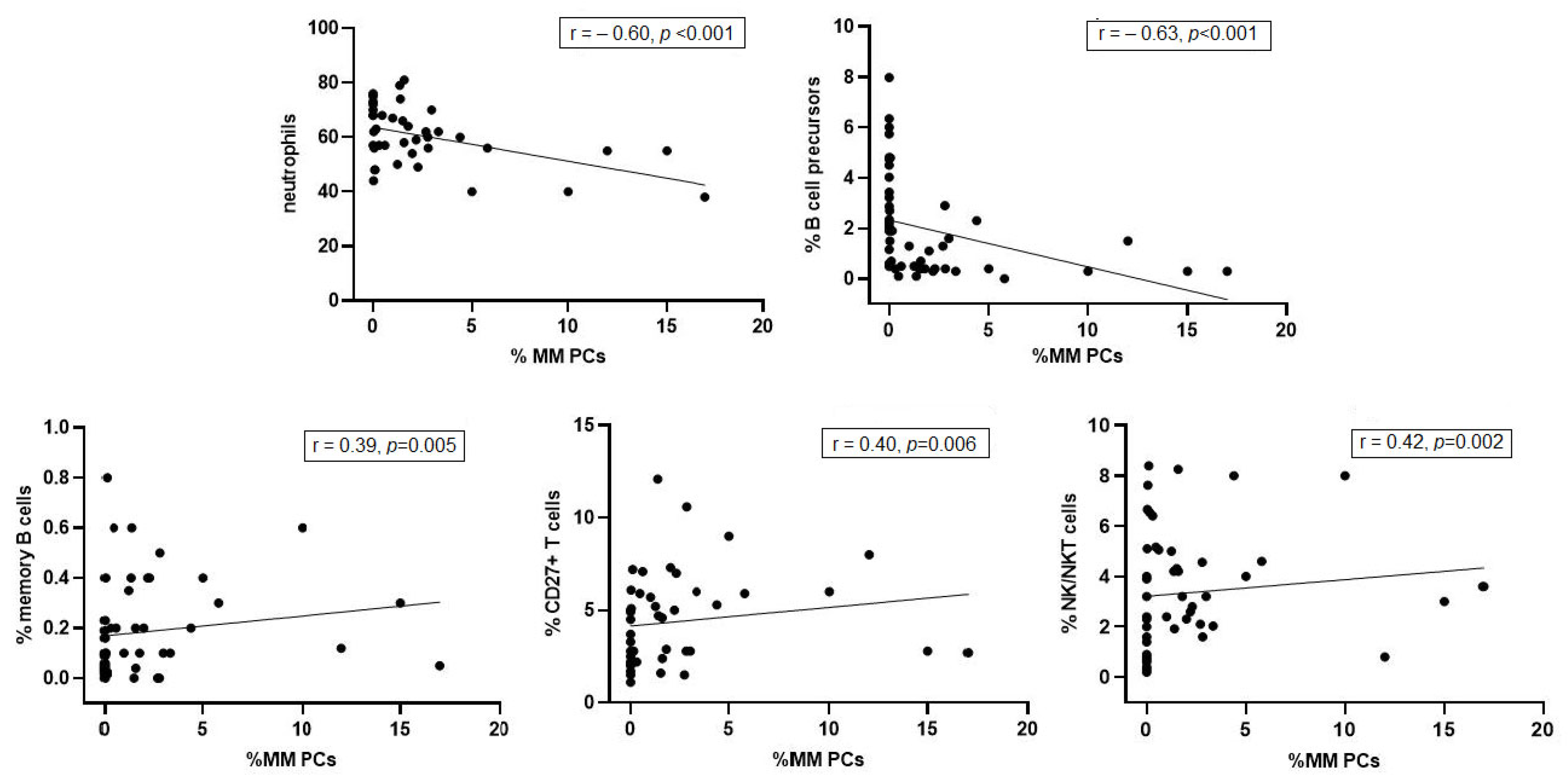
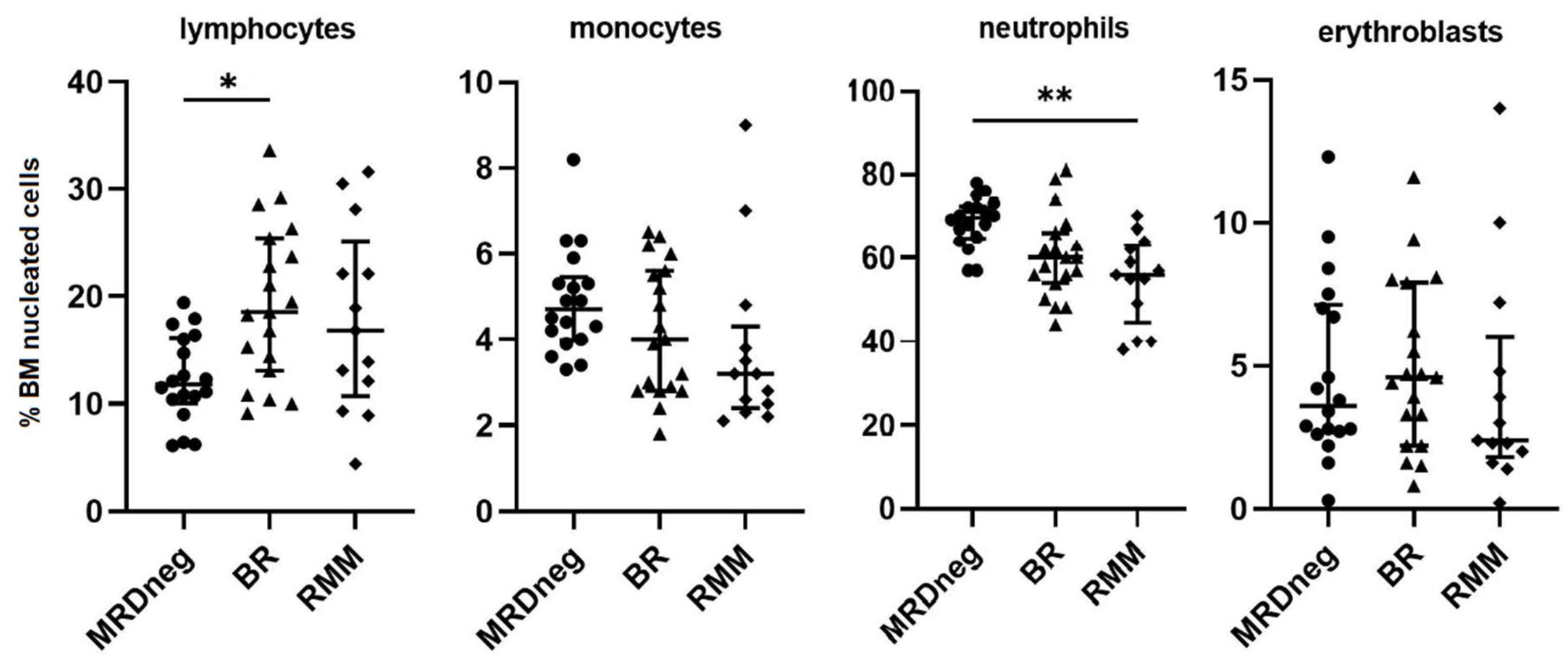
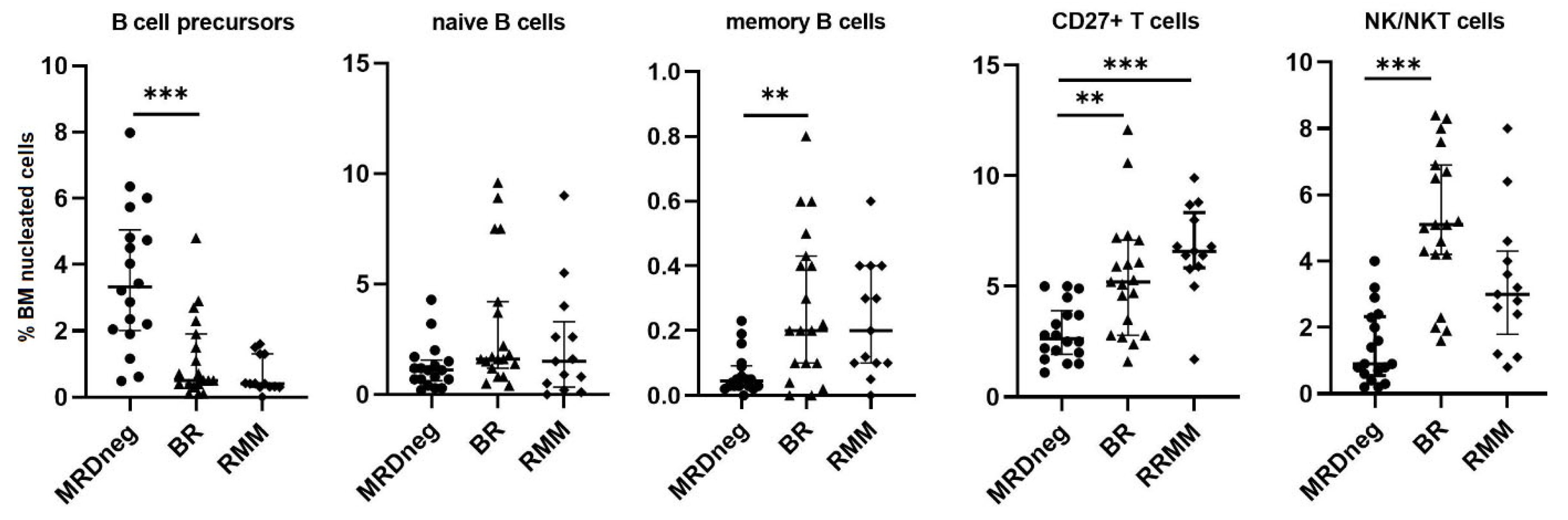
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef] [PubMed]
- Leone, P.; Solimando, A.G.; Malerba, E.; Fasano, R.; Buonavoglia, A.; Pappagallo, F.; De Re, V.; Argentiero, A.; Silvestris, N.; Vacca, A.; et al. Actors on the Scene: Immune cells in the myeloma niche. Front. Oncol. 2020, 10, 599098. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.; Ferreira, B.V.; Caetano, J.; Barahona, F.; Carneiro, E.A.; João, C. Boosting immunity against multiple myeloma. Cancers 2021, 13, 1221. [Google Scholar] [CrossRef] [PubMed]
- Flores-Montero, J.; Sanoja-Flores, L.; Paiva, B.; Puig, N.; García-Sánchez, O.; Böttcher, S.; van der Velden, V.H.J.; Pérez-Morán, J.J.; Vidriales, M.B.; García-Sanz, R.; et al. Next generation flow for highly sensitive and standardized detection of minimal residual disease in multiple myeloma. Leukemia 2017, 31, 2094–2103. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. Large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in multiple myeloma patients. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef]
- Paiva, B.; Puig, N.; Cedena, M.T.; Rosiñol, L.; Cordón, L.; Vidriales, M.B.; Burgos, L.; Flores-Montero, J.; Sanoja-Flores, L.; Lopez-Anglada, L.; et al. Measurable residual disease by Next-Generation Flow Cytometry in multiple myeloma. J. Clin. Oncol. 2020, 38, 784–792. [Google Scholar] [CrossRef]
- García-Sanz, R.; Oriol, A.; Moreno, M.J.; de la Rubia, J.; Payer, A.R.; Hernández, M.T.; Palomera, L.; Teruel, A.I.; Blanchard, M.J.; Gironella, M.; et al. Zoledronic acid as compared with observation in multiple myeloma patients at biochemical relapse: Results of the randomized AZABACHE Spanish trial. Haematologica 2015, 100, 1207–1213. [Google Scholar] [CrossRef]
- Paiva, B.; Pérez-Andrés, M.; Vídriales, M.B.; Almeida, J.; de las Heras, N.; Mateos, M.V.; López-Corral, L.; Gutiérrez, N.C.; Blanco, J.; Oriol, A.; et al. Competition between clonal plasma cells and normal cells for potentially overlapping bone marrow niches is associated with a progressively altered cellular distribution in MGUS vs. myeloma. Leukemia 2011, 25, 697–706. [Google Scholar] [CrossRef]
- Busch, A.; Zeh, D.; Janzen, V.; Mügge, L.O.; Wolf, D.; Fingerhut, L.; Hahn-Ast, C.; Maurer, O.; Brossart, P.; von Lilienfeld-Toal, M. Treatment with lenalidomide induces immunoactivating and counter-regulatory immunosuppressive changes in myeloma patients. Clin. Exp. Immunol. 2014, 177, 439–453. [Google Scholar] [CrossRef]
- Zhaoyun, L.; Rong, F. Predictive role of immune profiling for survival of multiple myeloma patients. Front. Immunol. 2021, 12, 663748. [Google Scholar] [CrossRef]
- Ho, C.M.; McCarthy, P.L.; Wallace, P.K.; Zhang, Y.; Fora, A.; Mellors, P.; Tario, J.D.; McCarthy, B.L.S.; Chen, G.L.; Holstein, S.A.; et al. Immune signatures associated with improved progression-free and overall survival for myeloma patients treated with AHSCT. Blood Adv. 2017, 1, 1056–1066. [Google Scholar] [CrossRef] [PubMed]
- Görgün, G.T.; Whitehill, G.; Anderson, J.L.; Hideshima, T.; Maguire, C.; Laubach, J.; Raje, N.; Munshi, N.C.; Richardson, P.G.; Anderson, K.C. Tumor-promoting immune-suppressive myeloid-derived suppressor cells in the multiple myeloma microenvironment in humans. Blood 2013, 121, 2975–2987. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; Ye, J.C.; Howard, A.; Bhutani, M.; Gormley, N.; Hahn, T.; Hillengass, J.; Krishnan, A.; Landgren, C.O.; Munshi, N.C.; et al. Summary of the Second Annual BMT CTN Myeloma Intergroup Workshop on minimal residual disease and immune profiling. Biol. Blood Marrow Transplant. 2019, 25, e89–e97. [Google Scholar] [CrossRef]
- Paiva, B.; Cedena, M.T.; Puig, N.; Arana, P.; Vidriales, M.B.; Cordon, L.; Flores-Montero, J.; Gutierrez, N.C.; Martín-Ramos, M.L.; Martinez-Lopez, J.; et al. Minimal residual disease monitoring and immune profiling in multiple myeloma in elderly patients. Blood 2016, 127, 3165–3174. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, K.; Tsakirakis, N.; Malandrakis, P.; Vitsos, P.; Metousis, A.; Orologas-Stavrou, N.; Ntanasis-Stathopoulos, I.; Kanellias, N.; Eleutherakis-Papaiakovou, E.; Pothos, P.; et al. Deep Phenotyping Reveals Distinct Immune Signatures Correlating with Prognostication, Treatment Responses, and MRD Status in Multiple Myeloma. Cancers 2020, 12, 3245. [Google Scholar] [CrossRef] [PubMed]
- Puig, N.; Paiva, B.; Lasa, M.; Burgos, L.; Perez, J.J.; Merino, J.; Moreno, C.; Vidriales, M.B.; Toboso, D.G.; Cedena, M.T.; et al. Flow cytometry for fast screening and automated risk assessment in systemic light-chain amyloidosis. Leukemia 2019, 33, 1256–1267. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406, Correction in Haematologica 2016, 101, 995. [Google Scholar] [CrossRef]
- Krzywdzińska, A.; Puła, B.; Czyż, A.; Krzymieniewska, B.; Kiernicka-Parulska, J.; Mierzwa, A.; Szymczak, D.; Milanowska, A.; Kiraga, A.; Kwiecień, I.; et al. Harmonization of flow cytometric minimal residual disease assessment in multiple myeloma in centers of Polish Myeloma Consortium. Diagnostics 2021, 11, 1872. [Google Scholar] [CrossRef]
- Stetler-Stevenson, M.; Paiva, B.; Stoolman, L.; Lin, P.; Jorgensen, J.L.; Orfao, A.; Van Dongen, J.; Rawstron, A.C. Consensus guidelines for myeloma minimal residual disease sample staining and data acquisition. Cytom. B Clin. Cytom. 2015, 90, 26–30. [Google Scholar] [CrossRef]
- Mendonça de Pontes, R.; Flores-Montero, J.; Sanoja-Flores, L.; Puig, N.; Pessoa de Magalhães, R.J.; Corral-Mateos, A.; Salgado, A.B.; García-Sánchez, O.; Pérez-Morán, J.; Mateos, M.V. B-Cell regeneration profile and minimal residual disease status in bone marrow of treated multiple myeloma patients. Cancers 2021, 13, 1704. [Google Scholar] [CrossRef] [PubMed]
- Guillerey, C.; Nakamura, K.; Vuckovic, S.; Hill, G.R.; Smyth, M.J. Immune responses in multiple myeloma: Role of the natural immune surveillance and potential of immunotherapies. Cell Mol. Life Sci. 2016, 73, 1569–1589. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Cai, Z.; Wang, S.; Zhang, X.; Qian, J.; Hong, S.; Li, H.; Wang, M.; Yang, J.; Yi, Q. Macrophages are an abundant component of myeloma microenvironment and protect myeloma cells from chemotherapy drug-induced apoptosis. Blood 2009, 114, 3625–3628. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Kostopoulos, I.V.; Kastritis, E.; Ntanasis-Stathopoulos, I.; Migkou, M.; Rousakis, P.; Argyriou, A.T.; Kanellias, N.; Fotiou, D.; Eleutherakis-Papaiakovou, E.; et al. Impact of minimal residual disease detection by Next-Generation Flow Cytometry in multiple myeloma patients with sustained complete remission after frontline therapy. HemaSphere 2019, 3, e300. [Google Scholar] [CrossRef]
- Mohan, M.; Kendrick, S.; Szabo, A.; Yarlagadda, N.; Atwal, D.; Pandey, Y.; Roy, A.; Parikh, R.; Lopez, J.; Thanendrarajan, S.; et al. Clinical implications of loss of bone marrow minimal residual disease negativity in multiple myeloma. Blood Adv. 2022, 6, 808–817. [Google Scholar] [CrossRef]
- Arteche-López, A.; Kreutzman, A.; Alegre, A.; Sanz Martín, P.; Aguado, B.; González-Pardo, M.; Espiño, M.; Villar, L.M.; García Belmonte, D.; de la Cámara, R.; et al. Multiple myeloma patients in long-term complete response after autologous stem cell transplantation express a particular immune signature with potential prognostic implication. Bone Marrow Transplant. 2017, 52, 832–838. [Google Scholar] [CrossRef]
- Perez, C.; Botta, C.; Zabaleta, A.; Puig, N.; Cedena, M.T.; Goicoechea, I.; Alameda, D.; San José-Eneriz, E.; Merino, J.; Rodríguez-Otero, P.; et al. Immunogenomic identification and characterization of granulocytic myeloid-derived suppressor cells in multiple myeloma. Blood 2020, 136, 199–209. [Google Scholar] [CrossRef]
- San Miguel, J.F.; Almeida, J.; Mateo, G.; Blade, J.; Lopez-Berges, C.; Caballero, D.; Hernandez, J.; Moro, M.J.; Fernandez-Calvo, J.; Diaz-Mediavilla, J.; et al. Immunophenotypic evaluation of the plasma cell compartment in multiple myeloma: A tool for comparing the efficacy of different treatment strategies and predicting outcome. Blood 2002, 99, 1853–1856. [Google Scholar] [CrossRef]
- Pessoa de Magalhães, R.J.; Vidriales, M.B.; Paiva, B.; Fernandez-Gimenez, C.; García-Sanz, R.; Mateos, M.V.; Gutierrez, N.C.; Lecrevisse, Q.; Blanco, J.F.; Hernández, J.; et al. Analysis of the immune system of multiple myeloma patients achieving long-term disease control by multidimensional flow cytometry. Haematologica 2013, 98, 79–86. [Google Scholar] [CrossRef]
- Botta, C.; Pérez Ruiz, C.; Goicoechea, I.; Puig, N.; Cedena, M.T.; Cordon, L.; Zabaleta, A.; Burgos, L.; Maia, C.; Rodríguez, S.; et al. Single-cell characterization of the multiple myeloma (MM) immune microenvironment identifies CD27-negative T cells as potential source of tumor-reactive lymphocytes. Blood 2019, 134 (Suppl. S1), 506. [Google Scholar] [CrossRef]
- Koehne, G.; Zeller, W.; Stockschlaeder, M.; Zander, A.R. Phenotype of lymphocyte subsets after autologous peripheral blood stem cell transplantation. Bone Marrow Transplant. 1997, 19, 149–156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dosani, T.; Carlsten, M.; Maric, I.; Landgren, O. The cellular immune system in myelomagenesis: NK cells and T cells in the development of myeloma [corrected] and their uses in immunotherapies. Blood Cancer J. 2015, 5, e306, Correction in Blood Cancer J. 2015, 5, e321. [Google Scholar] [CrossRef] [PubMed]
- Jurisic, V.; Srdic, T.; Konjevic, G.; Markovic, O.; Colovic, M. Clinical stage-depending decrease of NK cell activity in multiple myeloma patients. Med. Oncol. 2007, 24, 312–317. [Google Scholar] [CrossRef] [PubMed]
- García-Ortiz, A.; Rodríguez-García, Y.; Encinas, J.; Maroto-Martín, E.; Castellano, E.; Teixidó, J.; Martínez-López, J. The role of tumor microenvironment in multiple myeloma development and progression. Cancers 2021, 13, 217. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Hideshima, T.; Akiyama, M.; Podar, K.; Yasui, H.; Raje, N.; Kumar, S.; Chauhan, D.; Treon, S.P.; Richardson, P.; et al. Molecular mechanisms whereby immunomodulatory drugs activate natural killer cells: Clinical application. Br. J. Haematol. 2005, 128, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Giles, A.J.; Hutchinson, M.-K.; Sonnemann, H.M.; Jung, J.; Fecci, P.E.; Ratnam, N.M.; Zhang, W.; Song, H.; Bailey, R.; Davis, D.; et al. Dexamethasone-induced immunosuppression: Mechanisms and implications for immunotherapy. J. Immunother. Cancer. 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed]
- Holstein, S.A.; McCarthy, P.L. Immunomodulatory drugs in multiple myeloma: Mechanisms of action and clinical experience. Drugs 2017, 77, 505–520. [Google Scholar] [CrossRef]
- Firer, M.A.; Shapira, M.Y.; Luboshits, G. The impact of induction regimes on immune responses in patients with multiple myeloma. Cancers 2021, 13, 4090. [Google Scholar] [CrossRef]
- Lopes, R.; Caetano, J.; Ferreira, B.; Barahona, F.; Carneiro, E.A.; João, C. The immune microenvironment in multiple myeloma: Friend or foe? Cancers 2021, 13, 625. [Google Scholar] [CrossRef]
- Visram, A.; Dasari, S.; Anderson, E.; Kumar, S.; Kourelis, T.V. Relapsed multiple myeloma demonstrates distinct patterns of immune microenvironment and malignant cell-mediated immunosuppression. Blood Cancer J. 2021, 11, 45. [Google Scholar] [CrossRef]
- Hadjiaggelidou, C.; Katodritou, E. Regulatory T-cells and cultiple cyeloma: Implications in tumor immune biology and treatment. J. Clin. Med. 2021, 10, 4588. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Mathur, R.; Farooque, A.; Kaul, V.; Seema Gupta, S.; Dwarakanath, B.S. T-regulatory cells in tumor progression and therapy. Cancer Manag. Res. 2019, 11, 10731–10747. [Google Scholar] [CrossRef] [PubMed]
- Cencini, E.; Fabbri, A.; Sicuranza, A.; Gozzetti, A.; Bocchia, M. The role of tumor-associated macrophages in hematologic malignancies. Cancers 2021, 13, 3597. [Google Scholar] [CrossRef] [PubMed]
- Mina, R.; Belotti, A.; Petrucci, M.T.; Zambello, R.; Capra, A.; Di Lullo, G.; Ronconi, S.; Pescosta, N.; Grasso, M.; Monaco, F.; et al. Bortezomib-dexamethasone as maintenance therapy or early retreatment at biochemical relapse versus observation in relapsed/refractory multiple myeloma patients: A randomized phase II study. Blood Cancer J. 2020, 10, 58. [Google Scholar] [CrossRef]
| Parameter | CR MRDneg Group (n = 20) | BR Group (n = 20) | RMM Group (n = 12) |
|---|---|---|---|
| Age (years) | 58 (52–70) | 58 (51–69) | 59 (54–67) |
| Gender (male/female %) | 40/60 | 45/55 | 50/50 |
| MM type at diagnosis (%) | |||
| IgA kappa | 0 | 1/20 (5%) | 0 |
| IgA lambda | 4/20 (20%) | 2/20 (10%) | 5/12 (41.5%) |
| IgG kappa | 9/20 (45%) | 13/20 (65%) | 3/12 (25%) |
| IgG lambda | 5/20 (25%) | 3/20 (15%) | 2/12 (16.5%) |
| Kappa | 2/20 (10%) | 1/20 (5%) | 1/12 (8.5%) |
| Lambda | 0 | 0 | 1/12 (8.5%) |
| ISS stage at diagnosis (%) | |||
| I | 9/20 (45%) | 9/20 (45%) | 4/12 (33.5%) |
| II | 7/20 (35%) | 8/20 (40%) | 3/12 (25%) |
| III | 4/20 (20%) | 3/20 (25%) | 5/12 (41.5%) |
| Cytogenetics at diagnosis (%) | |||
| High risk * | 3/20 (20%) | 3/20 (15%) | 6/12 (50%) |
| Non-high risk | 7/20 (50%) | 12/20 (60%) | 3/12 (25%) |
| Not available | 10/20 (30%) | 5/20 (25%) | 3/12 (25%) |
| Number of previous lines of therapy, median (range) | 1 | 1 (1–3) | 2 (1–4) |
| Previous ASCT | 20/20 (100%) | 13/20 (65%) | 11/12 (91.5%) |
| Type of previous therapy | |||
| (%) of patient treated with | |||
| a given regimen: | |||
| VTD | 18/20 (90%) | 12/20 (60%) | 6/12 (50%) |
| VD | 1/20 (5%) | 0 | 3/12 (25%) |
| PAD | 1/20 (5%) | 1/20 (5%) | 2/12 (16.6%) |
| VMP | 0 | 1/20 (5%) | 0 |
| CTD | 0 | 6/20 (30%) | 3/12 (25%) |
| DVD | 0 | 1/20 (5%) | 1/12 (8.3%) |
| Rd | 0 | 0 | 3/12 (25%) |
| VCD | 0 | 0 | 4/12 (33.3%) |
| KD | 0 | 0 | 1/12 (8.3%) |
| PCD | 0 | 0 | 1/12 (8.3%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krzywdzińska, A.; Puła, B.; Szymczak, D.; Milanowska, A.; Szeremet, A.; Jamroziak, K. Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma. J. Clin. Med. 2022, 11, 3722. https://doi.org/10.3390/jcm11133722
Krzywdzińska A, Puła B, Szymczak D, Milanowska A, Szeremet A, Jamroziak K. Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma. Journal of Clinical Medicine. 2022; 11(13):3722. https://doi.org/10.3390/jcm11133722
Chicago/Turabian StyleKrzywdzińska, Agnieszka, Bartosz Puła, Donata Szymczak, Aneta Milanowska, Agnieszka Szeremet, and Krzysztof Jamroziak. 2022. "Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma" Journal of Clinical Medicine 11, no. 13: 3722. https://doi.org/10.3390/jcm11133722
APA StyleKrzywdzińska, A., Puła, B., Szymczak, D., Milanowska, A., Szeremet, A., & Jamroziak, K. (2022). Immunophenotypic Characteristics of Bone Marrow Microenvironment Cellular Composition at the Biochemical Progression of Multiple Myeloma. Journal of Clinical Medicine, 11(13), 3722. https://doi.org/10.3390/jcm11133722






