Solitary Extramedullary Plasmacytoma of the Larynx and Secondary Laryngeal Involvement in Plasma Cell Myeloma: Single-Centre Retrospective Analysis and Systematic Literature Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Retrospective Analysis
2.3. Systematic Review
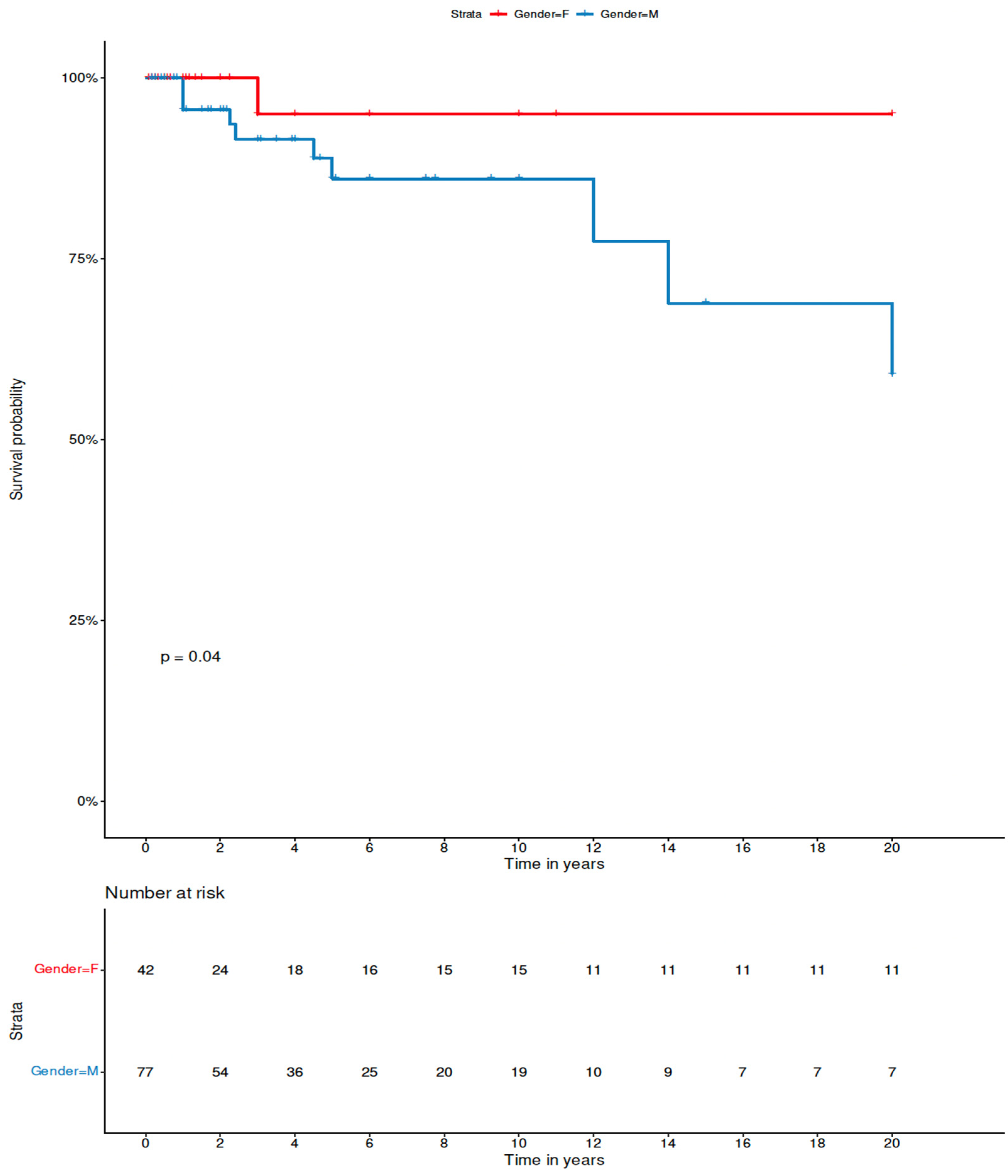
2.4. Statistical Analysis
3. Results
3.1. Results of Retrospective Analysis
3.2. Literature Review
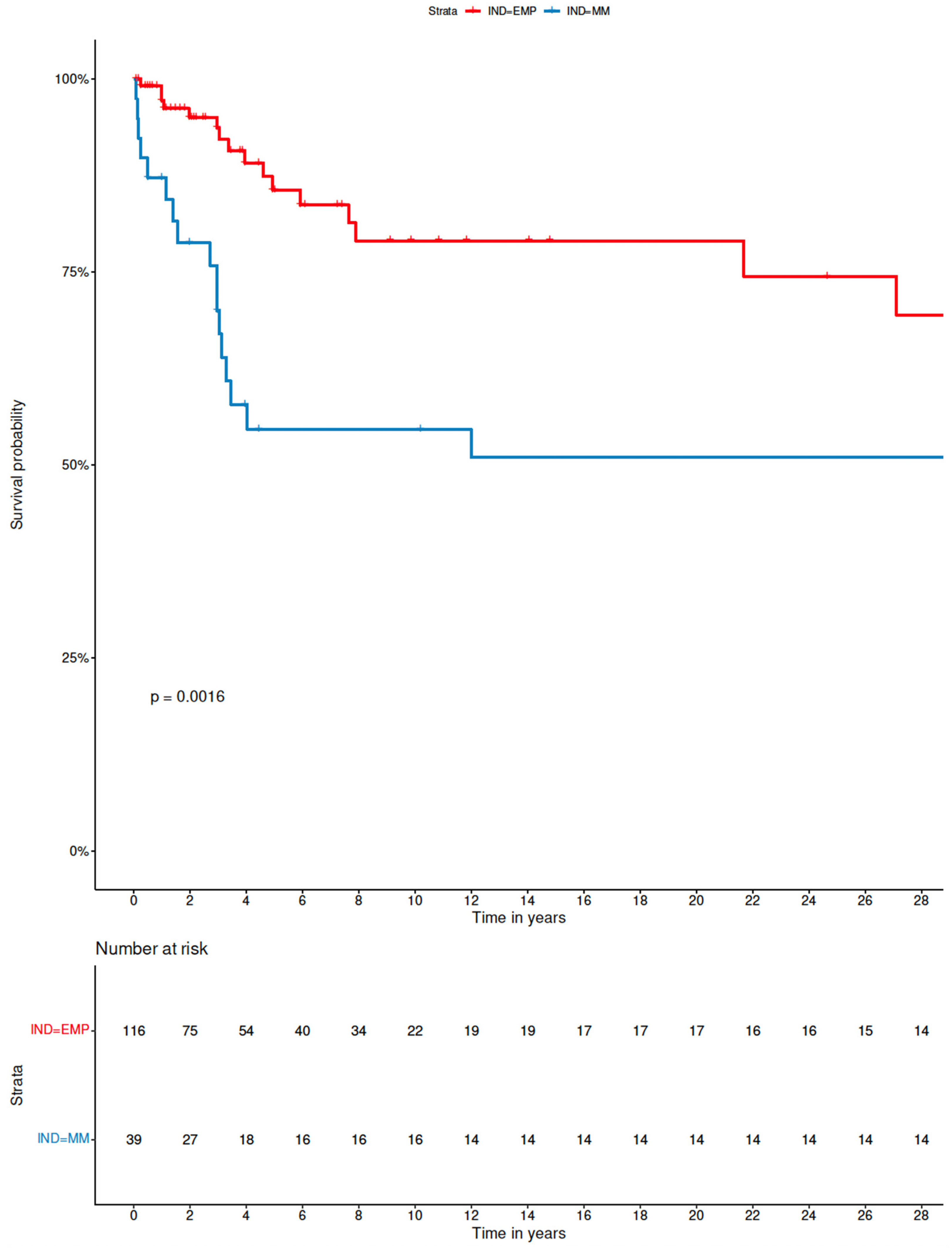
3.3. Analysis of Factors Influencing Outcome of sEMP-L
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- McKenna, R.W.; Kyle, R.A.; Kuehl, W.M.; Grogan, T.M.; Harris, N.L.; Coupland, R.W. Plasma cell neoplasms. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues, 4th ed.; Swerdlow, S.H., Campo, E., Lee Harris, N., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Vardiman, J.W., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2008; pp. 198–213. [Google Scholar]
- Ge, S.; Zhu, G.; Yi, Y. Extramedullary plasmacytoma of the larynx: Literature review and report of a case who subsequently developed acute myeloid leukemia. Oncol. Lett. 2018, 16, 2995–3004. [Google Scholar] [CrossRef] [PubMed]
- Brandt, H.H.; Brockmeier, S.J.; Tetter, N. Solitary extramedullary plasmacytoma of the larynx: A rare cause of dysphonia. BMJ Case Rep. 2020, 13, e234478. [Google Scholar] [CrossRef] [PubMed]
- Nocini, R.; Molteni, G.; Mattiuzzi, C.; Lippi, G. Updates on larynx cancer epidemiology. Chin. J. Cancer Res. 2020, 32, 18–25. [Google Scholar] [CrossRef]
- You, W.S.; Bhuta, S. Myeloma of Laryngeal Cartilage: Literature review and case study. Ear Nose Throat J. 2021, 100, 114–119. [Google Scholar] [CrossRef]
- Doğan, S.; Vural, A.; Kahriman, G.; İmamoğlu, H.; Abdülrezzak, Ü.; Öztürk, M. Non-squamous cell carcinoma diseases of the larynx: Clinical and imaging findings. Braz. J. Otorhinolaryngol. 2020, 86, 468–482. [Google Scholar] [CrossRef]
- Szczepanek, E.; Rzepakowska, A.; Końska, A. Extramedullary plasmacytoma of larynx manifesting as chronic hypertrophic laryngitis. AHP 2022, 3, 75–77. [Google Scholar] [CrossRef]
- Lu, G.; Zhang, Q. Extramedullary plasmacytoma of false vocal cord: Case report. Ear Nose Throat J. 2020. [Google Scholar] [CrossRef]
- Tanrivermis Sayit, A.; Elmali, M.; Gün, S. Evaluation of extramedullary plasmacytoma of the larynx with radiologic and histopathological findings. Radiologia 2020, 64, 69. [Google Scholar] [CrossRef]
- Ong, A.C.; Huh, E.H.; Moreland, A.J.; Rooper, L.M.; Aygun, N.; Akst, L.M.; Best, S.R.; Khan, M.A. Nonepithelial tumors of the larynx: Single-institution 13-year review with radiologic-pathologic correlation. Radiographics 2020, 40, 2011–2028. [Google Scholar] [CrossRef]
- Krebs, S.; Ganly, I.; Ghossein, R.; Yang, J.; Yahalom, J.; Schöder, H. Solitary extramedullary plasmacytoma of the cricoid cartilage-case report. Front. Oncol. 2017, 7, 284. [Google Scholar] [CrossRef]
- Wang, M.; Du, J.; Zou, J.; Liu, S. Extramedullary plasmacytoma of the cricoid cartilage progressing to multiple myeloma: A case report. Oncol. Lett. 2015, 9, 1764–1766. [Google Scholar] [CrossRef][Green Version]
- Haser, G.C.; Su, H.K.; Pitman, M.J.; Khorsandi, A.S. Extramedullary plasmacytoma of the cricoid cartilage with solitary plasmacytoma of the rib. Am. J. Otolaryngol. 2015, 36, 598–600. [Google Scholar] [CrossRef]
- Pino, M.; Farri, F.; Garofalo, P.; Taranto, F.; Toso, A.; Aluffi, P. Extramedullary plasmacytoma of the larynx treated by a surgical endoscopic approach and radiotherapy. Case Rep. Otolaryngol. 2015, 2015, 951583. [Google Scholar] [CrossRef]
- Xing, Y.; Qiu, J.; Zhou, M.L.; Zhou, S.H.; Bao, Y.Y.; Wang, Q.Y.; Zheng, Z.J. Prognostic factors of laryngeal solitary extramedullary plasmacytoma: A case report and review of literature. Int. J. Clin. Exp. Pathol. 2015, 8, 2415–2435. [Google Scholar]
- Abrari, A.; Bakshi, V. Anaplastic: Plasmablastic plasmacytoma of the vocal cord. Indian J. Pathol. Microbiol. 2014, 57, 659–660. [Google Scholar] [CrossRef]
- Loyo, M.; Baras, A.; Akst, L.M. Plasmacytoma of the larynx. Am. J. Otolaryngol. 2013, 34, 172–175. [Google Scholar] [CrossRef]
- Ghatak, S.; Dutta, M.; Kundu, I.; Ganguly, R.P. Primary solitary extramedullary plasmacytoma involving the true vocal cords in a pregnant woman. Tumori 2013, 99, e14–e18. [Google Scholar] [CrossRef]
- Ramírez-Anguiano, J.; Lara-Sánchez, H.; Martínez-Baños, D.; Martínez-Benítez, B. Extramedullary plasmacytoma of the larynx: A case report of subglottic localization. Case Rep. Otolaryngol. 2012, 2012, 437264. [Google Scholar] [CrossRef]
- Pinto, J.A.; Sônego, T.B.; Artico, M.S.; de Farias Aires Leal, C.; Bellotto, S. Extramedullary plasmacytoma of the larynx. Int. Arch. Otorhinolaryngol. 2012, 16, 410–413. [Google Scholar] [CrossRef]
- Kim, K.S.; Yang, H.S.; Park, E.S.; Bae, T.H. Solitary extramedullary plasmacytoma of the apex of arytenoid: Endoscopic, CT and pathologic findings. Clin. Exp. Otorhinolaryngol. 2012, 5, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Ravo, V.; Calvanese, M.G.; Manzo, R.; Cuomo, M.G.; Cammarota, F.; Murino, P.; Muto, P. Solitary plasmacytoma of the larynx treated with radiotherapy: A case report. Tumori 2012, 98, 35e–38e. [Google Scholar] [CrossRef] [PubMed]
- De Zoysa, N.; Sandler, B.; Amonoo-Kuofi, K.; Swamy, R.; Kothari, P.; Mochloulis, G. Extramedullary plasmacytoma of the true vocal fold. Ear Nose Throat J. 2012, 91, E23–E25. [Google Scholar] [PubMed]
- Pichi, B.; Terenzi, V.; Covello, R.; Spriano, G. Cricoid-based extramedullary plasmocytoma. J. Craniofac. Surg. 2011, 22, 2361–2363. [Google Scholar] [CrossRef]
- Zhang, X.L.; Li, D.Q.; Li, J.J.; Li, S.S.; Yang, X.M. Synchronous occurrence of extramedullary plasmacytoma and squamous cell carcinoma in situ in the larynx: A case report. Chin. J. Cancer 2010, 29, 1029–1034. [Google Scholar] [CrossRef]
- González Guijarro, I.; Díez González, L.; Rodriguez Acevedo, N.; Pallas Pallas, E. Extramedullary plasmacytoma of the larynx. A case report. Acta Otorrinolaringol. Esp. 2011, 62, 320–322. [Google Scholar] [CrossRef]
- Pratibha, C.B.; Sreenivas, V.; Babu, M.K.; Rout, P.; Nayar, R.C. Plasmacytoma of larynx—A case report. J. Voice 2009, 23, 735–738. [Google Scholar] [CrossRef]
- Fernández-Aceñero, M.J.; Larach, F.; Ortega-Fernández, C. Non-epithelial lesions of the larynx: Review of the 10-year experience in a tertiary Spanish hospital. Acta Otolaryngol. 2009, 129, 108–112. [Google Scholar] [CrossRef]
- Iseri, M.; Ozturk, M.; Ulubil, S.A. Synchronous presentation of extramedullary plasmacytoma in the nasopharynx and the larynx. Ear Nose Throat J. 2009, 88, E9–E12. [Google Scholar]
- Bilić, M.; Prgomet, D.; Kovac, L.; Topić, I.; Katić, V. Nonepidermoid carcinomas of the larynx—15 years experience in the single institution. Lijec. Vjesn. 2009, 131, 196–198. [Google Scholar]
- Rutherford, K.; Parsons, S.; Cordes, S. Extramedullary plasmacytoma of the larynx in an adolescent: A case report and review of the literature. Ear Nose Throat J. 2009, 88, E1–E7. [Google Scholar]
- Vanan, I.; Redner, A.; Atlas, M.; Marin, L.; Kadkade, P.; Bandovic, J.; Jaffe, E.S. Solitary extramedullary plasmacytoma of the vocal cord in an adolescent. J. Clin. Oncol. 2009, 27, e244–e247. [Google Scholar] [CrossRef]
- Straetmans, J.; Stokroos, R. Extramedullary plasmacytomas in the head and neck region. Eur. Arch. Otorhinolaryngol. 2008, 265, 1417–1423. [Google Scholar] [CrossRef]
- Ozbilen Acar, G.; Yilmaz, S.; Güven Güvenc, M.; Yilmaz, M.; Ozek, H.; Tüziner, N. Isolated extramedullary plasmacytoma of the true vocal cord. J. Otolaryngol. Head Neck Surg. 2008, 37, e129–e132. [Google Scholar]
- Lewis, K.; Thomas, R.; Grace, R.; Moffat, C.; Manjaly, G.; Howlett, D.C. Extramedullary plasmacytomas of the larynx and parapharyngeal space: Imaging and pathologic features. Ear Nose Throat J. 2007, 86, 567–569. [Google Scholar] [CrossRef]
- Kusunoki, T.; Ikeda, K.; Murata, K.; Nishida, S.; Tsubaki, M. Extramedullary plasmacytoma of the larynx: A case report from Japan. Ear Nose Throat J. 2007, 86, 763–764. [Google Scholar] [CrossRef]
- Velez, D.; Hinojar-Gutierrez, A.; Nam-Cha, S.; Acevedo-Barbera, A. Laryngeal plasmacytoma presenting as amyloid tumour: A case report. Eur. Arch. Otorhinolaryngol. 2007, 264, 959–961. [Google Scholar] [CrossRef]
- Nakashima, T.; Matsuda, K.; Haruta, A. Extramedullary plasmacytoma of the larynx. Auris Nasus Larynx 2006, 33, 219–222. [Google Scholar] [CrossRef]
- Mackiewicz-Nartowicz, H.; Garstecka, A.; Betlejewski, S.; Sinkiewicz, A.; Szukalski, J. Szpiczak krtani [Plasmacytoma of the larynx]. Otolaryngol. Pol. 2005, 59, 445–448. [Google Scholar]
- Coskun, H.S.; Er, O.; Soyuer, S.; Altinbas, M.; Eser, B.; Karahacioglu, E.; Altuntas, F. Solitary plasmacytoma: Experiences from Central Anatolia. Ir. J. Med. Sci. 2005, 174, 33–36. [Google Scholar] [CrossRef]
- Chao, M.W.; Gibbs, P.; Wirth, A.; Quong, G.; Guiney, M.J.; Liew, K.H. Radiotherapy in the management of solitary extramedullary plasmacytoma. Int. Med. J. 2005, 35, 211–215. [Google Scholar] [CrossRef]
- Sakiyama, S.; Kondo, K.; Mitsuteru, Y.; Takizawa, H.; Kenzaki, K.; Miyoshi, T.; Abe, M.; Wakatsuki, S.; Monden, Y. Extramedullary plasmacytoma immunoglobulin D (lambda) in the chest wall and the subglottic region. J. Thorac. Cardiovasc. Surg. 2005, 129, 1168–1169. [Google Scholar] [CrossRef][Green Version]
- Yavas, O.; Altundag, K.; Sungur, A. Extramedullary plasmacytoma of nasopharynx and larynx: Synchronous presentation. Am. J. Hematol. 2004, 75, 264–265. [Google Scholar] [CrossRef]
- Michalaki, V.J.; Hall, J.; Henk, J.M.; Nutting, C.M.; Harrington, K.J. Definitive radiotherapy for extramedullary plasmacytomas of the head and neck. Br. J. Radiol. 2003, 76, 738–741. [Google Scholar] [CrossRef]
- Kamijo, T.; Inagi, K.; Nakajima, M.; Motoori, T.; Tadokoro, K.; Nishiyama, S. A case of extramedullary plasmacytoma of the larynx. Acta Otolaryngol. Suppl. 2002, 547, 104–106. [Google Scholar] [CrossRef]
- Takeda, Y.; Ogawa, A.; Akagi, H.; Yuen, K.; Nishizaki, K.; Hattori, H. A case report of extramedullary plasmacytoma in the trachea and larynx. Otolaryngol. Head Neck Surg. 2002, 74, 383–387. [Google Scholar]
- Soni, N.K.; Trivedi, K.A.; Kumar, A.; Prajapati, J.A.; Goswami, J.V.; Patel, J.J.; Patel, D.D. Solitary extramedullary plasmacytoma - larynx. Indian J. Otolaryngol. Head Neck Surg. 2002, 54, 309–310. [Google Scholar] [CrossRef]
- Strojan, P.; Soba, E.; Lamovec, J.; Munda, A. Extramedullary plasmacytoma: Clinical and histopathologic study. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 692–701. [Google Scholar] [CrossRef]
- Maheshwari, G.K.; Baboo, H.A.; Gopal, U.; Shah, N.M. Extramedullary plasmacytoma of the larynx: A case report. J. Indian Med. Assoc. 2001, 99, 267–268. [Google Scholar]
- Nagasaka, T.; Lai, R.; Kuno, K.; Nakashima, T.; Nakashima, N. Localized amyloidosis and extramedullary plasmacytoma involving the larynx of a child. Hum. Pathol. 2001, 32, 132–134. [Google Scholar] [CrossRef]
- Abe, K.; Watanabe, H.; Etoh, T.; Akazawa, F.; Tarumura, K.; Tokushige, I. Two cases of extramedullary plasmacytoma. J. Hiroshima Med. Ass. 2001, 54, 750–753. [Google Scholar]
- Rakover, Y.; Bennett, M.; David, R.; Rosen, G. Isolated extramedullary plasmacytoma of the true vocal fold. J. Laryngol. Otol. 2000, 114, 540–542. [Google Scholar] [CrossRef] [PubMed]
- Rosenblatt, E.; Zidan, J.; Ben-Izhak, O.; Kuten, A. Radiotherapy for plasmocytoma of the larynx. Isr. Med. Assoc. J. 1999, 1, 125–126. [Google Scholar]
- Furukido, K.; Kawamoto, H.; Nagasawa, A.; Iwamoto, T.; Ueda, N.; Sumii, M. Plasmacytoma of the larynx—A case report. Pract. Otol. 1999, 102, 121–126. [Google Scholar] [CrossRef]
- Yasumatsu, R.; Shinzato, Y.; Tanimura, A.; Tomita, K.; Komiyama, S. Extramedullary plasmacytoma of the larynx—Report of a case. Jpn. J. Cancer Clin. 1999, 45, 1224–1228. [Google Scholar]
- Alexiou, C.; Kau, R.J.; Dietzfelbinger, H.; Kremer, M.; Spiess, J.C.; Schratzenstaller, B.; Arnold, W. Extramedullary plasmacytoma: Tumor occurrence and therapeutic concepts. Cancer 1999, 85, 2305–2314. [Google Scholar] [CrossRef]
- Hotz, M.A.; Schwaab, G.; Bosq, J.; Munck, J.N. Extramedullary solitary plasmacytoma of the head and neck. A clinicopathological study. Ann. Otol. Rhinol. Laryngol. 1999, 108, 495–500. [Google Scholar] [CrossRef]
- Nowak-Sadzikowska, J.; Weiss, M. Extramedullary plasmacytoma of the larynx. Analysis of 5 cases. Eur. J. Cancer 1998, 34, 1468. [Google Scholar] [CrossRef]
- Weiss, M.; Kowalska, T.; Walasek, T.; Reinfuss, M. Extramedullary plasmocytoma of the larynx. Analysis of four cases. Otolaryngol. Pol. 1998, 52, 605–606. [Google Scholar]
- Welsh, J.; Westra, W.H.; Eisele, D.; Hogan, R.; Lee, D.J. Solitary plasmacytoma of the epiglottis: A case report and review of the literature. J. Laryngol. Otol. 1998, 112, 174–176. [Google Scholar] [CrossRef]
- Bhattacharya, A.K.; Han, K.; Baredes, S. Extramedullary plasmacytoma of the head and neck associated with the human immunodeficiency virus. Ear Nose Throat J. 1998, 77, 61–62. [Google Scholar] [CrossRef]
- Sulzner, S.E.; Amdur, R.J.; Weider, D.J. Extramedullary and neck plasmacytoma of the head. Am. J. Otolaryngol. 1998, 19, 203–208. [Google Scholar] [CrossRef]
- Susnerwala, S.S.; Shanks, J.H.; Banerjee, S.S.; Scarffe, J.H.; Farrington, W.T.; Slevin, N.J. Extramedullary plasmacytoma of the head and neck region: Clinicopathological correlation in 25 cases. Br. J. Cancer 1997, 75, 921–927. [Google Scholar] [CrossRef]
- Han, S.; Kitamura, H.; Takagita, S.; Asato, R.; Iwahashi, Y.; Maetani, T. Multiple extramedullary plasmacytoma arising in the head and neck region; a case report. Pract. Otol. 1996, 89, 1377–1381. [Google Scholar] [CrossRef]
- Rodriguez-de-Velasquez, A.; Weber, A.L.; Montgomery, W. Extramedullary laryngeal plasmacytoma. Ann. Otol. Rhinol. Laryngol. 1996, 105, 483–486. [Google Scholar] [CrossRef]
- Abe, K.; Kiminaga, C.; Igarashi, M.; Miyata, M.; Morita, M.; Kitamura, K. Five cases with extramedullary plasmacytoma. Head Neck Cancer 1995, 21, 230–235. [Google Scholar] [CrossRef][Green Version]
- Narozny, W.; Stankiewicz, C.; Mikaszewski, B.; Kowalska, B. Extramedullary plasmacytomas of the larynx. Otolaryngol. Pol. 1995, 49, 265–268. [Google Scholar]
- Zbären, P.; Zimmermann, A. Solitary plasmocytoma of the larynx. ORL J. Otorhinolaryngol. Relat. Spec. 1995, 57, 50–53. [Google Scholar] [CrossRef]
- Rolins, H.; Levin, M.; Goldberg, S.; Mody, K.; Frank, J. Solitary extramedullary plasmacytoma of the epiglottis: A case report and review of the literature. Otolaryngol. Head Neck Surg. 1995, 112, 754–757. [Google Scholar] [CrossRef]
- Mochimatsu, I.; Tsukuda, M.; Sawaki, S.; Nakatani, Y. Extramedullary plasmacytoma of the larynx. J. Laryngol. Otol. 1993, 107, 1049–1051. [Google Scholar] [CrossRef]
- Weissman, J.L.; Myers, J.N.; Kapadia, S.B. Extramedullary plasmacytoma of the larynx. Am. J. Otolaryngol. 1993, 14, 128–131. [Google Scholar] [CrossRef]
- Jankowska-Kuc, M.; Rostkowska, B.; Jaworska, M. Multifocal extramedullary plasmocytoma of larynx. Otolaryngol. Pol. 1993, 47, 163–166. [Google Scholar] [PubMed]
- Barbu, R.R.; Khan, A.; Port, J.L.; Abramson, A.; Gartenhaus, W.S. Case report: Extramedullary plasmacytoma of the larynx. Comput. Med. Imaging Graph. 1992, 16, 359–361. [Google Scholar] [CrossRef]
- Tateno, K.; Kawashima, M.; Matuura, M.; Maehara, Y.; Sakaino, K.; Simizu, R.; Satake, B.; Makino, S.; Matuura, S.; Shimano, S. Four cases of an extramedullary plasmacytoma of the head and neck. J. Cancer Clin. 1990, 36, 81–86. [Google Scholar]
- Kost, K.M. Plasmacytomas of the larynx. J. Otolaryngol. 1990, 19, 141–146. [Google Scholar] [PubMed]
- Agatsuma, Y.; Iwata, K.; Sonobe, N.; Okada, T. A case of extramedullary plasmacytoma of the larynx. Kochi J. Med. 1989, 16, 63–69. [Google Scholar]
- Gambino, D.R. Pathologic quiz case 2. Extramedullary plasmacytoma. Arch. Otolaryngol. 1988, 114, 92–93, 95. [Google Scholar]
- Gaffney, C.C.; Dawes, P.J.; Jackson, D. Plasmacytoma of the head and neck. Clin. Radiol. 1987, 38, 385–388. [Google Scholar] [CrossRef]
- Gadomski, S.P.; Zwillenberg, D.; Choi, H.Y. Non-epidermoid carcinoma of the larynx: The Thomas Jefferson University experience. Otolaryngol. Head Neck Surg. 1986, 95, 558–565. [Google Scholar] [CrossRef]
- Burke, W.A.; Merritt, C.C.; Briggaman, R.A. Disseminated extramedullary plasmacytomas. J. Am. Acad. Dermatol. 1986, 14, 335–339. [Google Scholar] [CrossRef]
- Gormley, P.K.; Primrose, W.J.; Bharucha, H. Subglottic plasmacytoma of the larynx: An acute presentation. J. Laryngol. Otol. 1985, 99, 925–929. [Google Scholar] [CrossRef]
- Maniglia, A.J.; Xue, J.W. Plasmacytoma of the larynx. Laryngoscope 1983, 93, 741–744. [Google Scholar] [CrossRef]
- Ferlito, A.; Carbone, A.; Volpe, R.; Recher, G. Late occurrence of IgD myeloma in plasmacytoma of nasal cavity, cervical lymph node and larynx. J. Laryngol. Otol. 1982, 96, 759–766. [Google Scholar] [CrossRef]
- Bjelkenkrantz, K.; Lundgren, J.; Olofsson, J. Extramedullary plasmacytoma of the larynx. J. Otolaryngol. 1981, 10, 28–34. [Google Scholar]
- Bush, S.E.; Goffinet, D.R.; Bagshaw, M.A. Extramedullary plasmacytoma of the head and neck. Radiology 1981, 140, 801–805. [Google Scholar] [CrossRef]
- Endo, Y.; Saito, R.; Ukita, J.; Sezaki, T.; Kanataki, K.; Miki, M. Three cases of plasmacytoma in otolaryngological region. ORL 1979, 20, 286–293. [Google Scholar]
- Singh, B.; Lahiri, A.K.; Kakar, P.K. Extramedullary plasmacytoma. J. Laryngol. Otol. 1979, 93, 1239–1244. [Google Scholar] [CrossRef]
- Woodruff, R.K.; Whittle, J.M.; Malpas, J.S. Solitary plasmacytoma. I: Extramedullary soft tissue plasmacytoma. Cancer 1979, 43, 2340–2343. [Google Scholar] [CrossRef]
- Cohen, S.R.; Landing, B.H.; Isaacs, H.; King, K.K.; Hanson, V. Solitary plasmacytoma of the larynx and upper trachea associated with systemic lupus erythematosus. Ann. Otol. Rhinol. Laryngol. Suppl. 1978, 87, 11–14. [Google Scholar] [CrossRef]
- Pahor, A.L. Plasmacytoma of the larynx. J. Laryngol. Otol. 1978, 92, 223–232. [Google Scholar] [CrossRef]
- Gorenstein, A.; Neel, H.B.; Devine, K.D.; Weiland, L.H. Solitary extramedullary plasmacytoma of the larynx. Arch. Otolaryngol. 1977, 103, 159–161. [Google Scholar] [CrossRef]
- Petrovich, Z.; Fishkin, B.; Hittle, R.E.; Acquarelli, M.; Barton, R. Extramedullary plasmacytoma of the upper respiratory passages. Int. J. Radiat. Oncol. Biol. Phys. 1977, 2, 723–730. [Google Scholar] [CrossRef]
- Muller, S.P.; Fisher, G.H. Pathologic quiz case 1: Extramedullary plasmacytoma of the larynx. Arch. Otolaryngol. 1976, 102, 442–444. [Google Scholar]
- Horiuchi, M.; Murakami, Y.; Nameki, H.; Nishida, K.; Sakuma, A. Extramedullary plasmacytoma of the larynx. Report of a case. Jpn. J. Cancer Clin. 1973, 19, 953–958. [Google Scholar]
- Booth, J.B.; Cheesman, A.D.; Vincenti, N.H. Extramedullary plasmacytomata of the upper respiratory tract. Ann. Otol. Rhinol. Laryngol. 1973, 82, 709. [Google Scholar] [CrossRef]
- Stone, H.B.; Cole, T.B. Extramedullary plasmacytomas of the head and neck. South Med. J. 1971, 64, 1386–1388. [Google Scholar] [CrossRef]
- Touma, U.B. Extramedullary plasmocytoma of the head and neck. J. Laryngol. Otol. 1971, 85, 125. [Google Scholar] [CrossRef]
- Kakar, P.K.; Gupta, K.R.; Saharia, P.S. Solitary plasmacytoma larynx. J. Laryngol. Otol. 1970, 84, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Poole, A.G.; Marchetta, F.C. Extramedullary plasmacytoma of the head and neck. Cancer 1968, 22, 14–21. [Google Scholar] [CrossRef]
- Nabar, B.V. Plasmacytoma of the upper respiratory tract. J. Laryngol Otol. 1968, 82, 657. [Google Scholar] [CrossRef] [PubMed]
- Kraska, A. A case of isolated myeloma of the larynx. Otolaryngol. Pol. 1967, 21, 351–353. [Google Scholar]
- Studencki, E. On the problem of roentgenotherapeutic treatment of solitary plasmocytic reticulomas of the larynx. Otolaryngol. Pol. 1966, 20, 565–567. [Google Scholar]
- Todd, I.D. Treatment of solitary plasmacytoma. Clin. Radiol. 1965, 16, 395–399. [Google Scholar] [CrossRef]
- Webb, H.E.; Harrison, E.G.; Masson, J.K.; Remine, W.H. Solitary extramedullary myeloma (plasmacytoma) of the upper part of the respiratory tract and oropharynx. Cancer 1962, 15, 1142–1155. [Google Scholar] [CrossRef]
- Krotz, R.C.; Ritterhoff, R. Sacroma of the larynx. Ann. Otol. Rhinol. Laryngol. 1961, 70, 239. [Google Scholar] [CrossRef]
- Clark, G.O. Plasmocytoma of larynx. J. Laryngol. Otol. 1957, 71, 486–488. [Google Scholar] [CrossRef]
- Carson, C.P.; Ackerman, L.V.; Maltby, J.D. Plasma cell myeloma: A clinical, pathologic and roentgenologic review of 90 cases. Am. J. Clin. Pathol. 1955, 25, 849. [Google Scholar] [CrossRef]
- Korkis, F.B. Plasma cell tumours of the upper respiratory tract. J. Laryngol. Otol. 1954, 68, 517. [Google Scholar] [CrossRef]
- Priest, R.E. Extramedullary plasma cell tumors of the nose, pharynx and larynx: A case report. Laryngoscope 1952, 62, 277–283. [Google Scholar] [CrossRef]
- Ewing, M.R.; Foote, F.W., Jr. Plasma-cell tumors of the mouth and upper air passages. Cancer 1952, 5, 499–513. [Google Scholar] [CrossRef][Green Version]
- Costen, J.B. Plasmocytoma: A case with original lesion of the epiglottis and metastasis to the tibia. Laryngoscope 1951, 61, 266–270. [Google Scholar] [CrossRef]
- Mattick, W.L. Plasmocytoma: An unusual case with progressive involvement of Waldyer’s ring and the larynx, bronchus and pleura. Arch. Otolaryngol. 1950, 51, 263–271. [Google Scholar] [CrossRef]
- Rawson, A.J.; Eyler, P.W.; Horn, R.C., Jr. Plasma cell tumors of the upper respiratory tract; a clinico-pathologic study with emphasis on criteria for histologic diagnosis. Am. J. Pathol. 1950, 26, 445–461. [Google Scholar] [PubMed]
- Stout, A.P.; Kenney, F.R. Primary plasma-cell tumors of the upper air passages and oral cavity. Cancer 1949, 2, 261–278. [Google Scholar] [CrossRef]
- Hodge, G.E.; Wilson, T. Extramedullary plasmocytoma of the larynx. Can. Med. Assoc. J. 1948, 59, 165. [Google Scholar] [PubMed]
- Lumb, G.; Prossor, T.M. Plasma cell tumours. J. Bone Jt. Surg. Br. 1948, 30, 124–152. [Google Scholar] [CrossRef][Green Version]
- Clerf, C.H. Sarcoma of the larynx; report of eight cases. Arch. Otolaryngol. 1946, 44, 517. [Google Scholar] [CrossRef]
- Jaeger, E. Das extramedullare Plasmocytom. Ztschr. Krebsforsch. 1942, 52, 349–383. [Google Scholar] [CrossRef]
- Haven, F.Z.; Parkhill, E.M. Tumours of the larynx other than squamous cell epithelioma. Arch. Otolaryngol. 1941, 34, 1113. [Google Scholar]
- Ringertz, N. Pharyngeal and laryngeal plasmacytomas. Acta Otolaryngol. 1938, 26, 386–387. [Google Scholar]
- Heindl, A. Fall von Plasmozytom. Monatschr. Ohrenh. 1933, 67, 878–880. [Google Scholar]
- Blacklock, J.W.; McCartney, C.J. Plasmacytoma of the naso-pharynx. Pathol. Bacteriol. 1932, 35, 69. [Google Scholar] [CrossRef]
- Wachter, H. Ein Fall von multiplem Plasmazytom der oberen Luftwege. Arch. Laryng. Rhin. 1914, 28, 69–73. [Google Scholar]
- Steinke, K.V.; Schneider, B.K.; Welkoborsky, H.J. Rare Differential Diagnosis of Dyspnea: Extramedullary Plasmocytoma (EMP) of the larynx-case report and review of the latest literature of laryngeal EMP and laryngeal involvement of multiple myeloma. Case Rep. Otolaryngol. 2019, 2019, 5654014. [Google Scholar] [CrossRef]
- Allegra, E.; Marino, N.; Modica, D.; Emmanuele, C.; Saita, V. Primary laryngeal localization of multiple myeloma: A case report. Mol. Clin. Oncol. 2017, 6, 154–156. [Google Scholar] [CrossRef][Green Version]
- Nochikattil, S.K.; Iype, E.M.; Ramrao, S.K.; Nair, P.; Thomas, S.A. Case of multiple myeloma: Mimicking carcinoma larynx. Indian J. Otolaryngol. Head Neck Surg. 2016, 68, 534–536. [Google Scholar] [CrossRef][Green Version]
- Gan, Y.J.; Chopra, A.; Kanagalingam, J. Subglottic extramedullary plasmacytoma with light chain multiple myeloma masquerading as adult-onset asthma. J. Voice 2014, 28, 394.e1–394.e4. [Google Scholar] [CrossRef]
- Mitchell, H.K.; Garas, G.; Mazarakis, N.; McGlashan, J. Extramedullary relapse of multiple myeloma in the thyroid cartilage. BMJ Case Rep. 2013, 2013, bcr2013200689. [Google Scholar] [CrossRef]
- Alherabi, A.Z.; Khan, A.M.; Marglani, O.A.; Abdulfattah, T.A. Multiple myeloma presenting as dysphagia. Saudi Med. J. 2013, 34, 648–650. [Google Scholar]
- Grobman, A.B.; Vivero, R.J.; Campuzano-Zuluaga, G.; Ganjei-Azar, P.; Rosow, D.E. Laryngeal involvement of multiple myeloma. Case Rep. Oncol. Med. 2012, 2012, 257814. [Google Scholar] [CrossRef]
- Kumar, N.; Pandey, A.N.; Kashyap, R.; Agarwal, G. Extramedullary relapse in a case of multiple myeloma involving the thyroid cartilage: Case report and review of literature. Indian J. Surg. Oncol. 2011, 2, 313–315. [Google Scholar] [CrossRef]
- Öztürk, M.; Mavili, E.; Gorkem, S.B.; Cagli, S.; Yüce, I. An unusual cause of dyspnea: Myelomatous involvement of cricoid cartilage: A case report. Neuroradiol. J. 2008, 21, 584–586. [Google Scholar] [CrossRef]
- Dispenza, F.; Sciandra, D.; Saraniti, C. Thyroid cartilage involvement in patient affected by IgA multiple myeloma: Case report. Auris Nasus Larynx 2008, 35, 288–290. [Google Scholar] [CrossRef]
- Shimada, T.; Matsui, M.; Ikebuchi, K.; Nakano, H.; Shinomiya, T.; Nakai, S.; Hisa, Y. Multiple myeloma involving the thyroid cartilage. Auris Nasus Larynx 2007, 34, 277–279. [Google Scholar] [CrossRef]
- Nampoothiri, M.P.; Kumar, K.P.S.; Sajina, V.K. Multiple myeloma presenting as stridor: A case report. Indian J. Otolaryngol. Head Neck Surg. 2006, 58, 111–112. [Google Scholar] [CrossRef]
- Luppino, F.S.; Pameijer, F.A.; Balm, A.J.M. Radiology quiz case 2. Laryngeal plasmacytoma in a patient with known multiple myeloma (MM). Arch. Otolaryngol. Head Neck Surg. 2005, 131, 74, 77–78. [Google Scholar] [CrossRef]
- Gross, M.; Eliashar, R.; Petrova, P.; Goldfarb, A.; Sichel, J.Y. Neck mass as primary manifestation of multiple myeloma originating in the thyroid cartilage. Otolaryngol. Head Neck Surg. 2002, 126, 326–328. [Google Scholar] [CrossRef]
- Sosna, J.; Slasky, B.S.; Paltiel, O.; Pizov, G.; Libson, E. Multiple myeloma involving the thyroid cartilage: Case report. Am. J. Neuroradiol. 2002, 23, 316–318. [Google Scholar]
- Aslan, I.; Yenice, H.; Baserer, N. An indolent course of multiple myeloma mimicking a solitary thyroid cartilage plasmacytoma. Eur. Arch. Otorhinolaryngol. 2002, 259, 84–86. [Google Scholar] [CrossRef]
- Saad, R.; Raab, S.; Liu, Y.; Pollice, P.; Silverman, J.F. Plasmacytoma of the larynx diagnosed by fine-needle aspiration cytology: A case report. Diagn. Cytopathol. 2001, 24, 408–411. [Google Scholar] [CrossRef] [PubMed]
- Uppal, H.S.; Harrison, P. Extramedullary plasmacytoma of the larynx presenting with upper airway obstruction in a patient with long-standing IgD myeloma. J. Laryngol. Otol. 2001, 115, 745–746. [Google Scholar] [CrossRef] [PubMed]
- Nofsinger, Y.C.; Mirza, N.; Rowan, P.T.; Lanza, D.; Weinstein, G. Head and neck manifestations of plasma cell neoplasms. Laryngoscope 1997, 107, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Gabryś, K.; Mazur, G.; Rabczyński, J.; Krecicki, T. Involvement of the larynx during the course of plasmocytoma. Pol. Arch. Med. Wewn. 1996, 95, 73–77. [Google Scholar] [PubMed]
- Van Dyke, C.W.; Masaryk, T.J.; Lavertu, P. Multiple myeloma involving the thyroid cartilage. Am. J. Neuroradiol. 1996, 17, 570–572. [Google Scholar]
- Rabinov, R.C.; Castro, D.J.; Calcaterra, T.C.; Fu, Y.S.; Anderson, C.T.; Bates, E.; Soudant, J.; Saxton, R. Subglottic plasmacytoma: The use of jet ventilation and contact Nd: YAG laser for tissue diagnosis. J. Clin. Laser Med. Surg. 1993, 11, 131–134. [Google Scholar] [CrossRef]
- Werner, J.A.; Lippert, B.M.; Schmidt, D.; Rudert, H. Subglottic metastasis of multiple myeloma. Case report and review of the literature of laryngeal plasmacytoma. HNO 1991, 39, 405–409. [Google Scholar]
- Georghiou, P.A.; Hogg, M.L. Immunoglobulin A myeloma presenting with laryngeal obstruction. Med. J. Aust. 1988, 149, 447–449. [Google Scholar] [CrossRef]
- Jones, N.S.; Kenyon, G.S.; Mahy, N. Multiple myeloma in bony metaplasia of the cricoid cartilage (a rare cause of laryngeal obstruction). J. Laryngol. Otol. 1987, 101, 1301–1305. [Google Scholar] [CrossRef]
- East, D. Laryngeal involvement in multiple myeloma. J. Laryngol. Otol. 1978, 92, 61–65. [Google Scholar] [CrossRef]
- Pirkey, W.P. Metastatic multiple myeloma to the larynx. Laryngoscope 1957, 67, 85–87. [Google Scholar] [CrossRef]
- Shaw, H. Tumours of the Larynx. In Diseases of the Ear, Nose and Throat, 3rd ed.; Scott Brown, D., Ed.; Butterworth: London, UK, 1972; Volume 2, pp. 375–459. [Google Scholar]
- Holler, A.; Cicha, I.; Eckstein, M.; Haderlein, M.; Pöttler, M.; Rappl, A.; Iro, H.; Alexiou, C. Extramedullary plasmacytoma: Tumor occurrence and therapeutic concepts—A follow-up. Cancer Med. 2022, 1–13. [Google Scholar] [CrossRef]
- Bladé, J.; Beksac, M.; Caers, J.; Jurczyszyn, A.; von Lilienfeld-Toal, M.; Moreau, P.; Rasche, L.; Rosiñol, L.; Usmani, S.Z.; Zamagni, E.; et al. Extramedullary disease in multiple myeloma: A systematic literature review. Blood Cancer J. 2022, 12, 45. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Moreau, P.; Terpos, E.; Mateos, M.V.; Zweegman, S.; Cook, G.; Delforge, M.; Hájek, R.; Schjesvold, F.; Cavo, M.; et al. Multiple myeloma: EHA-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2021, 32, 309–322. [Google Scholar] [CrossRef]
- Żurek, M.; Jasak, K.; Niemczyk, K. Artificial Intelligence in laryngeal endoscopy: Systematic review and meta-analysis. J. Clin. Med. 2022, 11, 2752. [Google Scholar] [CrossRef]
- Galli, J.; Settimi, S.; Mele, D.A.; Salvati, A.; Schiavi, E.; Parrilla, C.; Paludetti, G. Role of narrow band imaging technology in the diagnosis and follow up of laryngeal lesions: Assessment of diagnostic accuracy and reliability in a large patient cohort. J. Clin. Med. 2021, 10, 1224. [Google Scholar] [CrossRef]
- Moreno, D.F.; Clapés, V.; Soler, J.A.; González-Montes, Y.; Gironella, M.; Motlló, C.; Granell, M.; Abella, E.; García-Pintos, M.; García-Guiñón, A.; et al. Real-world evidence of daratumumab monotherapy in relapsed/refractory multiple myeloma patients and efficacy on soft-tissue plasmacytomas. Clin. Lymphoma Myeloma Leuk. 2022, 22, 635–642. [Google Scholar] [CrossRef]
- Li, W.; Liu, M.; Yuan, T.; Yan, L.; Cui, R.; Deng, Q. Efficacy and follow-up of humanized anti-BCMA CAR-T cell therapy in relapsed/refractory multiple myeloma patients with extramedullary-extraosseous, extramedullary-bone related, and without extramedullary disease. Hematol. Oncol. 2022, 40, 223–232. [Google Scholar] [CrossRef]
- Varettoni, M.; Corso, A.; Pica, G.; Mangiacavalli, S.; Pascutto, C.; Lazzarino, M. Incidence, presenting features and outcome of extramedullary disease in multiple myeloma: A longitudinal study on 1003 consecutive patients. Ann. Oncol. 2010, 21, 325–330. [Google Scholar] [CrossRef]
- Gagelmann, N.; Eikema, D.J.; Iacobelli, S.; Koster, L.; Nahi, H.; Stoppa, A.M.; Masszi, T.; Caillot, D.; Lenhoff, S.; Udvardy, M.; et al. Impact of extramedullary disease in patients with newly diagnosed multiple myeloma undergoing autologous stem cell transplantation: A study from the Chronic Malignancies Working Party of the EBMT. Haematologica 2018, 103, 890–897. [Google Scholar] [CrossRef]
- Smedby, K.E.; Vajdic, C.M.; Falster, M.; Engels, E.A.; Martínez-Maza, O.; Turner, J.; Hjalgrim, H.; Vineis, P.; Costantini, A.S.; Bracci, P.M.; et al. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: A pooled analysis within the InterLymph Consortium. Blood 2008, 111, 4029–4038. [Google Scholar] [CrossRef]
- Sukswai, N.; Lyapichev, K.; Khoury, J.D.; Medeiros, L.J. Diffuse large B-cell lymphoma variants: An update. Pathology 2020, 52, 53–67. [Google Scholar] [CrossRef]
- Di Rocco, A.; Petrucci, L.; Assanto, G.M.; Martelli, M.; Pulsoni, A. Extranodal Marginal Zone Lymphoma: Pathogenesis, Diagnosis and Treatment. Cancers 2022, 14, 1742. [Google Scholar] [CrossRef]
- Biernat, M.M.; Wróbel, T. Bacterial infection and non-Hodgkin B-cell lymphoma: Interactions between pathogen, host and the tumor environment. Int. J. Mol. Sci. 2021, 22, 7372. [Google Scholar] [CrossRef]
- Odell, E.; Eckel, H.E.; Simo, R.; Quer, M.; Paleri, V.; Klussmann, J.P.; Remacle, M.; Sjögren, E.; Piazza, C. European Laryngological Society position paper on laryngeal dysplasia Part I: Aetiology and pathological classification. Eur. Arch. Otorhinolaryngol. 2021, 278, 1717–1722. [Google Scholar] [CrossRef]
- Desantis, V.; Savino, F.D.; Scaringella, A.; Potenza, M.A.; Nacci, C.; Frassanito, M.A.; Vacca, A.; Montagnani, M. The leading role of the immune microenvironment in multiple myeloma: A new target with a great prognostic and clinical value. J. Clin. Med. 2022, 11, 2513. [Google Scholar] [CrossRef]
- Hadjiaggelidou, C.; Katodritou, E. Regulatory T-cells and multiple myeloma: Implications in tumor immune biology and treatment. J. Clin. Med. 2021, 10, 4588. [Google Scholar] [CrossRef]
- Stessman, H.A.F.; Mansoor, A.; Zhan, F.; Janz, S.; Linden, M.A.; Baughn, L.B.; Van Ness, B. Reduced CXCR4 expression is associated with extramedullary disease in a mouse model of myeloma and predicts poor survival in multiple myeloma patients treated with bortezomib. Leukemia 2013, 27, 2075–2077. [Google Scholar] [CrossRef]
- Bila, J.; Katodritou, E.; Guenova, M.; Basic-Kinda, S.; Coriu, D.; Dapcevic, M.; Ibricevic-Balic, L.; Ivanaj, A.; Karanfilski, O.; Zver, S.; et al. Bone marrow microenvironment interplay and current clinical practice in multiple myeloma: A review of the Balkan myeloma study group. J. Clin. Med. 2021, 10, 3940. [Google Scholar] [CrossRef]
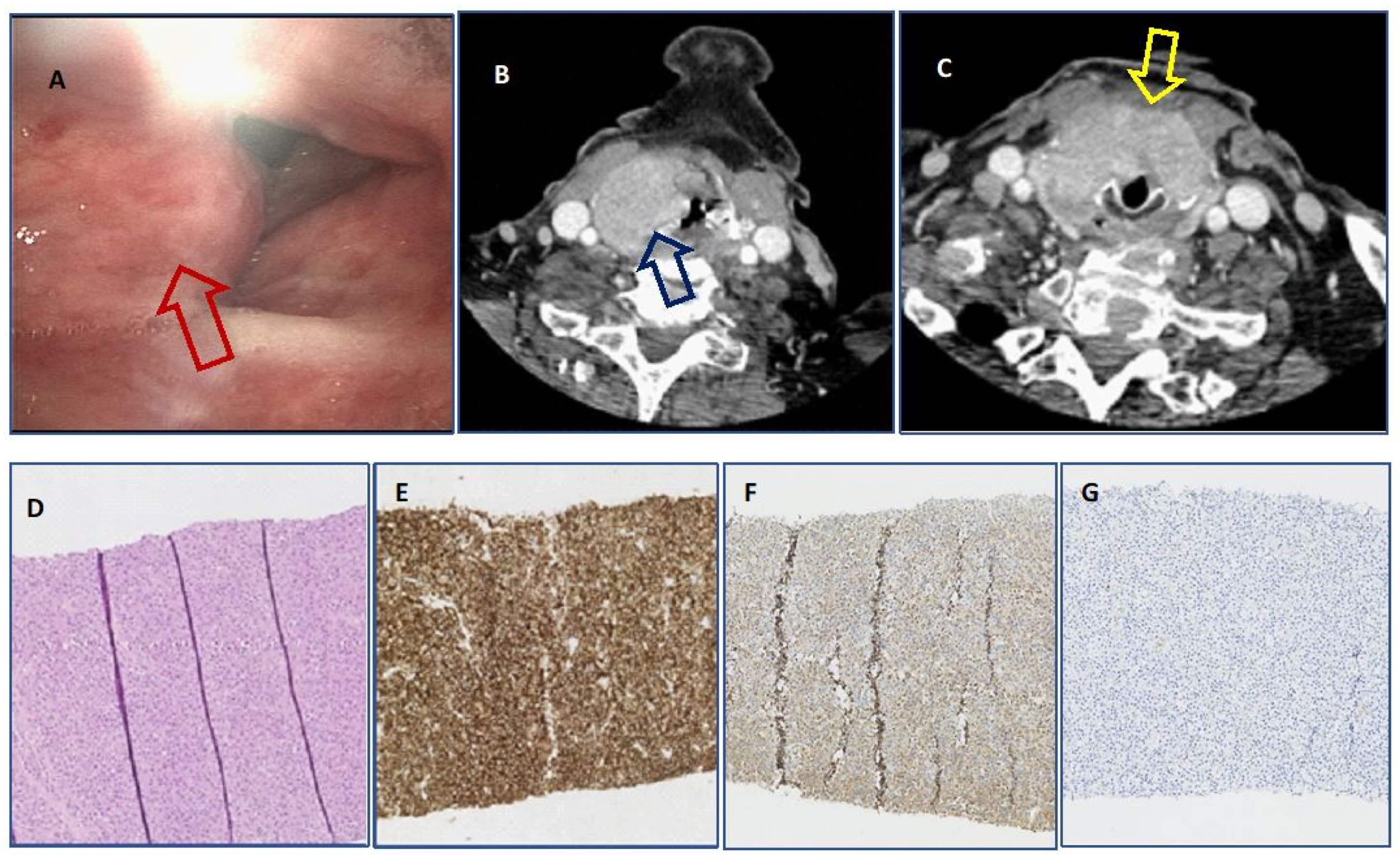
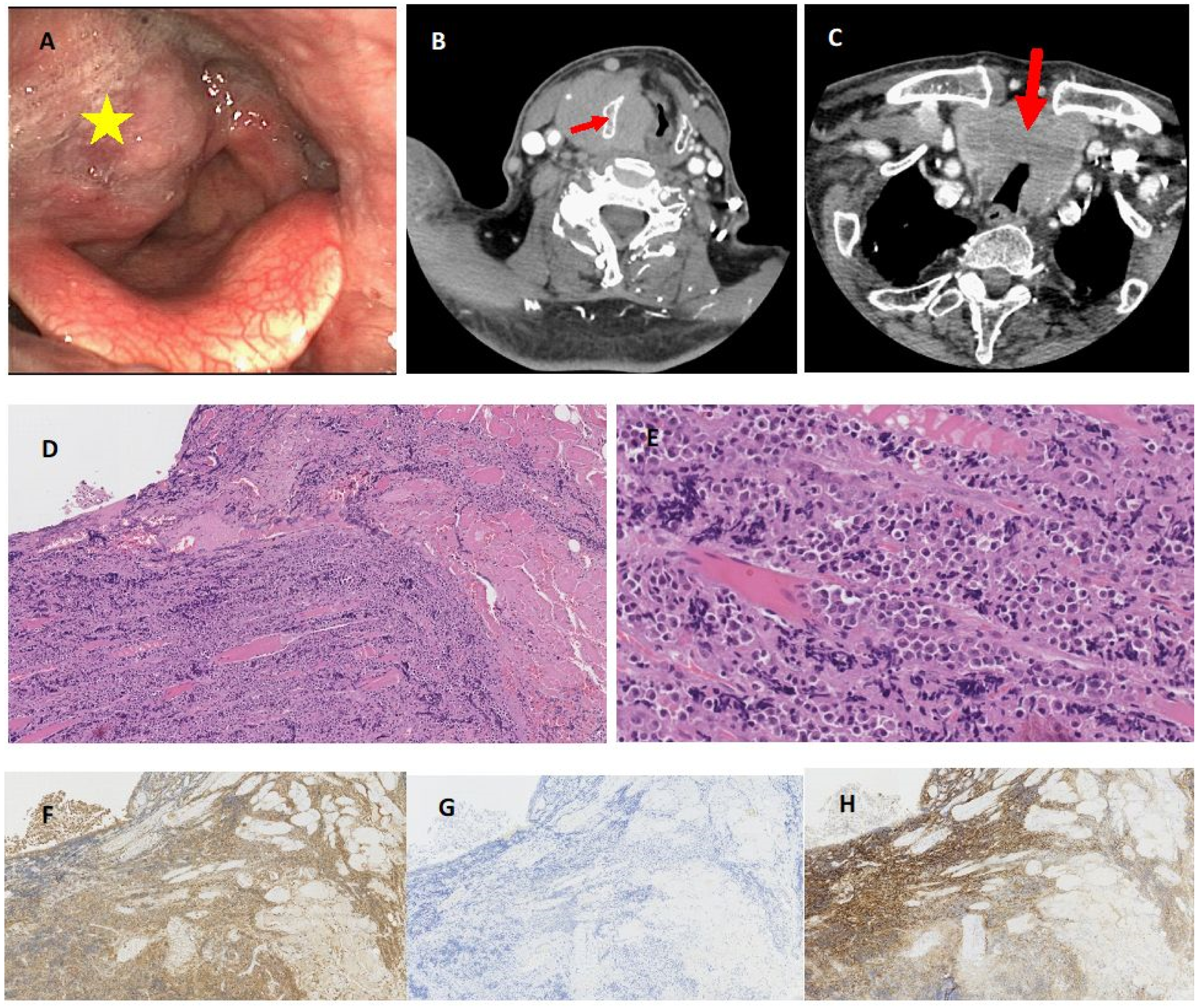
| Patient | Age (Years) | Sex | Type of Disease | Localisation | Symptoms | Surgery | RTX Dose (cGy) | CTH | PFS from Larynx Involvement (mo) | Survival Form Larynx Involvement (mo) | Death | OS (mo) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 70 | M | sEMPL | 1 | 1, 2, 3 | 0 | 5000 | 0 | 18 | 18 | 0 | NA |
| Patient 2 | 59 | M | sEMPL | 1 | 0 | 0 | 4000 | 0 | 111 | 111 | 0 | NA |
| Patient 3 | 72 | F | PCM-L | 5 | 1, 3 | 0 | N | 1 | 0.75 | 0.75 | 1 | 42 |
| Patient 4 | 84 | M | PCM-L | 1 | 4 | 0 | 4000 | 1 | 14 | 14 | 1 | 33 |
| Patient 5 | 78 | M | PCM-L | 5 | 3 | 0 | N | 0 | 1.5 | 1.5 | 1 | 1.5 |
| Patient 6 | 89 | M | PCM-L | 4 | 3 | 0 | N | 0 | 2 | 2 | 1 | 2 |
| No. | Author (Year) | Age (y) | Sex | Localisation | Symptoms | Surgery | RTX Dose (cGy) | CTH | Relapse in the Larynx | Progression to Systemic PCM | Death | PFS (mo) | OS (mo) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lu and Zhang (2020) | 57 | F | 1 | 1 | 1 | 5000 | 0 | 0 | 0 | 0 | 8 | 8 | [9] |
| 2 | Tanrivermis Sayit et al. (2020) | 74 | F | 4 | 1, 7 | 0 | Y | 1 | 0 | 0 | 0 | 3 | 3 | [10] |
| 3 | Ong et al. (2020) | 55 | M | 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | [11] |
| 4 | Brandt et al. (2020) | 54 | F | 1 | 1, 2 | 0 | 5000 | 0 | 0 | 0 | 0 | 18 | 18 | [4] |
| 5 | Ge et al. (2018) | 46 | M | 1 | 3, 6 | 0 | 5500 | 1 | 0 | 0 | 0 | 60 | 60 | [3] |
| 6 | Krebs et al. (2017) | 77 | M | 3 | 4, 5 | 0 | 4600 | 0 | 0 | 0 | 0 | 26 | 26 | [12] |
| 7 | Wang et al. (2015) | 43 | M | 6 | 1, 2, 4 | 1 | N | 0 | 1 | 1 | 0 | 12 | 60 | [13] |
| 8 | Haser et al. (2015) | 72 | M | 5 | 1, 4, 8 | 0 | Y | 0 | 0 | 0 | 0 | 12 | 12 | [14] |
| 9 | Pino et al. (2015) | 65 | M | 1 | 1, 2, 7 | 1 | 4600 | 0 | 0 | 0 | 0 | 54 | 54 | [15] |
| 10 | Xing et al. (2015) | 47 | F | 4 | 1 | 1 | 5000 | 0 | 0 | 0 | 0 | 18 | 18 | [16] |
| 11 | Abrari et al. (2014) | 56 | M | 2 | 4,10 | 0 | Y | 0 | 0 | 0 | 0 | NA | NA | [17] |
| 12 | Loyo et al. (2013) | 80 | F | 4 | 1, 2, 4, 8 | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [18] |
| 13 | Ghatak et al. (2013) | 29 | F | 4 | 1, 4, 7 | 0 | 5000 | 0 | 0 | 0 | 0 | 16 | 16 | [19] |
| 14 | Ramírez-Anguiano et al. (2012) | 57 | M | 5 | 2, 4, 8 | 0 | 5400 | 0 | 0 | 0 | 0 | 18 | 18 | [20] |
| 15 | Pinto et al. (2012) | 49 | F | 1 | 2 | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [21] |
| 16 | Kim et al. (2012) | 58 | M | 1 | 0 | 1 | N | 0 | 0 | 0 | 0 | 24 | 24 | [22] |
| 17 | Ravo et al. (2012) | 56 | M | 1 | 1, 4, 7 | 1 | 5000 | 0 | 0 | 0 | 0 | 5 | 5 | [23] |
| 18 | De Zoysa et al. (2012) | 62 | F | 2 | 2 | 0 | Y | 0 | 0 | 0 | 0 | 2 | 2 | [24] |
| 19 | Pichi et al. (2011) | 73 | M | 5 | 1, 4 | 0 | 4000 | 0 | 0 | 1 | 1 | 12 | 24 | [25] |
| 20 | Zhang et al. (2010) | 56 | F | 1 | 1, 2 | 1 | N | 0 | 0 | 0 | 0 | 24 | 24 | [26] |
| 21 | González Guijarro et al. (2010) | 11 | M | 4 | 2 | 1 | 4500 | 0 | 0 | 0 | 0 | 36 | 36 | [27] |
| 22 | Pratibha et al. (2009) | 49 | M | 6 | 1 | 0 | 4400 | 0 | 0 | 0 | 0 | 6 | 6 | [28] |
| 23 | Fernández-Aceñero et al. (2009) | 42 | F | 1 | 2, 7 | 0 | Y | 0 | 0 | 0 | 0 | NA | NA | [29] |
| 24 | Iseri et al. (2009) | 46 | F | 1 | NA | 1 | Y | 1 | 0 | 0 | 0 | 24 | 24 | [30] |
| 25 | Bilić et al. (2009) | 64 | M | 1 | NA | 0 | N | 1 | NA | NA | NA | 21 | 21 | [31] |
| 26 | Rutherford et al. (2009) | 13 | F | 3 | NA | 1 | Y | 0 | 0 | 0 | NA | 1 | 1 | [32] |
| 27 | Vanan et al. (2009) | 16 | F | 2 | 1 | 0 | 5040 | 0 | 0 | 0 | 0 | 12 | 12 | [33] |
| 28 | Straetmans and Stokroos (2008) | 57 | M | 1 | NA | 1 | 5000 | 0 | 1 | 0 | 0 | 2 | 24 | [34] |
| 29 | Ozbilean Acar et al. (2008) | 43 | F | 2 | NA | 1 | N | 0 | 0 | 0 | 0 | 24 | 24 | [35] |
| 30 | Lewis et al. (2007) | 71 | M | 1 | 1 | 1 | Y | 0 | 0 | 0 | 0 | 24 | 24 | [36] |
| 31 | Kusunoki et al. (2007) | 76 | F | 1 | 0 | 0 | N | 0 | 0 | 0 | 0 | 6 | 6 | [37] |
| 32 | Velez et al. (2007) | 64 | M | 4 | 1 | 1 | Y | 0 | 0 | 0 | 0 | 36 | 36 | [38] |
| 33 | Nakashima et al. (2006) | 39 | M | 1 | 0 | 1 | 6000 | 0 | 0 | 0 | 0 | 72 | 72 | [39] |
| 34 | Nakashima et al. (2006) | 59 | M | 1 | 0 | 1 | N | 0 | 0 | 0 | 0 | 180 | 180 | [39] |
| 35 | Mackiewicz-Nartowicz et al. (2005) | 79 | F | 2 | 1 | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [40] |
| 36 | Coskun et al. (2005) | NA | NA | NA | NA | 0 | Y | 0 | 0 | NA | 0 | NA | NA | [41] |
| 37 | Chao et al. (2005) | 60 | M | 1 | 5, 1 | 0 | 5000 | 0 | 0 | 0 | 1 | 37 | 37 | [42] |
| 38 | Sakiyama et al. (2005) | 47 | F | 3 | 0 | 0 | 5000 | 1 | 1 | 0 | 0 | 4 | 88 | [43] |
| 39 | Yavas et al. (2004) | 43 | F | 2 | 1 | 0 | N | 0 | 0 | 0 | 0 | NA | NA | [44] |
| 40 | Michalaki et al. (2003) | 46 | F | NA | NA | 0 | 4500 | 0 | 0 | 0 | 0 | 49 | 49 | [45] |
| 41 | Michalaki et al. (2003) | 59 | M | NA | NA | 0 | 4000 | 0 | 0 | 0 | 0 | 67 | 67 | [45] |
| 42 | Kamijo et al. (2002) | 84 | M | 1 | 1 | 1 | 6000 | 0 | 0 | 0 | 0 | 24 | 24 | [46] |
| 43 | Takeda et al. (2002) | 53 | F | 3 | NA | 0 | 4600 | 0 | NA | NA | NA | NA | NA | [47] |
| 44 | Soni et al. (2002) | 65 | M | 3 | 1, 3, 4, 7, 8 | 0 | 6000 | 0 | 0 | 0 | 0 | 25 | 25 | [48] |
| 45 | Strojan et al. (2002) | 65 | M | 1 | NA | 0 | 6000 | 0 | 0 | 0 | 1 | 93 | 93 | [49] |
| 46 | Strojan et al. (2002) | 72 | M | 2 | NA | 0 | 4600 | 0 | 0 | 0 | 1 | 56 | 56 | [49] |
| 47 | Strojan et al. (2002) | 50 | F | 1 | NA | 0 | 5000 | 0 | 0 | 0 | 0 | 27 | 27 | [49] |
| 48 | Maheshwari et al. (2001) | 65 | M | 3 | 3,4 | 0 | Y | 0 | 0 | 0 | 0 | 12 | 12 | [50] |
| 49 | Nagasaka et al. (2001) | 12 | F | 3 | 1,4 | 1 | 4320 | 0 | 0 | 0 | 0 | 48 | 48 | [51] |
| 50 | Abe et al. (2001) | 62 | F | NA | NA | 1 | 6500 | 0 | NA | NA | NA | NA | NA | [52] |
| 51 | Rakover et al. (2000) | 38 | M | 2 | 1, 5 | 1 | 5000 | 0 | 1 | 0 | 0 | 5 | 46 | [53] |
| 52 | Rosenblatt et al. (1999) | 40 | M | 2 | 1 | 0 | 5000 | 0 | 0 | 0 | 0 | 47 | 47 | [54] |
| 53 | Furukido et al. (1999) | 78 | F | 2 | NA | 1 | N | 0 | NA | NA | NA | NA | NA | [55] |
| 54 | Yusumatsu et al. (1999) | 77 | M | 1 | NA | 1 | 6000 | 0 | NA | NA | NA | NA | NA | [56] |
| 55 | Alexiou et al. (1999) | 69 | M | NA | NA | 1 | N | 0 | 0 | 0 | 0 | 62 | 62 | [57] |
| 56 | Alexiou et al. (1999) | 40 | M | 1 | NA | 1 | Y | 0 | 0 | 0 | 0 | 20 | 20 | [57] |
| 57 | Hotz et al. (1999) | 63 | M | NA | 4 | 1 | 4000 | 0 | 0 | 0 | 0 | 108 | 108 | [58] |
| 58 | Hotz et al. (1999) | 45 | M | NA | 7 | 1 | 5500 | 0 | NA | 0 | 0 | 108 | 108 | [58] |
| 59 | Nowak-Sadzikowska and Weiss (1998) | 34 | M | 1 | 1 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [59] |
| 60 | Nowak-Sadzikowska and Weiss (1998) | 50 | M | 2 | 1 | 0 | 6000 | 0 | 0 | 0 | 0 | 120 | 120 | [59] |
| 61 | Nowak-Sadzikowska and Weiss (1998) | 36 | M | 1 | 0 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [59] |
| 62 | Nowak-Sadzikowska and Weiss (1998) | 68 | F | 1 | 1 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [59] |
| 63 | Nowak-Sadzikowska and Weiss (1998) | 48 | M | 2 | 1 | 0 | 6000 | 0 | 0 | 0 | 0 | 120 | 120 | [59] |
| 64 | Weiss et al. (1998) | 34 | M | 1 | 1 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [60] |
| 65 | Weiss et al. (1998) | 50 | M | 2 | 1 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [60] |
| 66 | Weiss et al. (1998) | 36 | M | 1 | 0 | 0 | 6000 | 0 | 0 | 0 | 0 | 120 | 120 | [60] |
| 67 | Weiss et al. (1998) | 68 | F | 1 | 1 | 0 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [60] |
| 68 | Welsh et al. (1998) | 59 | M | 1 | 3,4 | 0 | 5000 | 0 | 0 | 0 | 0 | 42 | 42 | [61] |
| 69 | Bhattacharya et al. (1998) | 49 | F | 1 | 7,10 | 0 | NA | NA | 0 | 0 | 1 | 3 | 3 | [62] |
| 70 | Sulzner et al. (1998) | 49 | M | 1 | 3 | 0 | 4500 | 0 | 0 | 0 | 0 | 61 | 61 | [63] |
| 71 | Susnerwala et al. (1997) | 79 | F | NA | NA | 0 | 3500 | 0 | 0 | 0 | 0 | 132 | 132 | [64] |
| 72 | Susnerwala et al. (1997) | 65 | M | NA | NA | 0 | 4500 | 0 | 0 | 0 | 0 | 52 | 52 | [64] |
| 73 | Han et al. (1996) | 34 | M | 2 | NA | 1 | 5000 | 0 | NA | NA | NA | NA | NA | [65] |
| 74 | Rodriguez-de-Velasquez et al. (1996) | 33 | F | 5 | 2 | NA | NA | NA | NA | NA | NA | NA | NA | [66] |
| 75 | Abe et al. (1995) | 53 | F | 1 | NA | 0 | 4500 | 0 | NA | NA | NA | NA | NA | [67] |
| 76 | Narożny et al. (1995) | 51 | M | 1 | 1, 7, 10 | 0 | 6000 | 0 | 0 | 1 | 1 | 12 | 12 | [68] |
| 77 | Narożny et al. (1995) | 60 | M | 1 | 2, 4 | 1 | 5400 | 0 | 0 | 0 | 0 | 120 | 120 | [68] |
| 78 | Narożny et al. (1995) | 55 | F | 1 | 7 | 1 | N | 0 | 0 | 0 | 0 | 12 | 12 | [68] |
| 79 | Narożny et al. (1995) | 57 | M | 2 | 1 | 1 | N | 0 | 0 | 0 | 0 | 12 | 12 | [68] |
| 80 | Zbären and Zimmermann (1995) | 88 | M | 1 | 1, 3 | 1 | N | 0 | 0 | 0 | 0 | 72 | 72 | [69] |
| 81 | Zbären and Zimmermann (1995) | 71 | M | 1 | 1 | 1 | N | 0 | 0 | 0 | 0 | 12 | 12 | [69] |
| 82 | Rolins et al. (1995) | 43 | M | 1 | 0 | 0 | N | 0 | 0 | 0 | 0 | 36 | 36 | [70] |
| 83 | Mochimatsu et al. (1993) | 54 | M | 1 | NA | 1 | 5000 | 0 | NA | 1 | 0 | 144 | 144 | [71] |
| 84 | Weissmann et al. (1993) | 76 | M | 5 | 1, 3, 10 | 1 | Y | 0 | 0 | 0 | 0 | NA | NA | [72] |
| 85 | Jankowska-Kuc et al. (1993) | 58 | M | 4 | 1, 7 | 1 | 5950 | 0 | 0 | 0 | 0 | 36 | 36 | [73] |
| 86 | Barbu et al. (1992) | 69 | M | 1 | 2, 7 | 0 | Y | 0 | 0 | 0 | 0 | 36 | 36 | [74] |
| 87 | Tateno et al. (1990) | 54 | M | 1 | NA | 1 | N | 0 | NA | NA | NA | NA | NA | [75] |
| 88 | Kost (1990) | 43 | M | 6 | 1 | 1 | 7000 | 0 | 0 | 0 | NA | NA | 84 | [76] |
| 89 | Agatsuma et al. (1989) | 74 | M | 6 | NA | 1 | 4000 | 0 | NA | NA | NA | NA | NA | [77] |
| 90 | Gambino (1988) | 47 | M | 1 | 5, 7 | 1 | Y | 0 | NA | NA | 0 | NA | NA | [78] |
| 91 | Gaffney et al. (1987) | 80 | M | NA | NA | 0 | 4159 | 0 | 0 | 0 | 0 | 7 | 7 | [79] |
| 92 | Gadomski et al. (1986) | 54 | M | 2 | 1, 6, 7 | 1 | N | 1 | 0 | NA | 1 | 180 | 180 | [80] |
| 93 | Gadomski et al. (1986) | 51 | M | 1 | 1, 6, 7 | 0 | 4500 | 0 | 0 | NA | 0 | 60 | 60 | [80] |
| 94 | Burke et al. (1986) | 53 | M | 1 | 1, 3, 4 | 0 | N | 1 | 0 | 0 | 0 | 10 | 10 | [81] |
| 95 | Gormley et al. (1985) | 78 | F | 3 | 1, 4, 8 | 1 | 3250 | 0 | 0 | 0 | 0 | NA | NA | [82] |
| 96 | Maniglia and Xue (1983) | 64 | F | 5 | 1, 4 | 1 | 5000 | 0 | 0 | 0 | 1 | 12 | 12 | [83] |
| 97 | Ferlito et al. (1982) | 45 | M | 1 | 1 | 0 | N | 1 | 0 | 1 | 0 | 29 | 74 | [84] |
| 98 | Bjelkenkrantz et al. (1981) | NA | NA | 1 | NA | 1 | 5000 | 0 | 0 | 0 | 0 | 84 | 84 | [85] |
| 99 | Bush et al. (1981) | 52 | F | 1 | NA | 0 | 5500 | 0 | 0 | 0 | 1 | 36 | 36 | [86] |
| 100 | Bush et al. (1981) | 34 | F | NA | NA | 1 | 5500 | 0 | 0 | 0 | 0 | 42 | 42 | [86] |
| 101 | Endo et al. (1979) | 68 | M | 3 | NA | 0 | N | 1 | NA | NA | NA | NA | NA | [87] |
| 102 | Singh et al. (1979) | 42 | F | 1 | NA | 1 | Y | 0 | NA | 0 | NA | NA | 29 | [88] |
| 103 | Woodruff et al. (1979) | 64 | F | 1 | NA | 0 | Y | 0 | 0 | 0 | 1 | 72 | 72 | [89] |
| 104 | Woodruff et al. (1979) | 34 | F | 1 | NA | 0 | Y | 0 | 0 | 0 | 0 | NA | NA | [89] |
| 105 | Cohen et al. (1978) | 15 | F | 3 | 4 | 1 | 4000 | 0 | 1 | 0 | 0 | 3 | 22 | [90] |
| 106 | Pahor (1978) | 62 | M | 6 | 1, 4, 7 | 1 | 4500 | 1 | 1 | 0 | 0 | 21 | 31 | [91] |
| 107 | Gorenstein et al. (1977) | 58 | M | 4 | 1 | 1 | Y | 0 | NA | 0 | 0 | 36 | 36 | [92] |
| 108 | Gorenstein et al. (1977) | 63 | M | 4 | 1 | 1 | Y | 0 | NA | 0 | 0 | 300 | 300 | [92] |
| 109 | Gorenstein et al. (1977) | 59 | M | 3 | 4, 8 | 1 | N | 0 | NA | 0 | 1 | 60 | 60 | [92] |
| 110 | Gorenstein et al. (1977) | 42 | M | 2 | 1 | 1 | N | 0 | NA | 0 | 0 | 60 | 60 | [92] |
| 111 | Gorenstein et al. (1977) | 61 | M | 4 | 1, 7 | 0 | Y | 0 | NA | 0 | 0 | 72 | 72 | [92] |
| 112 | Petrovich et al. (1977) | 74 | M | 1 | 10 | 0 | 7312 | 0 | 0 | 0 | 0 | 24 | 88 | [93] |
| 113 | Muller and Fischer (1976) | 44 | M | 1 | 1, 8 | NA | NA | NA | NA | NA | NA | NA | NA | [94] |
| 114 | Horiuchi et al. (1973) | 51 | F | 1 | NA | 1 | N | 0 | NA | NA | NA | NA | NA | [95] |
| 115 | Booth et al. (1973) | 54 | F | 4 | NA | 1 | N | 0 | 1 | 0 | 1 | NA | 264 | [96] |
| 116 | Stone and Cole (1971) | 67 | M | 1 | NA | 0 | Y | 1 | 0 | 0 | 0 | 10 | 10 | [97] |
| 117 | Touma (1971) | 65 | M | 4 | NA | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [98] |
| 118 | Kakar et al. (1970) | 45 | F | 1 | 1, 4 | 1 | N | 0 | 0 | 0 | 0 | 13 | 13 | [99] |
| 119 | Poole and Marchetta (1968) | 41 | M | NA | NA | 1 | Y | 0 | NA | 0 | 1 | NA | 41 | [100] |
| 120 | Nabar (1968) | 60 | F | 1 | NA | 1 | N | 0 | 0 | 0 | 0 | 132 | 132 | [101] |
| 121 | Nabar (1968) | 55 | M | 1 | NA | 0 | Y | 0 | 0 | 0 | 1 | NA | NA | [101] |
| 122 | Nabar (1968) | 46 | F | 1 | NA | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [101] |
| 123 | Kraska (1967) | 40 | F | 1 | 1, 4 | 0 | 7290 | 0 | 0 | 0 | 0 | 14 | 14 | [102] |
| 124 | Studencki (1966) | 54 | F | 4 | 1, 4, 10 | 0 | 5500 | 0 | 0 | 0 | 0 | 7 | 7 | [103] |
| 125 | Todd (1965) | 65 | M | 1 | NA | 0 | 2500 | 0 | 1 | 1 | 1 | 60 | 96 | [104] |
| 126 | Todd (1965) | 43 | M | 1 | NA | 0 | 4800 | 0 | 0 | 0 | 0 | 60 | 60 | [104] |
| 127 | Webb et al. (1962) | 55 | F | 4 | 1 | 1 | N | 0 | 0 | 0 | 0 | 132 | 132 | [105] |
| 128 | Webb et al. (1962) | 32 | M | 3 | 1 | 1 | Y | 0 | 0 | 0 | 0 | 120 | 120 | [105] |
| 129 | Webb et al. (1962) | 46 | M | 1 | 1, 9 | 1 | Y | 0 | 1 | 1 | 1 | 168 | 330 | [105] |
| 130 | Krotz and Ritterhoff (1961) | 75 | M | NA | NA | 0 | Y | 0 | 1 | NA | 1 | NA | NA | [106] |
| 131 | Clark (1957) | 60 | F | 1 | 1, 4, 7 | 1 | N | 0 | 0 | 0 | 0 | 1 | 1 | [107] |
| 132 | Carson et al. (1955) | 52 | M | 1 | NA | 1 | Y | 0 | 0 | 1 | 0 | 54 | 54 | [108] |
| 133 | Carson et al. (1955) | 73 | M | 1 | NA | 1 | N | 0 | NA | NA | 1 | 156 | 156 | [108] |
| 134 | Korkis (1954) | 42 | F | 1 | NA | 1 | N | 0 | 0 | 0 | 0 | NA | NA | [109] |
| 135 | Priest (1952) | 50 | M | 4 | 2, 10 | 1 | N | 0 | 1 | 0 | 0 | 9 | 48 | [110] |
| 136 | Ewing and Foote (1952) | 76 | M | 1 | 1 | NA | Y | NA | 0 | 0 | 0 | 6 | 6 | [111] |
| 137 | Costen (1951) | 52 | M | 1 | NA | 0 | Y | 0 | 0 | 1 | 0 | NA | 12 | [112] |
| 138 | Mattick (1950) | 41 | M | 4 | 1, 3, 4, 7, 10 | 1 | N | 0 | 1 | 1 | 1 | 27 | 41 | [113] |
| 139 | Rawson et al. (1950) | 59 | F | 1 | NA | 1 | Y | 0 | 0 | 1 | 0 | 36 | NA | [114] |
| 140 | Stout and Kenney (1949) | 46 | M | 1 | 4, 6 | 1 | 8400 | 0 | 1 | 0 | 0 | 3 | 171 | [115] |
| 141 | Hodge and Wilson (1948) | 53 | M | 4 | 1, 3, 5, 10 | 1 | N | 0 | 0 | 0 | 0 | 12 | 12 | [116] |
| 142 | Lumb and Prossor (1948) | 34 | M | 3 | NA | 0 | Y | 0 | 1 | 0 | 0 | 24 | 30 | [117] |
| 143 | Lumb and Prossor (1948) | 20 | M | 7 | NA | 1 | Y | 0 | 0 | 0 | 0 | 90 | 90 | [117] |
| 144 | Clerf (1946) | 75 | M | 1 | NA | 1 | N | 0 | 0 | 0 | 1 | 13 | 13 | [118] |
| 145 | Jaeger (1942) | 67 | F | NA | NA | 0 | Y | 0 | 0 | 0 | 1 | 6 | 6 | [119] |
| 146 | Haven and Parkhill (1941) | 62 | M | 1 | NA | 1 | Y | 0 | 0 | 0 | 0 | 54 | 54 | [120] |
| 147 | Ringertz (1938) | 59 | M | 2 | 1 | 0 | Y | 0 | 0 | 0 | 0 | 48 | 48 | [121] |
| 148 | Ringertz (1938) | 73 | M | 1 | NA | 1 | N | 0 | 0 | 0 | 0 | 12 | 12 | [121] |
| 149 | Ringertz (1938) | 57 | F | 1 | NA | 1 | Y | 0 | 0 | 0 | 1 | 48 | 48 | [121] |
| 150 | Heindl (1933) | NA | NA | NA | NA | 1 | N | 0 | 0 | 0 | 0 | 36 | NA | [122] |
| 151 | Blacklock and Macartney (1932) | 64 | M | NA | NA | 1 | N | 0 | 0 | 0 | 0 | NA | 24 | [123] |
| 152 | Wachter (1914) | 48 | F | NA | NA | 1 | N | 0 | 0 | 0 | 0 | 5 | 142 | [124] |
| No. | Author (Year) | Age (y) | Sex | Localisation in the Larynx | Symptoms at Larynx Involvement | Involvement of Larynx at PCM Diagnosis | Surgery | RTX Dose (cGy) | CTH | PFS (mo) | OS (mo) | Death | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | You and Bhuta (2019) | 68 | M | 4 | 2, 5 | 1 | 0 | N | 1 | 132 | 146 | 1 | [6] |
| 2 | Doğan et al. (2019) | 44 | M | 3 | 4 | NA | 0 | N | 1 | NA | NA | NA | [7] |
| 3 | Doğan et al. (2019) | 55 | M | 6 | 1, 4 | NA | 0 | N | 1 | NA | NA | NA | [7] |
| 4 | Doğan et al. (2019) | 70 | M | 3 | 4, 6 | NA | 0 | N | 1 | NA | NA | NA | [7] |
| 5 | Steinke et al. (2019) | 81 | M | 4 | 2, 4, 7 | 1 | 0 | 6000 | 0 | NA | NA | 0 | [125] |
| 6 | Allegra et al. (2017) | 68 | M | 4 | 2, 4, 6 | 0 | 0 | N | 1 | 6 | 6 | 0 | [126] |
| 7 | Nochikattil et al. (2016) | 44 | F | 1 | 1, 7 | 0 | NA | NA | NA | NA | NA | 0 | [127] |
| 8 | Gan et al. (2014) | 55 | M | 3 | 2, 4, 7, 10 | 0 | 1 | N | 1 | 24 | 24 | 0 | [128] |
| 9 | Mitchell et al. (2013) | 63 | M | 4 | 1, 5 | 1 | 0 | Y | 1 | 36 | NA | 0 | [129] |
| 10 | Alherabi et al. (2013) | 77 | M | 1 | 6 | 0 | NA | NA | 0 | NA | NA | 0 | [130] |
| 11 | Grobman et al. (2012) | 58 | M | 1 | 2 | 1 | 0 | 3000 | 0 | 120 | 124 | 0 | [131] |
| 12 | Kumar et al. (2011) | 63 | M | 2 | 1, 4, 6, 9 | 1 | 1 | N | 1 | 48 | 49 | 1 | [132] |
| 13 | Straetmans and Stokroos (2008) | 62 | F | 3 | 7 | 1 | 0 | N | 1 | 36 | 48 | 0 | [34] |
| 14 | Oztürk et al. (2008) | 65 | M | 3 | 4 | 1 | 0 | NA | 1 | NA | NA | 0 | [133] |
| 15 | Dispenza et al. (2007) | 69 | F | 4 | 1, 6 | 0 | 0 | N | 1 | 12 | 12 | 0 | [134] |
| 16 | Shimada et al. (2006) | 72 | M | NA | 0 | 0 | 1 | 5000 | 0 | 36 | 36 | 0 | [135] |
| 17 | Nampoothiri et al. (2006) | 65 | M | 3 | 7 | 0 | 0 | N | 1 | NA | NA | 0 | [136] |
| 18 | Luppino et al. (2005) | NA | NA | 2 | 1, 6 | 1 | NA | NA | NA | NA | NA | NA | [137] |
| 19 | Gross et al. (2002) | 50 | M | 4 | 1, 4, 5, 7 | 0 | 0 | 4500 | 1 | 3 | 3 | 1 | [138] |
| 20 | Sosna et al. (2002) | 54 | M | 4 | 1, 4, 10 | 0 | 0 | 3600 | 1 | NA | NA | 1 | [139] |
| 21 | Aslan et al. (2001) | 70 | M | 3 | 1, 10 | 0 | 0 | 5000 | 0 | 6 | 6 | 0 | [140] |
| 22 | Saad et al. (2001) | 79 | M | 6 | 8 | 1 | 0 | N | 1 | 30 | 36 | 1 | [141] |
| 23 | Uppal et al. (2001) | 54 | M | 4 | 7,10 | 1 | 0 | Y | 1 | 36 | 37 | 1 | [142] |
| 24 | Nofsinger et al. (1997) | 72 | M | 3 | 1, 4 | 1 | 0 | 6300 | 1 | 6 | NA | 1 | [143] |
| 25 | Gabryś et al. (1996) | 63 | M | 3 | NA | 1 | 0 | Y | 1 | 40 | 40 | 1 | [144] |
| 26 | Van Dyke et al. (1996) | 62 | M | 4 | 1, 10 | 1 | 0 | 4000 | 1 | 12 | 54 | 0 | [145] |
| 27 | Rabinov et al. (1993) | 48 | M | 3 | 1, 7 | 1 | 1 | Y | 0 | NA | NA | 0 | [146] |
| 28 | Werner et al. (1991) | 48 | F | 3 | NA | 0 | 0 | N | 1 | 14 | 14 | 1 | [147] |
| 29 | Georghiou and Hogg (1988) | 71 | M | 3 | 1, 3, 7, 10 | 0 | 0 | Y | 1 | 1 | 1 | 1 | [148] |
| 30 | Jones et al. (1988) | 73 | M | 6 | 4, 6, 7 | 1 | 0 | N | 1 | 18 | 19 | 1 | [149] |
| 31 | Maniglia and Xue (1983) | 61 | F | 1 | 1, 3, 4 | 0 | 0 | Y | 0 | 2 | 38 | 1 | [83] |
| 32 | Maniglia and Xue (1983) | 73 | M | 5 | 1, 4 | 0 | 0 | 5600 | 0 | NA | NA | 0 | [83] |
| 33 | East (1978) | 47 | F | 1 | 1, 2, 3, 6 | 1 | 0 | 3600 | 1 | 14 | 17 | 1 | [150] |
| 34 | Pirkey (1957) | 49 | M | 3 | 1, 3, 4, 7, 10 | 0 | 0 | Y | 0 | 3 | 6 | 1 | [151] |
| 35 | Shaw (1972) | 56 | M | 1 | NA | NA | 1 | 6500 | 1 | NA | 36 | 1 | [152] |
| Parameter | Hazard Ratio | 95% Confidence Interval | p |
|---|---|---|---|
| Involvement of cartilage | 19.51 | 1.46–259.66 | 0.024 |
| Male gender | 8.00 | 0.88–72.55 | 0.065 |
| Radiotherapy | 0.14 | 0.01–1.52 | 0.106 |
| Surgery | 0.23 | 0.02–2.66 | 0.237 |
| Combined surgery with radiotherapy | 0.24 | 0.02–242 | 0.227 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanek, E.; Drozd-Sokołowska, J.; Sokołowski, J.; Rzepakowska, A.; Moskwa, A.; Pachla, J.; Grzybowski, J.; Woźnica, K.; Niemczyk, K.; Jamroziak, K. Solitary Extramedullary Plasmacytoma of the Larynx and Secondary Laryngeal Involvement in Plasma Cell Myeloma: Single-Centre Retrospective Analysis and Systematic Literature Review. J. Clin. Med. 2022, 11, 4390. https://doi.org/10.3390/jcm11154390
Szczepanek E, Drozd-Sokołowska J, Sokołowski J, Rzepakowska A, Moskwa A, Pachla J, Grzybowski J, Woźnica K, Niemczyk K, Jamroziak K. Solitary Extramedullary Plasmacytoma of the Larynx and Secondary Laryngeal Involvement in Plasma Cell Myeloma: Single-Centre Retrospective Analysis and Systematic Literature Review. Journal of Clinical Medicine. 2022; 11(15):4390. https://doi.org/10.3390/jcm11154390
Chicago/Turabian StyleSzczepanek, Elżbieta, Joanna Drozd-Sokołowska, Jacek Sokołowski, Anna Rzepakowska, Arkadiusz Moskwa, Jakub Pachla, Jakub Grzybowski, Katarzyna Woźnica, Kazimierz Niemczyk, and Krzysztof Jamroziak. 2022. "Solitary Extramedullary Plasmacytoma of the Larynx and Secondary Laryngeal Involvement in Plasma Cell Myeloma: Single-Centre Retrospective Analysis and Systematic Literature Review" Journal of Clinical Medicine 11, no. 15: 4390. https://doi.org/10.3390/jcm11154390
APA StyleSzczepanek, E., Drozd-Sokołowska, J., Sokołowski, J., Rzepakowska, A., Moskwa, A., Pachla, J., Grzybowski, J., Woźnica, K., Niemczyk, K., & Jamroziak, K. (2022). Solitary Extramedullary Plasmacytoma of the Larynx and Secondary Laryngeal Involvement in Plasma Cell Myeloma: Single-Centre Retrospective Analysis and Systematic Literature Review. Journal of Clinical Medicine, 11(15), 4390. https://doi.org/10.3390/jcm11154390






