Ferritin Trajectories over Repeated Whole Blood Donations: Results from the FIND+ Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Ferritin Measurements
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spencer, B. Blood donor iron status: Are we bleeding them dry? Curr. Opin. Hematol. 2013, 20, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Zalpuri, S.; Schotten, N.; Baart, A.M.; van de Watering, L.M.; van den Hurk, K.; van Kraaij, M.G. Iron deficiency-related symptoms in whole blood donors: A systematic review. Transfusion 2019, 59, 3275–3287. [Google Scholar] [CrossRef] [PubMed]
- Kiss, J.E.; Vassallo, R.R. How do we manage iron deficiency after blood donation? Br. J. Haematol. 2018, 181, 590–603. [Google Scholar] [CrossRef]
- Zalpuri, S.; Romeijn, B.; Allara, E.; Goldman, M.; Kamel, H.; Gorlin, J.; Vassallo, R.; Grégoire, Y.; Goto, N.; Flanagan, P.; et al. Variations in hemoglobin measurement and eligibility criteria across blood donation services are associated with differing low-hemoglobin deferral rates: A BEST Collaborative study. Transfusion 2020, 60, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Schotten, N.; Pasker-de Jong, P.C.; Moretti, D.; Zimmermann, M.B.; Geurts-Moespot, A.J.; Swinkels, D.W.; van Kraaij, M.G. The donation interval of 56 days requires extension to 180 days for whole blood donors to recover from changes in iron metabolism. Blood 2016, 128, 2185–2188. [Google Scholar] [CrossRef]
- Timmer, T.C.; de Groot, R.; Rijnhart, J.J.; Lakerveld, J.; Brug, J.; Perenboom, C.W.; Baart, A.M.; Prinsze, F.J.; Zalpuri, S.; van der Schoot, C.E.; et al. Dietary intake of heme iron is associated with ferritin and hemoglobin levels in Dutch blood donors: Results from Donor InSight. Haematologica 2020, 105, 2400–2406. [Google Scholar] [CrossRef] [PubMed]
- Sweegers, M.G.; Zalpuri, S.; Quee, F.A.; Prinsze, F.J.; Hoogendijk, E.O.; Twisk, J.W.; van Weert, A.W.; de Kort, W.L.; van den Hurk, K. Ferritin measurement IN Donors-Effectiveness of iron Monitoring to diminish iron deficiency and low haemoglobin in whole blood donors (FIND’EM): Study protocol for a stepped wedge cluster randomised trial. Trials 2020, 21, 823. [Google Scholar] [CrossRef]
- Baart, A.M.; van Noord, P.A.; Vergouwe, Y.; Moons, K.G.; Swinkels, D.W.; Wiegerinck, E.T.; de Kort, W.L.; Atsma, F. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion 2013, 53, 1670–1677. [Google Scholar] [CrossRef]
- Vinkenoog, M.; van den Hurk, K.; van Kraaij, M.; van Leeuwen, M.; Janssen, M.P. First results of a ferritin-based blood donor deferral policy in the Netherlands. Transfusion 2020, 60, 1785–1792. [Google Scholar] [CrossRef]
- Fillet, A.M.; Martinaud, C.; Malard, L.; Le Cam, S.; Hejl, C.; Chenus, F.; Woimant, G.; Chueca, M.; Jacquot, E.; Besiers, C.; et al. Iron deficiency among French whole-blood donors: First assessment and identification of predictive factors. Vox Sang. 2021, 116, 42–52. [Google Scholar] [CrossRef]
- Terada, C.T.; Santos, P.C.J.D.L.; Cançado, R.D.; Rostelato, S.; Lopreato, F.R.; Chiattone, C.S.; Guerra-Shinohara, E.M. Iron deficiency and frequency of HFE C282Y gene mutation in Brazilian blood donors. Transfus. Med. 2009, 19, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Spencer, B.R.; Guo, Y.; Cable, R.G.; Kiss, J.E.; Busch, M.P.; Page, G.P.; Endres-Dighe, S.M.; Kleinman, S.; Glynn, S.A.; Mast, A.E.; et al. Iron status and risk factors for iron depletion in a racially/ethnically diverse blood donor population. Transfusion 2019, 59, 3146–3156. [Google Scholar] [CrossRef]
- Prinsze, F.J.; de Groot, R.; Timmer, T.C.; Zalpuri, S.; van den Hurk, K. Donation-induced iron depletion is significantly associated with low hemoglobin at subsequent donations. Transfusion 2021, 61, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Nasserinejad, K.; van Rosmalen, J.; van den Hurk, K.; Baart, M.; Hoekstra, T.; Rizopoulos, D.; Lesaffre, E.; de Kort, W. Prevalence and determinants of declining versus stable hemoglobin levels in whole blood donors. Transfusion 2015, 55, 1955–1963. [Google Scholar] [CrossRef]
- Baart, A.M.; de Kort, W.L.; Atsma, F.; Moons, K.G.; Vergouwe, Y. Development and validation of a prediction model for low hemoglobin deferral in a large cohort of whole blood donors. Transfusion 2012, 52, 2559–2569. [Google Scholar] [CrossRef] [PubMed]
- Mast, A.E.; Schlumpf, K.S.; Wright, D.J.; Johnson, B.; Glynn, S.A.; Busch, M.P.; Olbina, G.; Westerman, M.; Nemeth, E.; Ganz, T. Hepcidin level predicts hemoglobin concentration in individuals undergoing repeated phlebotomy. Haematologica 2013, 98, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; Bodner, T.E. Growth Mixture Modeling: Identifying and Predicting Unobserved Subpopulations With Longitudinal Data. Organ. Res. Methods 2007, 10, 635–656. [Google Scholar]
- Nagin, D.S.; Odgers, C.L. Group-based trajectory modeling in clinical research. Annu. Rev. Clin. Psychol. 2010, 6, 109–138. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L.; Nagin, D.S.; Roeder, K. A SAS Procedure Based on Mixture Models for Estimating Developmental Trajectories. Sociol. Methods Res. 2001, 29, 374–393. [Google Scholar] [CrossRef]
- Nagin, D.S. Analyzing developmental trajectories: A semiparametric, group-based approach. Psychol. Methods 1999, 4, 139–157. [Google Scholar] [CrossRef]
- Niyonkuru, C.; Wagner, A.K.; Ozawa, H.; Amin, K.; Goyal, A.; Fabio, A. Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: A practical example. J. Neurotrauma 2013, 30, 938–945. [Google Scholar] [CrossRef]
- Schwartz, G. Estimating the dimension of a model. Ann. Stat. 1978, 6, 461–464. [Google Scholar] [CrossRef]
- Wit, E.; Heuvel, E.V.D.; Romeijn, J.W. All models are wrong...: An introduction to model uncertainty. Am. J. Bot. 2012, 66, 217–236. [Google Scholar] [CrossRef]
- Mantilla-Gutiérrez, C.Y.; Cardona-Arias, J.A. Iron deficiency prevalence in blood donors: A systematic review, 2001–2011. Rev. Esp. Salud Publica 2012, 86, 357–369. [Google Scholar] [PubMed]
- Nasserinejad, K.; de Kort, W.; Baart, M.; Komárek, A.; van Rosmalen, J.; Lesaffre, E. Predicting hemoglobin levels in whole blood donors using transition models and mixed effects models. BMC Med. Res. Methodol. 2013, 13, 62. [Google Scholar] [CrossRef][Green Version]
- Yook, J.S.; You, M.; Kim, J.; Toney, A.M.; Fan, R.; Puniya, B.L.; Helikar, T.; Vaulont, S.; Deschemin, J.C.; Okla, M.; et al. Essential role of systemic iron mobilization and redistribution for adaptive thermogenesis through HIF2-α/hepcidin axis. Proc. Natl. Acad. Sci. USA 2021, 118, e2109186118. [Google Scholar] [CrossRef]
- Baart, A.M.; de Kort, W.L.; Moons, K.G.; Vergouwe, Y. Prediction of low haemoglobin levels in whole blood donors. Vox Sang. 2011, 100, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Brittenham, G.M. Iron deficiency in whole blood donors. Transfusion 2011, 51, 458–461. [Google Scholar] [CrossRef]
- Schiepers, O.J.; van Boxtel, M.P.; de Groot, R.H.; Jolles, J.; de Kort, W.L.; Swinkels, D.W.; Kok, F.J.; Verhoef, P.; Durga, J. Serum iron parameters, HFE C282Y genotype, and cognitive performance in older adults: Results from the FACIT study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2010, 65, 1312–1321. [Google Scholar] [CrossRef]
- Dallman, P.R. Biochemical basis for the manifestations of iron deficiency. Annu. Rev. Nutr. 1986, 6, 13–40. [Google Scholar] [CrossRef] [PubMed]
- Newman, B.H.; Newman, D.T.; Ahmad, R.; Roth, A.J. The effect of whole-blood donor adverse events on blood donor return rates. Transfusion 2006, 46, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Popovsky, M.A. Anemia, iron depletion, and the blood donor: It’s time to work on the donor’s behalf. Transfusion 2012, 52, 688–692. [Google Scholar] [CrossRef]
- Ulfberg, J.; Nyström, B. Restless legs syndrome in blood donors. Sleep Med. 2004, 5, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Birgegård, G.; Schneider, K.; Ulfberg, J. High incidence of iron depletion and restless leg syndrome (RLS) in regular blood donors: Intravenous iron sucrose substitution more effective than oral iron. Vox Sang. 2010, 99, 354–361. [Google Scholar] [CrossRef]
- Bryant, B.J.; Yau, Y.Y.; Arceo, S.M.; Hopkins, J.A.; Leitman, S.F. Ascertainment of iron deficiency and depletion in blood donors through screening questions for pica and restless legs syndrome. Transfusion 2013, 53, 1637–1644. [Google Scholar] [CrossRef]
- Spencer, B.R.; Kleinman, S.; Wright, D.J.; Glynn, S.A.; Rye, D.B.; Kiss, J.E.; Mast, A.E.; Cable, R.G.; REDS-II RISE Analysis Group. Restless legs syndrome, pica, and iron status in blood donors. Transfusion 2013, 53, 1645–1652. [Google Scholar] [CrossRef]
- Soranzo, N.; Spector, T.D.; Mangino, M.; Kühnel, B.; Rendon, A.; Teumer, A.; Willenborg, C.; Wright, B.; Chen, L.; Li, M.; et al. A genome-wide meta-analysis identifies 22 loci associated with eight hematological parameters in the HaemGen consortium. Nat. Genet. 2009, 41, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Van Der Harst, P.; Zhang, W.; Mateo Leach, I.; Rendon, A.; Verweij, N.; Sehmi, J.; Paul, D.S.; Elling, U.; Allayee, H.; Li, X.; et al. Seventy-five genetic loci influencing the human red blood cell. Nature 2012, 492, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Cvejic, A.; Haer-Wigman, L.; Stephens, J.C.; Kostadima, M.; Smethurst, P.A.; Frontini, M.; van den Akker, E.; Bertone, P.; Bielczyk-Maczyńska, E.; Farrow, S.; et al. SMIM1 underlies the Vel blood group and influences red blood cell traits. Nat. Genet. 2013, 45, 542–545. [Google Scholar] [CrossRef]
- Kiss, J.E.; Brambilla, D.; Glynn, S.A.; Mast, A.E.; Spencer, B.R.; Stone, M.; Kleinman, S.H.; Cable, R.G.; National Heart, Lung, and Blood Institute. Oral iron supplementation after blood donation: A randomized clinical trial. JAMA 2015, 313, 575–583. [Google Scholar] [CrossRef]
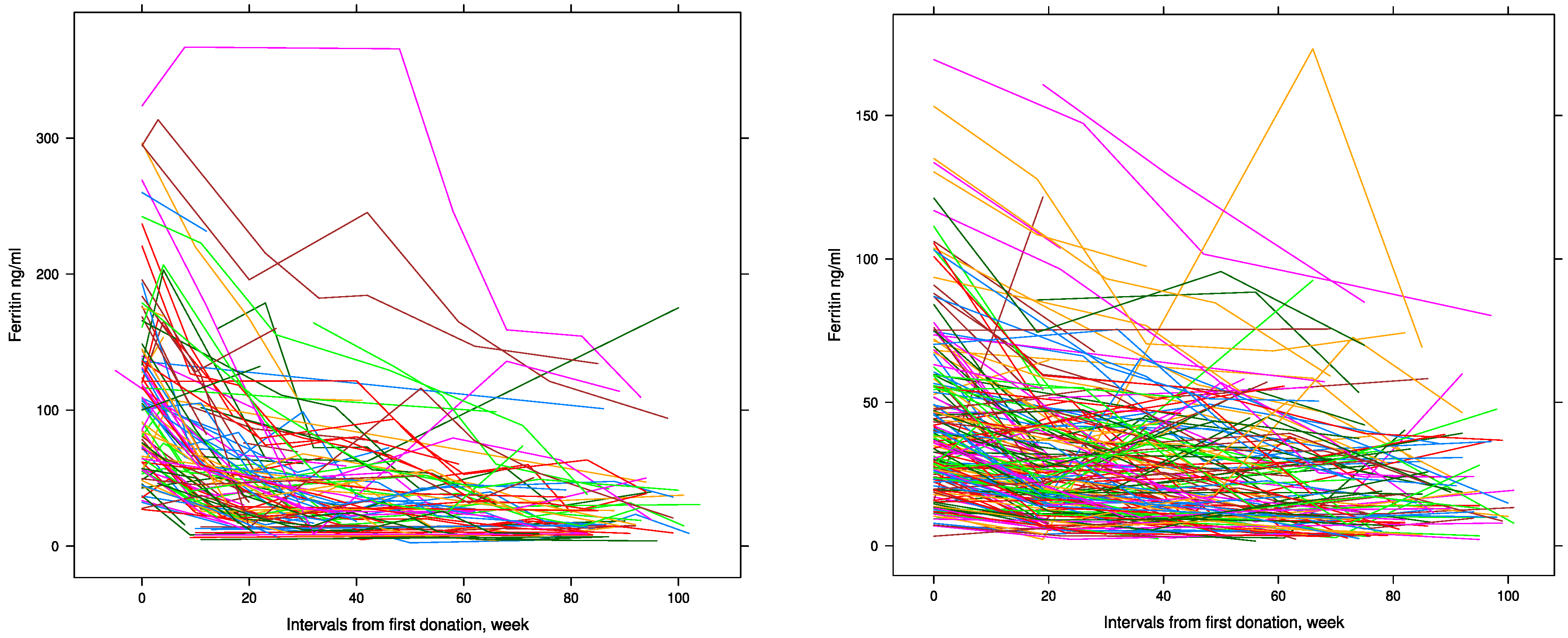

| Variables | Male Donors (n = 101) | Female Donors (n = 199) |
|---|---|---|
| Age (years) | 29.96 (25.08–38.12) | 24.62 (22.58–29.60) |
| BMI (kg/m2) | 24.22 (22.48–26.10) | 23.14 (21.55–26.44) |
| Number of donations | 4 (2–6) | 3 (2–4) |
| Hb at screening visit (mmol/L) | 9.30 (8.90–9.60) | 8.30 (8.00–8.70) |
| Baseline ferritin levels (ng/mL) Ferritin at last donation 2 (ng/mL) | 103.35 (56.77–137.76) 28.13 (17.80–43.24) | 36.66 (23.21–56.59) 13.04 (10.63–29.30) |
| Inter-donation interval (weeks) | 37.00 (19.00–63.00) | 46.00 (24.00–69.00) |
| Donation in cold seasons (%) | 47.1 | 48.2 |
| Male Donors | Female Donors | |||
|---|---|---|---|---|
| Variables | Class 1 (n = 87) | Class 2 (n = 14) | Class 1 (n = 181) | Class 2 (n = 18) |
| Age (years) * | 28.11 (24.82–34.27) | 39.43 (31.74–47.64) | 24.42 (22.36–27.98) | 35.00 (27.28–47.59) |
| BMI (kg/m2) * | 23.86 (22.15–25.69) | 27.46 (24.81–28.34) | 22.85 (21.48–26.23) | 25.18 (23.23–29.04) |
| Number of donations | 4 (2–6) | 4 (2–6) | 3 (2–4) | 3 (2–5) |
| Hb at screening visit (ng/mL) | 9.40 (8.90–9.80) | 9.05 (8.82–9.65) | 8.30 (8.00–8.80) | 8.35 (8.17–8.55) |
| Ferritin at screening visit (ng/mL) * Ferritin at last donation 2 (ng/mL) * | 85.40 (56.10–120.24) 24.89 (14.16–31.10) | 239.62 (181.63–294.61) 53.26 (43.78–133.74) | 32.69 (20.34–48.90) 13.52 (6.30–28.46) | 114.13 (102.55–135.31) 53.45 (39.01–68.89) |
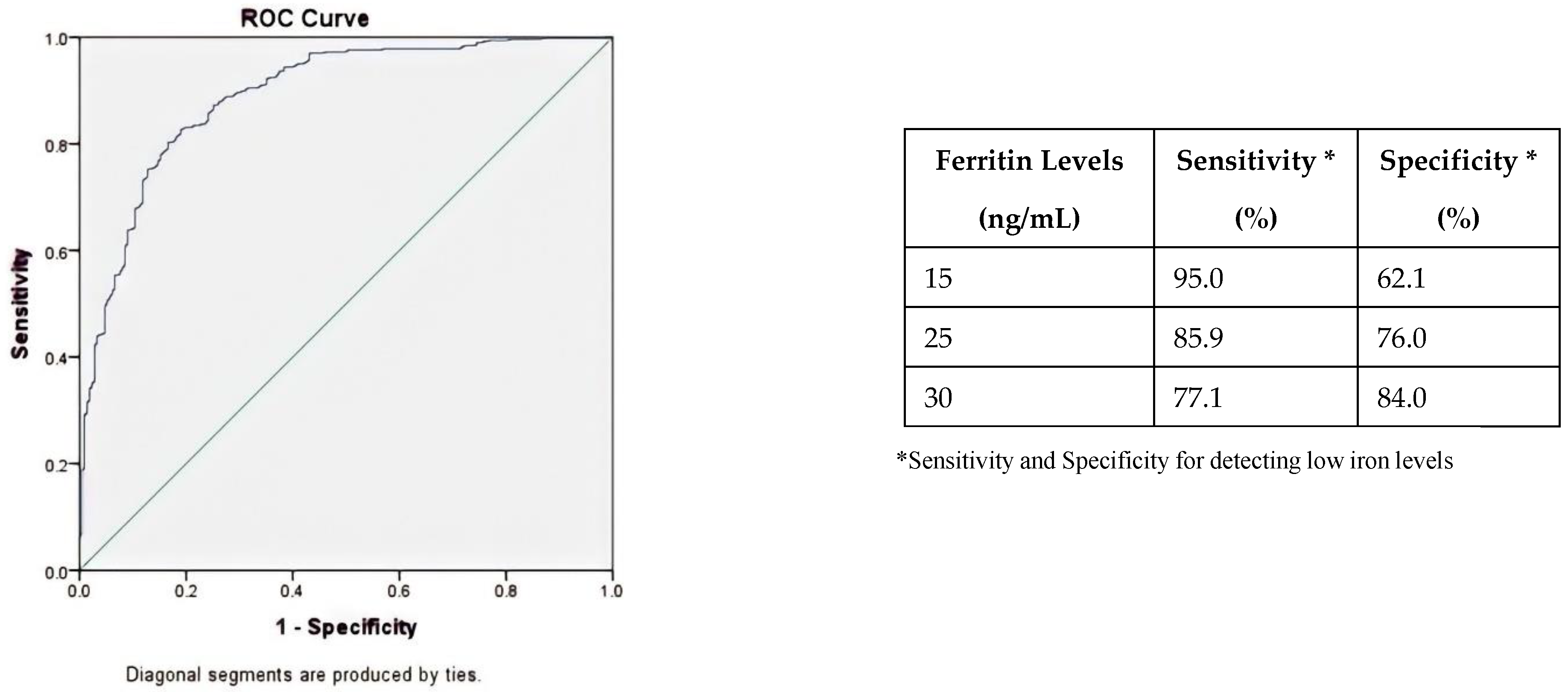
| Donor Ferritin Levels (ng/mL) * | N ** | Odds Ratio | 95% Confidence Interval | p-Value |
|---|---|---|---|---|
| Ferritin levels <15 | 99/193 | 3.83 | 3.19 to 4.48 | <0.001 |
| Ferritin levels 15–19.9 | 26/82 | 2.44 | 1.73 to 3.15 | <0.001 |
| Ferritin levels 20.1–24.9 | 15/62 | 2.10 | 1.31 to 2.89 | <0.001 |
| Ferritin levels 25–29.9 | 16/85 | 1.45 | 0.71 to 2.22 | 0.08 |
| Ferritin levels >30 | 20/473 | Ref | Ref | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moazzen, S.; Sweegers, M.G.; Janssen, M.; Hogema, B.M.; Hoekstra, T.; Van den Hurk, K. Ferritin Trajectories over Repeated Whole Blood Donations: Results from the FIND+ Study. J. Clin. Med. 2022, 11, 3581. https://doi.org/10.3390/jcm11133581
Moazzen S, Sweegers MG, Janssen M, Hogema BM, Hoekstra T, Van den Hurk K. Ferritin Trajectories over Repeated Whole Blood Donations: Results from the FIND+ Study. Journal of Clinical Medicine. 2022; 11(13):3581. https://doi.org/10.3390/jcm11133581
Chicago/Turabian StyleMoazzen, Sara, Maike G. Sweegers, Mart Janssen, Boris M. Hogema, Trynke Hoekstra, and Katja Van den Hurk. 2022. "Ferritin Trajectories over Repeated Whole Blood Donations: Results from the FIND+ Study" Journal of Clinical Medicine 11, no. 13: 3581. https://doi.org/10.3390/jcm11133581
APA StyleMoazzen, S., Sweegers, M. G., Janssen, M., Hogema, B. M., Hoekstra, T., & Van den Hurk, K. (2022). Ferritin Trajectories over Repeated Whole Blood Donations: Results from the FIND+ Study. Journal of Clinical Medicine, 11(13), 3581. https://doi.org/10.3390/jcm11133581






