Oral Valganciclovir Therapy in Infants Aged ≤2 Months with Congenital Cytomegalovirus Disease: A Multicenter, Single-Arm, Open-Label Clinical Trial in Japan
Abstract
:1. Introduction
2. Patients and Methods
2.1. Study Design and Procedure
2.2. Endpoints
2.3. Statistical Analysis of Endpoints
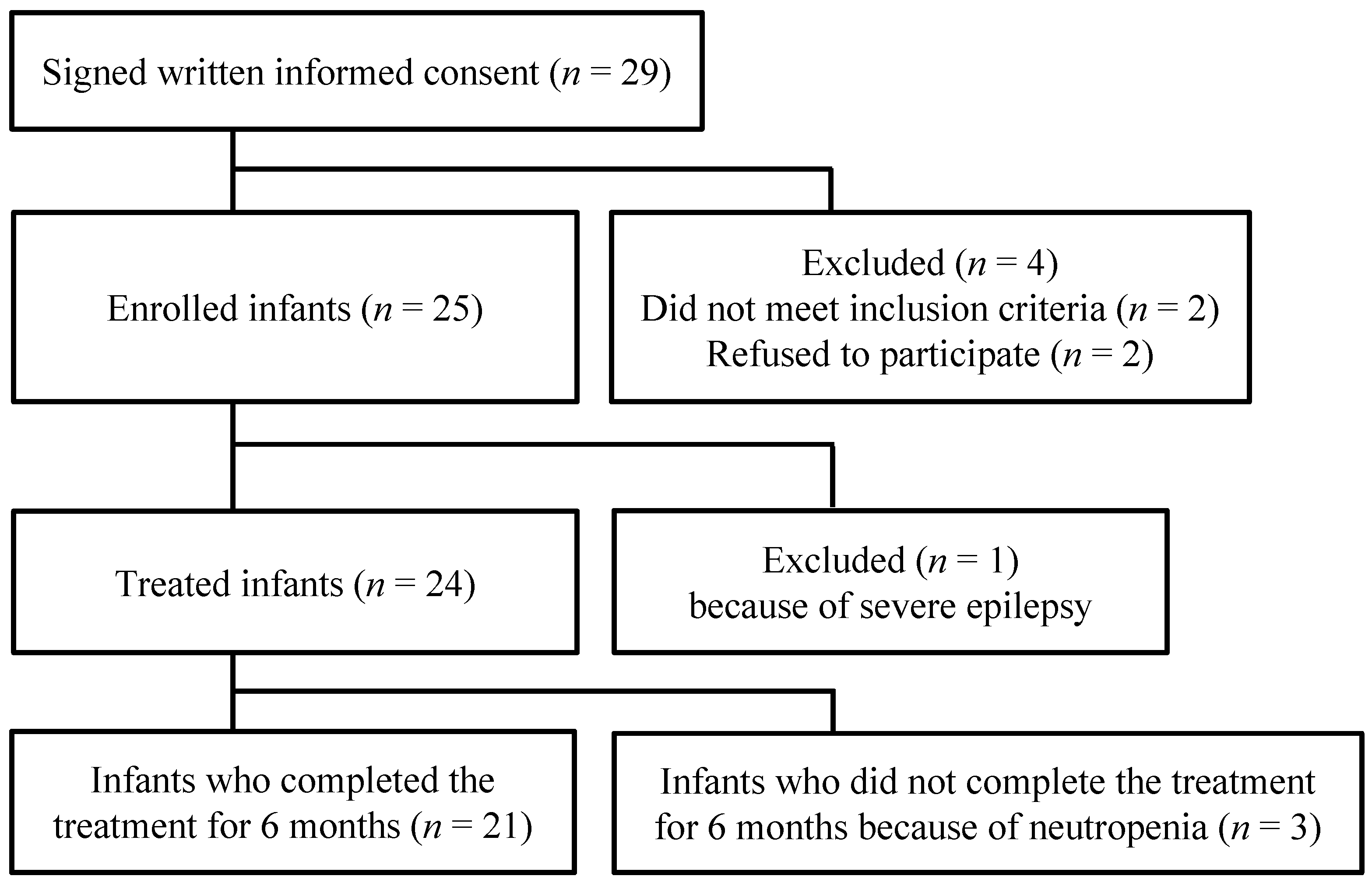
3. Results
3.1. Participant Selection, Baseline Demographics, and Clinical Characteristics
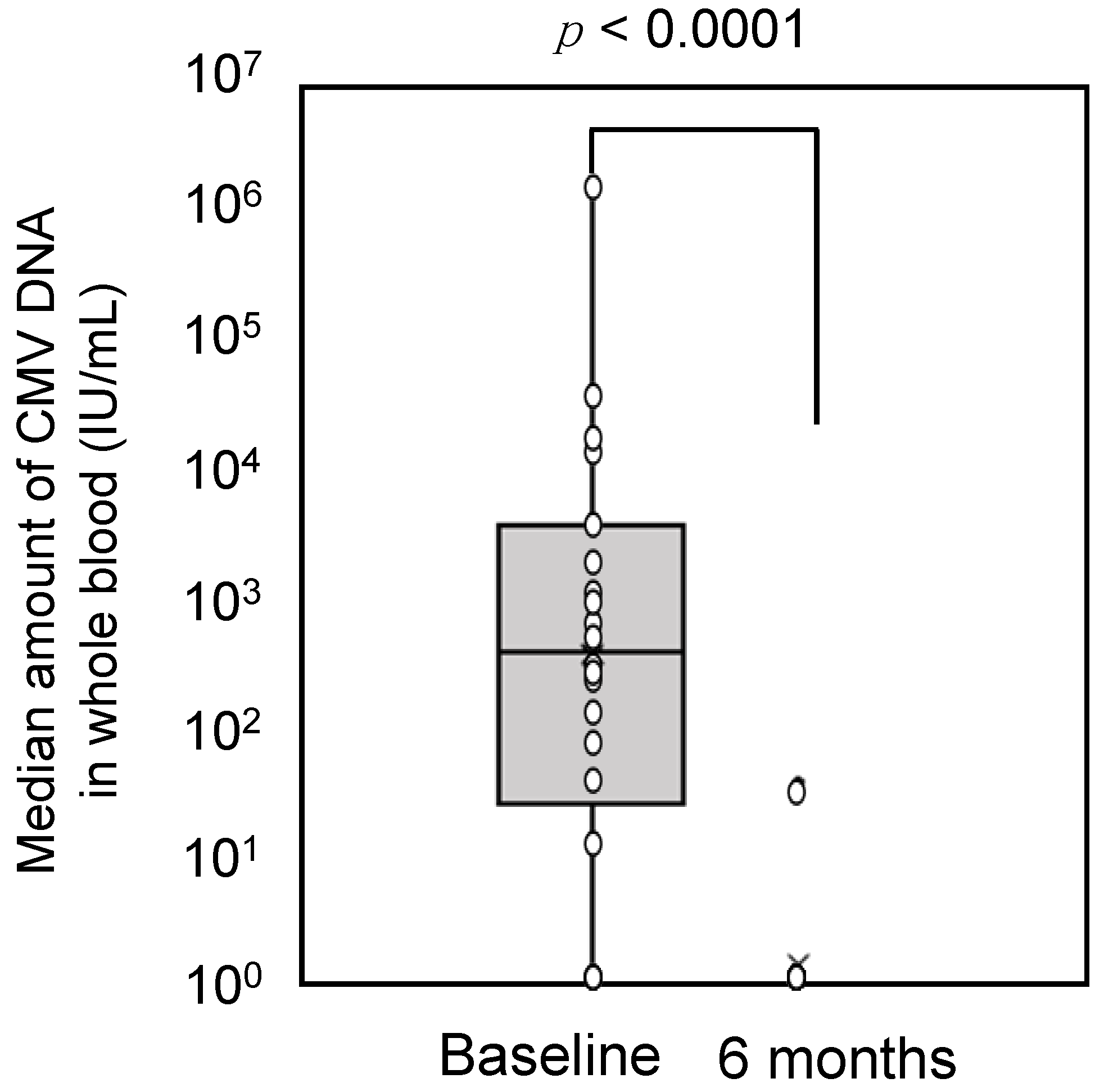
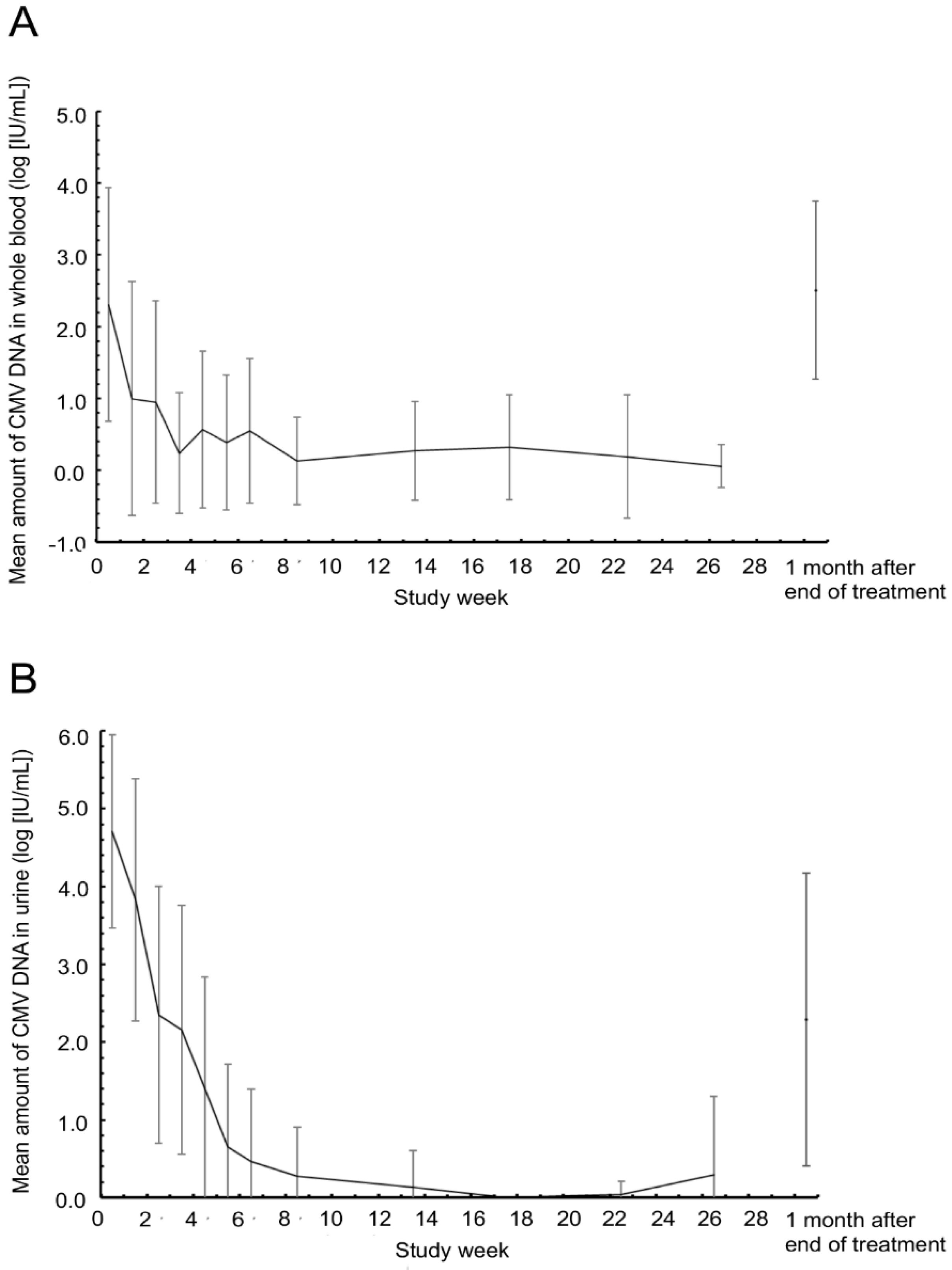
3.2. Primary Endpoint
3.3. Secondary Endpoints
3.4. Exploratory Endpoints
3.5. Safety Evaluations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mestas, E. Congenital cytomegalovirus. Adv. Neonatal Care 2016, 16, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Nagano, N.; Morioka, I. Congenital cytomegalovirus infection: Epidemiology, prediction, diagnosis, and emerging treatment options for symptomatic infants. Expert Opin. Orphan Drugs 2020, 8, 1–9. [Google Scholar] [CrossRef]
- Koyano, S.; Inoue, N.; Oka, A.; Moriuchi, H.; Asano, K.; Ito, Y.; Yamada, H.; Yoshikawa, T.; Suzutani, T.; Japanese Congenital Cytomegalovirus Study Group. Screening for congenital cytomegalovirus infection using newborn urine samples collected on filter paper: Feasibility and outcomes from a multicentre study. BMJ Open 2011, 1, e000118. [Google Scholar] [CrossRef]
- Yamada, H.; Tanimura, K.; Fukushima, S.; Fujioka, K.; Deguchi, M.; Sasagawa, Y.; Tairaku, S.; Funakoshi, T.; Morioka, I. A cohort study of the universal neonatal urine screening for congenital cytomegalovirus infection. J. Infect. Chemother. 2020, 26, 790–794. [Google Scholar] [CrossRef]
- Kimberlin, D.W.; Lin, C.Y.; Sánchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Kiell, J.M.; et al. Effect of ganciclovir therapy on hearing in symptomatic congenital cytomegalovirus disease involving the central nervous system: A randomized, controlled trial. J. Pediatr. 2003, 14, 16–25. [Google Scholar] [CrossRef]
- Oliver, S.E.; Cloud, G.A.; Sánchez, P.J.; Demmler, G.J.; Dankner, W.; Shelton, M.; Jacobs, R.F.; Vaudry, W.; Pass, R.F.; Soong, S.J.; et al. Neurodevelopmental outcomes following ganciclovir therapy in symptomatic congenital cytomegalovirus infections involving the central nervous system. J. Clin. Virol. 2009, 46, S22–S26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimberlin, D.W.; Jester, P.M.; Sánchez, P.J.; Ahmed, A.; Arav-Boger, R.; Michaels, M.G.; Ashouri, N.; Englund, J.A.; Estrada, B.; Jacobs, R.F.; et al. Valganciclovir for symptomatic congenital cytomegalovirus disease. N. Engl. J. Med. 2015, 372, 933–943. [Google Scholar] [CrossRef] [Green Version]
- Luck, S.E.; Wieringa, J.W.; Blázquez-Gamero, D.; Henneke, P.; Schuster, K.; Butler, K.; Capretti, M.G.; Cilleruelo, M.J.; Curtis, N.; Garofoli, F.; et al. Congenital cytomegalovirus: A European expert consensus statement on diagnosis and management. Pediatr. Infect. Dis. J. 2017, 36, 1205–1213. [Google Scholar] [CrossRef]
- Rawlinson, W.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutré, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Kawada, J.; Torii, Y.; Kawano, Y.; Suzuki, M.; Kamiya, Y.; Kotani, T.; Kikkawa, F.; Kimura, H.; Ito, Y. Viral load in children with congenital cytomegalovirus infection identified on newborn hearing screening. J. Clin. Virol. 2015, 65, 41–45. [Google Scholar] [CrossRef]
- Nishida, K.; Morioka, I.; Nakamachi, Y.; Kobayashi, Y.; Imanishi, T.; Kawano, S.; Iwatani, S.; Koda, T.; Deguchi, M.; Tanimura, K.; et al. Neurological outcomes in symptomatic congenital cytomegalovirus-infected infants after introduction of newborn urine screening and antiviral treatment. Brain Dev. 2016, 38, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Koyano, S.; Morioka, I.; Oka, A.; Moriuchi, H.; Asano, K.; Ito, Y.; Yoshikawa, T.; Yamada, H.; Suzutani, T.; Inoue, N.; et al. Congenital cytomegalovirus in Japan: More than 2 year follow up of infected newborns. Pediatr. Int. 2018, 60, 57–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohyama, S.; Morioka, I.; Fukushima, S.; Yamana, K.; Nishida, K.; Iwatani, S.; Fujioka, K.; Matsumoto, H.; Imanishi, T.; Nakamachi, Y.; et al. Efficacy of valganciclovir treatment depends on the severity of hearing dysfunction in symptomatic infants with congenital cytomegalovirus infection. Int. J. Mol. Sci. 2019, 20, 1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukushima, S.; Morioka, I.; Ohyama, S.; Nishida, K.; Iwatani, S.; Fujioka, K.; Mandai, T.; Matsumoto, H.; Nakamachi, Y.; Deguchi, M.; et al. Prediction of poor neurological development in patients with symptomatic congenital cytomegalovirus diseases after oral valganciclovir treatment. Brain Dev. 2019, 41, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Suganuma, E.; Sakata, H.; Adachi, N.; Asanuma, S.; Furuichi, M.; Uejima, Y.; Sato, S.; Abe, T.; Matsumoto, D.; Takahashi, R.; et al. Efficacy, safety, and pharmacokinetics of oral valganciclovir in patients with congenital cytomegalovirus infection. J. Infect. Chemother. 2021, 27, 185–191. [Google Scholar] [CrossRef]
- Lombardi, G.; Garofoli, F.; Villani, P.; Tizzoni, M.; Angelini, M.; Cusato, M.; Bollani, L.; De Silvestri, A.; Regazzi, M.; Stronati, M. Oral valganciclovir treatment in newborns with symptomatic congenital cytomegalovirus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2009, 28, 1465–1470. [Google Scholar] [CrossRef]
- Bilavsky, E.; Shahar-Nissan, K.; Pardo, J.; Attias, J.; Amir, J. Hearing outcome of infants with congenital cytomegalovirus and hearing impairment. Arch. Dis. Child. 2016, 101, 433–438. [Google Scholar] [CrossRef]
- McCrary, H.; Sheng, X.; Greene, T.; Park, A. Long-term hearing outcomes of children with symptomatic congenital CMV treated with valganciclovir. Int. J. Pediatr. Otorhinolaryngol. 2019, 118, 124–127. [Google Scholar] [CrossRef]
- Ziv, L.; Yacobovich, J.; Pardo, J.; Yarden-Bilavsky, H.; Amir, J.; Osovsky, M.; Bilavsky, E. Hematologic adverse events associated with prolonged valganciclovir treatment in congenital cytomegalovirus infection. Pediatr. Infect. Dis. J. 2019, 38, 127–130. [Google Scholar] [CrossRef]
- Turriziani Colonna, A.; Buonsenso, D.; Pata, D.; Salerno, G.; Chieffo, D.P.; Romeo, D.M.; Faccia, V.; Conti, G.; Molle, F.; Baldascino, A.; et al. Long-term clinical, audiological, visual, neurocognitive and behavioral outcome in children with symptomatic and asymptomatic congenital cytomegalovirus infection treated with valganciclovir. Front. Med. 2020, 7, 268. [Google Scholar] [CrossRef]
- Dorfman, L.; Amir, J.; Attias, J.; Bilavsky, E. Treatment of congenital cytomegalovirus beyond the neonatal period: An observational study. Eur. J. Pediatr. 2020, 179, 807–812. [Google Scholar] [CrossRef] [PubMed]
- Morioka, I.; Kakei, Y.; Omori, T.; Nozu, K.; Fujioka, K.; Yoshikawa, T.; Moriuchi, H.; Ito, Y.; Oka, A. Efficacy and safety of valganciclovir in patients with symptomatic congenital cytomegalovirus disease: Study Protocol Clinical Trial (SPIRIT Compliant). Medicine 2020, 99, e19765. [Google Scholar] [CrossRef] [PubMed]
- Revello, M.; Gerna, G. Diagnosis and management of human cytomegalovirus infection in the mother, fetus, and newborn infant. Clin. Microbiol. Rev. 2002, 15, 680–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Infants | ||
| Height at birth (cm) | Mean | 45.86 |
| SD | 3.64 | |
| Min. | 37.0 | |
| Median | 46.85 | |
| Max. | 51.0 | |
| Body weight at birth (g) | Mean | 2367.9 |
| SD | 521.7 | |
| Min. | 1304 | |
| Median | 2437 | |
| Max. | 3306 | |
| Gestational age at birth (weeks) | Mean | 37.95 |
| SD | 2.18 | |
| Min. | 34.0 | |
| Median | 38.43 | |
| Max. | 41.7 | |
| Central nervous system disorder (n (%)) | Microcephaly | 2 (8.3) |
| Hydrocephalus or ventricular enlargement | 12 (50.0) | |
| Periventricular calcification | 3 (12.5) | |
| Cortical hypoplasia | 7 (29.2) | |
| White matter injury | 8 (33.3) | |
| Retinal choroiditis | 1 (4.2) | |
| Abnormal auditory brainstem response | 21(87.5) | |
| Whole blood CMV load (IU/mL) | Mean | 93,739.8 |
| SD | 389,400.6 | |
| Min. | 11 | |
| Median | 470.0 | |
| Max. | 1,701,276 | |
| Level of best ear assessment (n (%)) | Normal | 5 (20.8) |
| Mild | 13 (54.2) | |
| Moderate | 5 (20.8) | |
| Severe | 1 (4.2) | |
| Age at the time of the first dose of medication (days) | Mean | 38.2 |
| SD | 13.3 | |
| Min. | 14 | |
| Median | 35.5 | |
| Max. | 66 | |
| Mothers | ||
| Age (years) | Mean | 29.9 |
| SD | 6.2 | |
| Min. | 17 | |
| Median | 28.5 | |
| Max. | 39 | |
| Childbirth status (n (%)) | Primipara | 11(45.8) |
| Multipara | 13 (54.2) | |
| Occupation (n (%)) | Childcare-giver at nursery | 1 (4.2) |
| Nurse and health care workers | 3 (12.5) | |
| Others | 13 (54.2) | |
| Unemployed | 7 (29.2) |
| A. Best Ear (n = 24) | Cases (%) | |
| Change in ABR before and after 6 months of treatment | (a) Improved hearing | 14 (58.3) |
| (b) No change (normal hearing) | 4 (16.7) | |
| (c) No change (same degree of hearing impairment) | 6 (25.0) | |
| (d) Hearing deteriorated | 0 (0.0) | |
| (a) + (b) + (c) | 24 (100.0) | |
| 95% CI 1 | 86.2–100.0 | |
| (a) + (b) | 18 (75.0) | |
| 95% CI 1 | 55.1–88.0 | |
| B. Total Ear (n = 48) | Ears (%) | |
| Change in ABR before and after 6 months of treatment | (a) Improved hearing | 19 (39.6) |
| (b) No change (normal hearing) | 6 (12.5) | |
| (c) No change (same degree of hearing impairment) | 20 (41.7) | |
| (d) Hearing deteriorated | 3 (6.3) | |
| (a) + (b) + (c) | 45 (93.8) | |
| 95% CI 2 | 87.1–100.0 | |
| (a) + (b) | 25 (52.1) | |
| 95% CI 2 | 37.4–66.8 |
| A. Best Ear | Younger Age Group (14–28 Days of Age) | Older Age Group (31–66 Days of Age) | p-Value | |
| n = 7 | n = 17 | |||
| Cases (%) | Cases (%) | |||
| Change in ABR before and after 6 months of treatment | (a) Improved hearing | 4 (57.1) | 10 (58.8) | |
| (b) No change (normal hearing) | 1 (14.3) | 3 (17.6) | ||
| (c) No change (same degree of hearing impairment) | 2 (28.6) | 4 (23.5) | ||
| (d) Hearing deteriorated | 0 (0.0) | 0 (0.0) | ||
| (a) + (b) + (c) | 7 (100.0) | 17 (100.0) | – | |
| 95% CI 1 | 64.6–100.0 | 81.6–100.0 | ||
| (a) + (b) | 5 (71.4) | 13 (76.5) | 1.000 2 | |
| 95% CI 1 | 35.9–91.8 | 52.7–90.4 | ||
| B. Total Ear | Younger Age Group | Older Age Group | p-Value | |
| n = 14 | n = 34 | |||
| Ears (%) | Ears (%) | |||
| Change in ABR before and after 6 months of treatment | (a) Improved hearing | 4 (28.6) | 15 (44.1) | |
| (b) No change (normal hearing) | 1 (7.1) | 5 (14.7) | ||
| (c) No change (same degree of hearing impairment) | 7 (50.0) | 13 (38.2) | ||
| (d) Hearing deteriorated | 2 (14.3) | 1 (2.9) | ||
| (a) + (b) + (c) | 12 (85.7) | 33 (97.1) | 0.208 4 | |
| 95% CI 3 | 69.0–100.0 | 91.5–100.0 | ||
| (a) + (b) | 5 (35.7) | 20 (58.8) | 0.071 4 | |
| 95% CI 3 | 19.0–52.5 | 40.2–77.5 |
| A. Best Ear | 6 Months n = 21 | 6 Weeks n = 21 | p-Value | ||
| Cases (%) | Cases (%) | ||||
| Change in ABR before and after treatment | (a) Improved hearing | 12 (57.1) | 10 (47.6) | ||
| (b) No change (normal hearing) | 4 (19.1) | 4 (19.1) | |||
| (c) No change (same degree of hearing impairment) | 5 (23.8) | 7 (33.3) | |||
| (d) Hearing deteriorated | 0 (0.0) | 0 (0.0) | |||
| (a) + (b) + (c) | 21 (100.0) | 21 (100.0) | – | ||
| 95% CI 1 | 84.5–100.0 | 84.5–100.0 | |||
| (a) + (b) | 16 (76.2) | 14 (66.7) | 0.414 2 | ||
| 95% CI 1 | 54.9–89.4 | 45.4–82.8 | |||
| B. Total Ear | 6 Months n = 42 | 6 Weeks n = 42 | p-Value | ||
| Ears (%) | Ears (%) | ||||
| Change in ABR before and after treatment | (a) Improved hearing | 16 (38.1) | 14 (33.3) | ||
| (b) No change (normal hearing) | 6 (14.3) | 7 (16.7) | |||
| (c) No change (same degree of hearing impairment) | 17 (40.5) | 20 (47.6) | |||
| (d) Hearing deteriorated | 3 (7.1) | 1 (2.4) | |||
| (a) + (b) + (c) | 39 (92.9) | 41 (97.6) | 0.273 4 | ||
| 95% CI 3 | 85.4–100.0 | 93.1–100.0 | |||
| (a) + (b) | 22 (52.4) | 21 (50.0) | 0.801 4 | ||
| 95% CI 3 | 36.9–67.8 | 32.5–67.5 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morioka, I.; Kakei, Y.; Omori, T.; Nozu, K.; Fujioka, K.; Takahashi, N.; Yoshikawa, T.; Moriuchi, H.; Ito, Y.; Oka, A., on behalf of the Japanese Congenital Cytomegalovirus Study Group. Oral Valganciclovir Therapy in Infants Aged ≤2 Months with Congenital Cytomegalovirus Disease: A Multicenter, Single-Arm, Open-Label Clinical Trial in Japan. J. Clin. Med. 2022, 11, 3582. https://doi.org/10.3390/jcm11133582
Morioka I, Kakei Y, Omori T, Nozu K, Fujioka K, Takahashi N, Yoshikawa T, Moriuchi H, Ito Y, Oka A on behalf of the Japanese Congenital Cytomegalovirus Study Group. Oral Valganciclovir Therapy in Infants Aged ≤2 Months with Congenital Cytomegalovirus Disease: A Multicenter, Single-Arm, Open-Label Clinical Trial in Japan. Journal of Clinical Medicine. 2022; 11(13):3582. https://doi.org/10.3390/jcm11133582
Chicago/Turabian StyleMorioka, Ichiro, Yasumasa Kakei, Takashi Omori, Kandai Nozu, Kazumichi Fujioka, Naoto Takahashi, Tetsushi Yoshikawa, Hiroyuki Moriuchi, Yoshinori Ito, and Akira Oka on behalf of the Japanese Congenital Cytomegalovirus Study Group. 2022. "Oral Valganciclovir Therapy in Infants Aged ≤2 Months with Congenital Cytomegalovirus Disease: A Multicenter, Single-Arm, Open-Label Clinical Trial in Japan" Journal of Clinical Medicine 11, no. 13: 3582. https://doi.org/10.3390/jcm11133582
APA StyleMorioka, I., Kakei, Y., Omori, T., Nozu, K., Fujioka, K., Takahashi, N., Yoshikawa, T., Moriuchi, H., Ito, Y., & Oka, A., on behalf of the Japanese Congenital Cytomegalovirus Study Group. (2022). Oral Valganciclovir Therapy in Infants Aged ≤2 Months with Congenital Cytomegalovirus Disease: A Multicenter, Single-Arm, Open-Label Clinical Trial in Japan. Journal of Clinical Medicine, 11(13), 3582. https://doi.org/10.3390/jcm11133582







