The Effect of Endurance Training on Serum BDNF Levels in the Chronic Post-Stroke Phase: Current Evidence and Qualitative Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Search Strategy
2.4. Data Extraction
2.5. Data Analysis: Quality Assessment
3. Results
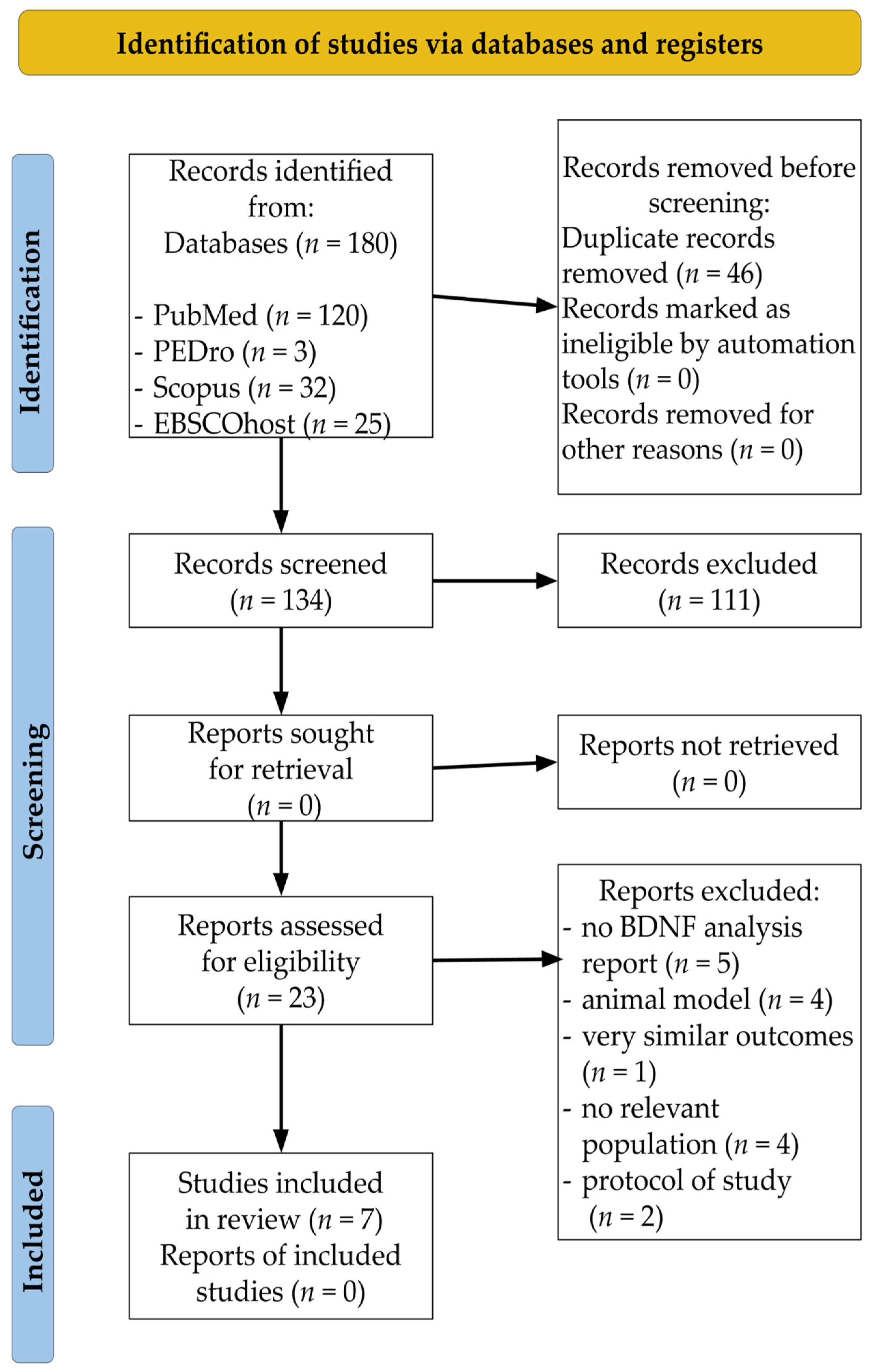
3.1. Study Selection
3.2. Study Characteristics
| No. | Type | Study | Country | Design | Patients (n) | Female (%) | Age (Years) | Post-Stroke Period (Months) |
|---|---|---|---|---|---|---|---|---|
| 1. | Single bout | Charalambos et al., 2018 [65] | The United States | RCT | 34 | 32.35% | 60.47 ± 12.42 | 53.82 ± 57.42 |
| 2. | Morais et al., 2018 [63] | Brazil | CCT | 10 | 50% | 58.0 ± 12.8 | 110.4 ± 69.6 | |
| 3. | Boyne et al., 2019 [61] | The United States | RCT | 16 | 43.8% | 57.4 ± 9.7 | 78 ± 49.2 | |
| 4. | King et al., 2019 [66] | Canada | CCT | 35 | 34.29% | 65.2 ± 9.4 | 31.5 ± 26.7 | |
| 5. | Long term | El-Tamawy et al., 2014 [64] | Egypt | PCT | 30 | 30% | 48.4 ± 6.39 | 3–18 |
| 6. | Hsu et al., 2020 [62] | Taiwan | RCT | 23 | 13.04% | HIIT 58.5 (49.8–67.2) MICT 53.1 (46.2–60.0) | HIIT 38.5 (19.1–57.9) MICT 28.8 (3.35–54.2) | |
| 7. | Single bout and long term | Ploughman et al., 2019 [60] | Canada | RCT | 52 | 3.77% | 63.4 ± 11.3 | 41.0 ± 39.8 |
3.3. PEDro Assessment and Study Quality
| No. | Type | Study | Journal | 2020 Journal Impact Factor | 5 year Journal Impact Factor | TC | AC |
|---|---|---|---|---|---|---|---|
| 1. | Single bout | Charalambos et al., 2018 [65] | Top Stroke Rehabil. | 2.119 | 2.797 | 8 | 2.0 |
| 2. | Morais et al., 2018 [63] | Top Stroke Rehabil. | 2.119 | 2.797 | 14 | 3.5 | |
| 3. | Boyne et al., 2019 [60] | J Appl Physiol. | 3.531 | 4.006 | 14 | 4.67 | |
| 4. | King et al., 2019 [66] | Neurol Res. | 2.448 | 2.480 | 7 | 2.33 | |
| 5. | Long term | El-Tamawy et al., 2014 [64] | Neuro-Rehabilitation | 2.138 | 2.501 | 61 | 7.63 |
| 6. | Hsu et al., 2020 [62] | Ann Phys Rehabil Med. | 4.919 | 5.622 | 1 | 1.0 | |
| 7. | Single bout and long term | Ploughman et al., 2019 [60] | Neurorehabil Neural Repair. | 3.919 | 5.378 | 15 | 5.0 |
| No. | Type | Study | PEDro Criteria | EL | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C1 | C2 | C3 | C4 | C5 | C6 | C7 | C8 | C9 | C10 | C11 | TS * | ||||
| 1. | Single bout | Charalambos et al., 2018 [65] | + | + | - | - | - | - | - | + | - | + | + | 4 | 2 |
| 2. | de Morais et al., 2018 [63] | + | - | - | - | - | - | - | + | - | - | + | 2 | 2 | |
| 3. | Boyne et al., 2019 [61] | + | + | - | - | - | - | + | + | - | + | + | 5 | 2 | |
| 4. | King et al., 2019 [66] | - | - | - | - | - | - | - | + | - | - | + | 2 | 2 | |
| 5. | Long-term | El-Tamawy et al., 2014 [64] | + | - | - | + | - | - | - | - | - | + | + | 3 | 2 |
| 6. | Hsu et al., 2020 [62] | + | + | - | + | + | - | + | - | - | + | + | 6 | 1 | |
| 7. | Single bout and long-term | Ploughman et al., 2019 [60] | + | + | + | + | - | - | + | + | + | + | + | 8 | 1 |
3.4. Effect of a Single Bout of Endurance Activity on Serum BDNF Concentrations
3.5. Effect of Long-Term Endurance Activity on Serum BDNF Concentrations
| No. | Type | Study | Protocol | Results | |||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome Measures | Groups | Active Phase of Exercise | Dose, Intensity | Type of Intensity | Serum BDNF (ng/mL) | Clinical MeasuresScale | |||
| 1. | Single bout | Charalambos et al., 2018 [65] | BDNF Lactate | (1) CON (2) TMW (3) TBE | 5 min | (1) Walking at 25% of their fastest comfortable speed (2) High-intensity range (70–85% HRmax) or 13–15 RPE speed increased gradually, 0.05m/sek every 15 s (3) High-intensity range (70–85% HRmax) or 13–15 RPE speed increased gradually, 0.05m/sek every 15 s | (1) Treadmill (2) Treadmill (3) Cycled on a total body exerciser | (1) NS (2) NS (3) NS No significance within or between groups | Lactate (mM/L) (1) ↑Δ = 0.16 (2) ↑Δ = 2.22 (3) ↑Δ = 6.10 A significant increase between post and pre levels |
| 2. | Single bout | Morais et al., 2018 [63] | BDNF | (1) Mild intensity (2) Moderate intensity | 30 min | (1) 50–63% HRmax (2) 64–76% HRma | (1) Walk (2) Walk | (1) Δ = −0.04 (2) ↑Δ = +0.05 Significant differences between post and pre at moderate intensity. No significance between post vs. pre levels at mild intensity. | - |
| 3. | Single bout | Boyne et al., 2019 [61] | BDNF CSP | (1) GXT (2) HIT—treadmill (3) HIT—stepper (4) MCT—treadmill | (1) Symptom-limited (2) 20 min (3) 20 min (4) 20 min | (1) Incline increased 2–4% every 2 min; (2) and (3) 30 s burst of max speed walking alternated with 30-to-60-s recovery periods; (4) 45 ± 5% HRR. | (1) Treadmill (2) Treadmill (3) Stepper (4) Treadmill | (1) Δ = +4.6 (2) Δ = +3.2 (3) Δ = +2.1 (4) Δ = −0.7 Statistically differences between post levels at 2 vs. 4 Δ= +3.9 | CSP response from T0 to T20 (1) NS (2) ↑Δ = −0.1% (3) ↑Δ = +0.2% (4) ↑Δ = +2.9% |
| 4. | Single bout | King et al., 2019 [66] | BDNF IGF-1 | GXT | Symptom-limited | 0% treadmill grade for the initial 2 min, followed by a 2.5% increase in grade every 2 min until an incline of 10% was reached and a 0.05 m/s increase in speed every 2 min thereafter until the test was terminated | Body-weight-supported treadmill or total-body recumbent stepper | Δ = +2.0 | IGF-1 ↓Δ = −0.98 ng/mL |
| 5. | Long term | El-Tamawy Long termet al., 2014 [64] | BDNF ACER | (1) Control group (2) Study group | 8 weeks (1) 25–30 min 3 days/wk (2) 30 min 3 days/wk; | (1) Physiotherapy program (stretching and strengthening exercises, facilitation for each muscle, postural control, balance, functional and gait training) (2) and physiotherapy program + aerobic exercise; intensity not stated | (1) - (2) Bicycle ergometer | (2) post vs. pre ↑Δ = +4.65 post (2) vs. (1) ↑Δ = +3.17 | ACER post (2) vs. (1) ↑Δ = 5.14 |
| 6. | Long term | Hsu et al., 2020 [62] | BDNF VO2peak CO AVO2diff O2Hb HHb THb MMSE cell-bearing neurities | (1) HIIT (2) MICT | 36 sessions 2–3 sessions week | (1) Five 3 min intervals at 80% VO2peak with each interval separated by 3 min of exercise at 40% of VO2peak (2) 60% VO2peak | Bicycle ergometer | (1) ↑Δ= + 1.85 (2) ↓Δ = −1.42 | VO2peak (1) ↑Δ = +17.2% (2) ↑Δ = +8.09% CO (1) ↑Δ = +14.8% (2) NS AVO2diff NS O2Hb NS HHb (1) ↑Δ = +55.8% (2) NS THb (1) ↑Δ = +47.0% (2) NS MMSE NS cell-bearing neurities (1) ↑Δ = +14.2% (2) NS |
| 7. | Single bout and long term | Ploughman et al., 2019 [60] | BDNF IGF-1 RPMT | (1) Aerobic + COG (2) Aerobic + Games (3) Activity + COG (4) Activity + Games | 10 weeks 20–30 min aerobic or activity; 20–30 min COG or games | (1) and (2) 60–80% of VO2peak (3) and (4) therapeutic activity, functional task training, intensity not stated | (1) and (2) Treadmill with body weight support (3) - (4) - | pre vs. post NS pre vs. follow-up NS | IGF-1 NS across groups RPMP pre vs. follow-up (1) ↑Δ = +5.7 vs. (4) ↓Δ = −2.1 |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AC | average citations |
| BDNF | brain-derived neurotrophic factor |
| BHB | beta-hydroxybutyrate |
| BWS | body-weight support |
| CCT | controlled clinical trial |
| EA | endurance activity |
| GXT | graded exercise test |
| HIIT | high-intensity interval training |
| HRmax | maximum heart rate |
| IF | impact factor |
| MICT | moderate-intensity continuous training |
| PA | physical activity |
| PEDro | physiotherapy evidence database |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | the International Prospective Register of Systematic Reviews |
| RCT | randomised clinical trial |
| SARS-CoV-2 | severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) |
| TC | total citations |
| VO2max | maximum oxygen consumption rate |
| VO2 peak | peak oxygen consumption |
References
- Krishnamurthi, R.V.; Ikeda, T.; Feigin, V.L. Global, Regional and Country-Specific Burden of Ischaemic Stroke, Intracerebral Haemorrhage and Subarachnoid Haemorrhage: A Systematic Analysis of the Global Burden of Disease Study 2017. Neuroepidemiology 2020, 54, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update from the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Statystyczny, R. Statistical Yearbook of the Republic of Poland; Statistical Publishing Establishment: Warszawa, Poland, 2016. [Google Scholar]
- Ntaios, G.; Michel, P.; Georgiopoulos, G.; Guo, Y.; Li, W.; Xiong, J.; Calleja, P.; Ostos, F.; González-Ortega, G.; Fuentes, B.; et al. Characteristics and Outcomes in Patients With COVID-19 and Acute Ischemic Stroke. Stroke 2020, 51. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, R.G.; Abdalkader, M.; Qureshi, M.M.; Frankel, M.R.; Mansour, O.Y.; Yamagami, H.; Qiu, Z.; Farhoudi, M.; Siegler, J.E.; Yaghi, S.; et al. Global impact of COVID-19 on stroke care. Int. J. Stroke 2021, 16, 573–584. [Google Scholar] [CrossRef] [PubMed]
- Domaszewska, K.; Boraczyński, M.; Tang, Y.-Y.; Gronek, J.; Wochna, K.; Boraczyński, T.; Wieliński, D.; Gronek, P. Protective Effects of Exercise Become Especially Important for the Aging Immune System in The Covid-19 Era. Aging Dis. 2022, 13, 129. [Google Scholar] [CrossRef]
- Ferro, J.; Caeiro, L.; Figueira, M.L. Neuropsychiatric sequelae of stroke. Nat. Rev. Neurol. 2016, 12, 269–280. [Google Scholar] [CrossRef]
- Kalaria, R.N.; Akinyemi, R.; Ihara, M. Stroke injury, cognitive impairment and vascular dementia. Biochim. Biophys. Acta (BBA) Mol. Basis Dis. 2016, 1862, 915–925. [Google Scholar] [CrossRef]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef]
- Tomasi, D.; Volkow, N.D. Aging and functional brain networks. Mol. Psychiatry 2011, 17, 549–558. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Constans, A.; Pin-Barre, C.; Temprado, J.-J.; Decherchi, P.; Laurin, J. Influence of Aerobic Training and Combinations of Interventions on Cognition and Neuroplasticity after Stroke. Front. Aging Neurosci. 2016, 8, 164. [Google Scholar] [CrossRef] [PubMed]
- Curuk, E.; Goyal, N.; Aruin, A.S. The Effect of Motor and Cognitive Tasks on Gait in People with Stroke. J. Stroke Cerebrovasc. Dis. 2019, 28, 104330. [Google Scholar] [CrossRef] [PubMed]
- Kirk-Sanchez, N.; McGough, E. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2013, 9, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Penna, L.G.; Pinheiro, J.P.; Ramalho, S.H.R.; Ribeiro, C.F. Effects of aerobic physical exercise on neuroplasticity after stroke: Systematic review. Arq. Neuro-Psiquiatr. 2021, 79, 832–843. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Colcombe, S.J.; Wadhwa, R.; Bherer, L.; Peterson, M.S.; Scalf, P.E.; Kim, J.; Alvarado, M.; Kramer, A. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiol. Aging 2007, 28, 272–283. [Google Scholar] [CrossRef]
- Seidler, R.D.; Bernard, J.A.; Burutolu, T.B.; Fling, B.W.; Gordon, M.T.; Gwin, J.T.; Kwak, Y.; Lipps, D.B. Motor control and aging: Links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev. 2010, 34, 721–733. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Tsai, H.-H.; Fu, T.-C.; Wang, J.-S. Exercise Training Enhances Platelet Mitochondrial Bioenergetics in Stroke Patients: A Randomized Controlled Trial. J. Clin. Med. 2019, 8, 2186. [Google Scholar] [CrossRef]
- Di Raimondo, D.; Rizzo, G.; Musiari, G.; Tuttolomondo, A.; Pinto, A.A. Role of Regular Physical Activity in Neuroprotection against Acute Ischemia. Int. J. Mol. Sci. 2020, 21, 9086. [Google Scholar] [CrossRef]
- Cobianchi, S.; Arbat-Plana, A.; López-Álvarez, V.M.; Navarro, X. Neuroprotective Effects of Exercise Treatments After Injury: The Dual Role of Neurotrophic Factors. Curr. Neuropharmacol. 2017, 15, 495–518. [Google Scholar] [CrossRef]
- Pin-Barre, C.; Hugues, N.; Constans, A.; Berton, E.; Pellegrino, C.; Laurin, J. Effects of Different High-Intensity Interval Training Regimens on Endurance and Neuroplasticity After Cerebral Ischemia. Stroke 2021, 52, 1109–1114. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Carl, D.; Khoury, J.C.; Gerson, M.; Kissela, B.; et al. Effects of Exercise Intensity on Acute Circulating Molecular Responses Poststroke. Neurorehabilit. Neural Repair 2020, 34, 222–234. [Google Scholar] [CrossRef] [PubMed]
- Pin-Barre, C.; Constans, A.; Brisswalter, J.; Pellegrino, C.; Laurin, J. Effects of High- Versus Moderate-Intensity Training on Neuroplasticity and Functional Recovery After Focal Ischemia. Stroke 2017, 48, 2855–2864. [Google Scholar] [CrossRef] [PubMed]
- Severinsen, M.C.K.; Pedersen, B.K. Muscle–Organ Crosstalk: The Emerging Roles of Myokines. Endocr. Rev. 2020, 41, 594–609. [Google Scholar] [CrossRef] [PubMed]
- Rosenthal, A.; Goeddel, D.V.; Nguyen, T.; Martin, E.; Burton, L.E.; Shih, A.; Laramee, G.R.; Wurm, F.; Mason, A.; Nikolics, K.; et al. Primary Structure and Biological Activity of Human Brain-Derived Neurotrophic Factor. Endocrinology 1991, 129, 1289–1294. [Google Scholar] [CrossRef]
- Hanson, I.M.; Seawright, A.; van Heyningen, V. The human BDNF gene maps between FSHB and HVBS1 at the boundary of 11p13–p14. Genomics 1992, 13, 1331–1333. [Google Scholar] [CrossRef]
- Dinoff, A.; Herrmann, N.; Swardfager, W.; Lanctôt, K.L. The effect of acute exercise on blood concentrations of brain-derived neurotrophic factor in healthy adults: A meta-analysis. Eur. J. Neurosci. 2017, 46, 1635–1646. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Schwald, M.; Cisse, M. Postnatal developmental profile of brain-derived neurotrophic factor in rat brain and platelets. Neurosci. Lett. 2002, 328, 261–264. [Google Scholar] [CrossRef]
- Klein, A.B.; Williamson, R.; Santini, M.A.; Clemmensen, C.; Ettrup, A.; Rios, M.; Knudsen, G.M.; Aznar, S. Blood BDNF concentrations reflect brain-tissue BDNF concentrations across species. Int. J. Neuropsychopharmacol. 2011, 14, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, P.; Brassard, P.; Adser, H.; Pedersen, M.V.; Leick, L.; Hart, E.; Secher, N.H.; Pedersen, B.K.; Pilegaard, H. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Exp. Physiol. 2009, 94, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, E.J.; Reichardt, L.F. Neurotrophins: Roles in Neuronal Development and Function. Annu. Rev. Neurosci. 2001, 24, 677–736. [Google Scholar] [CrossRef]
- Lommatzsch, M.; Zingler, D.; Schuhbaeck, K.; Schloetcke, K.; Zingler, C.; Schuff-Werner, P.; Virchow, J.C. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol. Aging 2005, 26, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.-T.; Luo, L.; Wu, Y.-J.; Liu, B.-B.; Liu, X.-L.; Geng, D.; Liu, Q. Circadian variations in behaviors, BDNF and cell proliferation in depressive mice. Metab. Brain Dis. 2015, 30, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Jamal, M.; Van der Does, W.; Elzinga, B.M.; Molendijk, M.L.; Penninx, B.W. Association Between Smoking, Nicotine Dependence, and BDNF Val66Met Polymorphism with BDNF Concentrations in Serum. Nicotine Tob. Res. 2014, 17, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Hebisz, P.; Hebisz, R.; Murawska-Ciałowicz, E.; Zatoń, M. Changes in exercise capacity and serum BDNF following long-term sprint interval training in well-trained cyclists. Appl. Physiol. Nutr. Metab. 2019, 44, 499–506. [Google Scholar] [CrossRef]
- Murawska-Ciałowicz, E.; de Assis, G.G.; Clemente, F.M.; Feito, Y.; Stastny, P.; Zuwała-Jagiełło, J.; Bibrowicz, B.; Wolański, P. Effect of four different forms of high intensity training on BDNF response to Wingate and Graded Exercise Test. Sci. Rep. 2021, 11, 8599. [Google Scholar] [CrossRef]
- Nicolini, C.; Toepp, S.; Harasym, D.; Michalski, B.; Fahnestock, M.; Gibala, M.J.; Nelson, A.J. No changes in corticospinal excitability, biochemical markers, and working memory after six weeks of high-intensity interval training in sedentary males. Physiol. Rep. 2019, 7, e14140. [Google Scholar] [CrossRef]
- Huat, T.J.; Khan, A.A.; Pati, S.; Mustafa, Z.; Abdullah, J.M.; Jaafar, H. IGF-1 enhances cell proliferation and survival during early differentiation of mesenchymal stem cells to neural progenitor-like cells. BMC Neurosci. 2014, 15, 91. [Google Scholar] [CrossRef]
- El-Sayes, J.; Harasym, D.; Turco, C.V.; Locke, M.B.; Nelson, A.J. Exercise-Induced Neuroplasticity: A Mechanistic Model and Prospects for Promoting Plasticity. Neuroscientist 2018, 25, 65–85. [Google Scholar] [CrossRef]
- Mattson, M.P.; Maudsley, S.; Martin, B. BDNF and 5-HT: A dynamic duo in age-related neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2004, 27, 589–594. [Google Scholar] [CrossRef]
- Maass, A.; Düzel, S.; Brigadski, T.; Goerke, M.; Becke, A.; Sobieray, U.; Neumann, K.; Lövdén, M.; Lindenberger, U.; Bäckman, L.; et al. Relationships of peripheral IGF-1, VEGF and BDNF levels to exercise-related changes in memory, hippocampal perfusion and volumes in older adults. NeuroImage 2016, 131, 142–154. [Google Scholar] [CrossRef]
- Whiteman, A.S.; Young, D.E.; He, X.; Chen, T.C.; Wagenaar, R.C.; Stern, C.E.; Schon, K. Interaction between serum BDNF and aerobic fitness predicts recognition memory in healthy young adults. Behav. Brain Res. 2013, 259, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Rex, C.S.; Lauterborn, J.C.; Lin, C.-Y.; Kramár, E.A.; Rogers, G.A.; Gall, C.M.; Lynch, G. Restoration of Long-Term Potentiation in Middle-Aged Hippocampus After Induction of Brain-Derived Neurotrophic Factor. J. Neurophysiol. 2006, 96, 677–685. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Miller, D.L.; Roecklein, K.A. The Aging Hippocampus. Neuroscientist 2011, 18, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Colardo, M.; Martella, N.; Pensabene, D.; Siteni, S.; Di Bartolomeo, S.; Pallottini, V.; Segatto, M. Neurotrophins as Key Regulators of Cell Metabolism: Implications for Cholesterol Homeostasis. Int. J. Mol. Sci. 2021, 22, 5692. [Google Scholar] [CrossRef]
- Rosas-Vargas, H.; Martínez-Ezquerro, J.D.; Bienvenu, T. Brain-Derived Neurotrophic Factor, Food Intake Regulation, and Obesity. Arch. Med. Res. 2011, 42, 482–494. [Google Scholar] [CrossRef]
- Davarpanah, M.; Shokri-Mashhadi, N.; Ziaei, R.; Saneei, P. A systematic review and meta-analysis of association between brain-derived neurotrophic factor and type 2 diabetes and glycemic profile. Sci. Rep. 2021, 11, 13773. [Google Scholar] [CrossRef]
- Sharma, N.; Castorena, C.M.; Cartee, G.D. Greater insulin sensitivity in calorie restricted rats occurs with unaltered circulating levels of several important myokines and cytokines. Nutr. Metab. 2012, 9, 90. [Google Scholar] [CrossRef]
- Marosi, K.; Mattson, M.P. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol. Metab. 2013, 25, 89–98. [Google Scholar] [CrossRef]
- Li, J.; Zhao, Y.-D.; Zeng, J.-W.; Chen, X.-Y.; Wang, R.-D.; Cheng, S.-Y. Serum Brain-derived neurotrophic factor levels in post-stroke depression. J. Affect. Disord. 2014, 168, 373–379. [Google Scholar] [CrossRef]
- Xu, H.-B.; Xu, Y.-H.; He, Y.; Xue, F.; Wei, J.; Zhang, H.; Wu, J. Decreased Serum Brain-Derived Neurotrophic Factor May Indicate the Development of Poststroke Depression in Patients with Acute Ischemic Stroke: A Meta-Analysis. J. Stroke Cerebrovasc. Dis. 2017, 27, 709–715. [Google Scholar] [CrossRef]
- Terroni, L.M.N.; Mattos, P.F.; Sobreiro, M.F.M.; Guajardo, V.D.; Fráguas, R. Post-stroke depression: Psychological, neuropsychological, HHA axis, localization of stroke aspects and treatment. Rev. Psiquiatr. Clin. 2009, 36, 100–108. [Google Scholar] [CrossRef][Green Version]
- McKenzie, J.E.; Brennan, S.E.; Ryan, R.E.; Thomson, H.J.; Johnston, R.V.; Thomas, J. Chapter 3: Defining the criteria for including studies and how they will be grouped for the synthesis. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 88, 105906. [Google Scholar] [CrossRef]
- Huang, X.; Lin, J.; Demner-Fushman, D. Evaluation of PICO as a knowledge representation for clinical questions. AMIA Annu. Symp. Proc. 2006, 2006, 359–363. [Google Scholar]
- Macedo, L.G.; Elkins, M.; Maher, C.; Moseley, A.M.; Herbert, R.; Sherrington, C. There was evidence of convergent and construct validity of Physiotherapy Evidence Database quality scale for physiotherapy trials. J. Clin. Epidemiol. 2010, 63, 920–925. [Google Scholar] [CrossRef]
- de Morton, N.A. The PEDro scale is a valid measure of the methodological quality of clinical trials: A demographic study. Aust. J. Physiother. 2009, 55, 129–133. [Google Scholar] [CrossRef]
- Verhagen, A.P.; de Vet, H.C.; de Bie, R.A.; Kessels, A.G.; Boers, M.; Bouter, L.M.; Knipschild, P.G. The Delphi List: A Criteria List for Quality Assessment of Randomized Clinical Trials for Conducting Systematic Reviews Developed by Delphi Consensus. J. Clin. Epidemiol. 1998, 51, 1235–1241. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef]
- Ploughman, M.; Eskes, G.A.; Kelly, L.P.; Kirkland, M.C.; Devasahayam, A.J.; Wallack, E.M.; Abraha, B.; Hasan, S.M.M.; Downer, M.B.; Keeler, L.; et al. Synergistic Benefits of Combined Aerobic and Cognitive Training on Fluid Intelligence and the Role of IGF-1 in Chronic Stroke. Neurorehabilit. Neural Repair 2019, 33, 199–212. [Google Scholar] [CrossRef]
- Boyne, P.; Meyrose, C.; Westover, J.; Whitesel, D.; Hatter, K.; Reisman, D.S.; Cunningham, D.; Carl, D.; Jansen, C.; Khoury, J.C.; et al. Exercise intensity affects acute neurotrophic and neurophysiological responses poststroke. J. Appl. Physiol. 2019, 126, 431–443. [Google Scholar] [CrossRef]
- Hsu, C.-C.; Fu, T.-C.; Huang, S.-C.; Chen, C.P.-C.; Wang, J.-S. Increased serum brain-derived neurotrophic factor with high-intensity interval training in stroke patients: A randomized controlled trial. Ann. Phys. Rehabil. Med. 2020, 64, 101385. [Google Scholar] [CrossRef]
- De Morais, V.A.C.; Tourino, M.F.D.S.; Almeida, A.C.D.S.; Albuquerque, T.B.D.; Linhares, R.C.; Christo, P.P.; Martinelli, P.M.; Scalzo, P.L. A single session of moderate intensity walking increases brain-derived neurotrophic factor (BDNF) in the chronic post-stroke patients. Top. Stroke Rehabil. 2017, 25, 1–5. [Google Scholar] [CrossRef] [PubMed]
- El-Tamawy, M.S.; Abd-Allah, F.; Ahmed, S.M.; Darwish, M.H.; Khalifa, H.A. Aerobic exercises enhance cognitive functions and brain derived neurotrophic factor in ischemic stroke patients. NeuroRehabilitation 2014, 34, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Charalambous, C.C.; Helm, E.E.; Lau, K.A.; Morton, S.M.; Reisman, D.S. The feasibility of an acute high-intensity exercise bout to promote locomotor learning after stroke. Top. Stroke Rehabil. 2017, 25, 83–89. [Google Scholar] [CrossRef] [PubMed]
- King, M.; Kelly, L.P.; Wallack, E.M.; Hasan, S.M.M.; Kirkland, M.C.; Curtis, M.E.; Chatterjee, T.; McCarthy, J.; Ploughman, M. Serum levels of insulin-like growth factor-1 and brain-derived neurotrophic factor as potential recovery biomarkers in stroke. Neurol. Res. 2019, 41, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The effects of physical activity and exercise on brain-derived neurotrophic factor in healthy humans: A review. Scand. J. Med. Sci. Sports 2013, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Einstad, M.S.; Saltvedt, I.; Lydersen, S.; Ursin, M.H.; Munthe-Kaas, R.; Ihle-Hansen, H.; Knapskog, A.-B.; Askim, T.; Beyer, M.K.; Næss, H.; et al. Associations between post-stroke motor and cognitive function: A cross-sectional study. BMC Geriatr. 2021, 21, 103. [Google Scholar] [CrossRef]
- Tang, A.; Eng, J.; Krassioukov, A.; Tsang, T.; Liu-Ambrose, T. High- and low-intensity exercise do not improve cognitive function after stroke: A randomized controlled trial. J. Rehabil. Med. 2016, 48, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Bo, W.; Lei, M.; Tao, S.; Jie, L.T.; Qian, L.; Lin, F.Q.; Ping, W.X. Effects of combined intervention of physical exercise and cognitive training on cognitive function in stroke survivors with vascular cognitive impairment: A randomized controlled trial. Clin. Rehabil. 2018, 33, 54–63. [Google Scholar] [CrossRef]
- Moore, S.; Hallsworth, K.; Jakovljevic, D.; Blamire, A.; He, J.; Ford, G.A.; Rochester, L.; Trenell, M.I. Effects of Community Exercise Therapy on Metabolic, Brain, Physical, and Cognitive Function Following Stroke. Neurorehabilit. Neural Repair 2014, 29, 623–635. [Google Scholar] [CrossRef]
- Quaney, B.M.; Boyd, L.A.; McDowd, J.M.; Zahner, L.H.; He, J.; Mayo, M.S.; Macko, R.F. Aerobic Exercise Improves Cognition and Motor Function Poststroke. Neurorehabilit. Neural Repair 2009, 23, 879–885. [Google Scholar] [CrossRef]
- Mang, C.; Campbell, K.L.; Ross, C.; Boyd, L.A. Promoting Neuroplasticity for Motor Rehabilitation After Stroke: Considering the Effects of Aerobic Exercise and Genetic Variation on Brain-Derived Neurotrophic Factor. Phys. Ther. 2013, 93, 1707–1716. [Google Scholar] [CrossRef]
- Gagrani, M.; Faiq, M.A.; Sidhu, T.; Dada, R.; Yadav, R.K.; Sihota, R.; Kochhar, K.P.; Verma, R.; Dada, T. Meditation enhances brain oxygenation, upregulates BDNF and improves quality of life in patients with primary open angle glaucoma: A randomized controlled trial. Restor. Neurol. Neurosci. 2018, 36, 741–753. [Google Scholar] [CrossRef] [PubMed]
- Alcantara, C.C.; García-Salazar, L.-F.; Silva-Couto, M.A.; Santos, G.L.; Reisman, D.S.; Russo, T. Post-stroke BDNF Concentration Changes Following Physical Exercise: A Systematic Review. Front. Neurol. 2018, 9, 637. [Google Scholar] [CrossRef]
- Shih, P.-C.; Yang, Y.-R.; Wang, R.-Y. Effects of Exercise Intensity on Spatial Memory Performance and Hippocampal Synaptic Plasticity in Transient Brain Ischemic Rats. PLoS ONE 2013, 8, e78163. [Google Scholar] [CrossRef]
- Shimada, H.; Hamakawa, M.; Ishida, A.; Tamakoshi, K.; Nakashima, H.; Ishida, K. Low-speed treadmill running exercise improves memory function after transient middle cerebral artery occlusion in rats. Behav. Brain Res. 2013, 243, 21–27. [Google Scholar] [CrossRef]
- Soya, H.; Nakamura, T.; Deocaris, C.C.; Kimpara, A.; Iimura, M.; Fujikawa, T.; Chang, H.; McEwen, B.S.; Nishijima, T. BDNF induction with mild exercise in the rat hippocampus. Biochem. Biophys. Res. Commun. 2007, 358, 961–967. [Google Scholar] [CrossRef]
- Ploughman, M.; Austin, M.W.; Glynn, L.; Corbett, D. The Effects of Poststroke Aerobic Exercise on Neuroplasticity: A Systematic Review of Animal and Clinical Studies. Transl. Stroke Res. 2014, 6, 13–28. [Google Scholar] [CrossRef]
- Kuhne, L.A.; Ksiezarczyk, A.-M.; Braumann, K.-M.; Reer, R.; Jacobs, T.; Röder, B.; Hötting, K. The Effects of Acute Cardiovascular Exercise on Memory and Its Associations with Exercise-Induced Increases in Neurotrophic Factors. Front. Aging Neurosci. 2021, 13, 750401. [Google Scholar] [CrossRef] [PubMed]
- Skriver, K.; Roig, M.; Lundbye-Jensen, J.; Pingel, J.; Helge, J.; Kiens, B.; Nielsen, J.B. Acute exercise improves motor memory: Exploring potential biomarkers. Neurobiol. Learn. Mem. 2014, 116, 46–58. [Google Scholar] [CrossRef]
- Winter, B.; Breitenstein, C.; Mooren, F.C.; Voelker, K.; Fobker, M.; Lechtermann, A.; Krueger, K.; Fromme, A.; Korsukewitz, C.; Floel, A.; et al. High impact running improves learning. Neurobiol. Learn. Mem. 2007, 87, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.; Johnsen, L.K.; Geertsen, S.; Christiansen, L.; Ritz, C.; Roig, M.; Lundbye-Jensen, J. Acute Exercise and Motor Memory Consolidation: The Role of Exercise Intensity. PLoS ONE 2016, 11, e0159589. [Google Scholar] [CrossRef]
- Pyke, W.; Ifram, F.; Coventry, L.; Sung, Y.; Champion, I.; Javadi, A.-H. The effects of different protocols of physical exercise and rest on long-term memory. Neurobiol. Learn. Mem. 2019, 167, 107128. [Google Scholar] [CrossRef] [PubMed]
- Hasan, S.M.M.; Rancourt, S.N.; Austin, M.W.; Ploughman, M. Defining Optimal Aerobic Exercise Parameters to Affect Complex Motor and Cognitive Outcomes after Stroke: A Systematic Review and Synthesis. Neural Plast. 2016, 2016, 2961573. [Google Scholar] [CrossRef] [PubMed]
- Knaepen, K.; Goekint, M.; Heyman, E.; Meeusen, R. Neuroplasticity—Exercise-Induced Response of Peripheral Brain-Derived Neurotrophic Factor. Sports Med. 2010, 40, 765–801. [Google Scholar] [CrossRef]
- Hugues, N.; Pellegrino, C.; Rivera, C.; Berton, E.; Pin-Barre, C.; Laurin, J. Is High-Intensity Interval Training Suitable to Promote Neuroplasticity and Cognitive Functions after Stroke? Int. J. Mol. Sci. 2021, 22, 3003. [Google Scholar] [CrossRef] [PubMed]
- Limaye, N.S.; Carvalho, L.B.; Kramer, S. Effects of Aerobic Exercise on Serum Biomarkers of Neuroplasticity and Brain Repair in Stroke: A Systematic Review. Arch. Phys. Med. Rehabil. 2021, 102, 1633–1644. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.C.; Eng, J.J.; Dawson, A.S.; Gylfadóttir, S. The use of aerobic exercise training in improving aerobic capacity in individuals with stroke: A meta-analysis. Clin. Rehabil. 2006, 20, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.Y.; Charlesworth, S.A.; Lau, R.W.; Chung, R.C. Using Aerobic Exercise to Improve Health Outcomes and Quality of Life in Stroke: Evidence-Based Exercise Prescription Recommendations. Cerebrovasc. Dis. 2013, 35, 7–22. [Google Scholar] [CrossRef]
- Luo, L.; Li, C.; Deng, Y.; Wang, Y.; Meng, P.; Wang, Q. High-Intensity Interval Training on Neuroplasticity, Balance between Brain-Derived Neurotrophic Factor and Precursor Brain-Derived Neurotrophic Factor in Poststroke Depression Rats. J. Stroke Cerebrovasc. Dis. 2018, 28, 672–682. [Google Scholar] [CrossRef]
- Oberlin, L.E.; Waiwood, A.M.; Cumming, T.B.; Marsland, A.L.; Bernhardt, J.; Erickson, K.I. Effects of Physical Activity on Poststroke Cognitive Function. Stroke 2017, 48, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Saunders, D.H.; Sanderson, M.; Hayes, S.; Johnson, L.; Kramer, S.; Carter, D.D.; Jarvis, H.; Brazzelli, M.; E Mead, G. Physical fitness training for stroke patients. Cochrane Database Syst. Rev. 2020, 2020, CD003316. [Google Scholar] [CrossRef]
- Feter, N.; Alt, R.; Dias, M.G.; Rombaldi, A.J. How do different physical exercise parameters modulate brain-derived neurotrophic factor in healthy and non-healthy adults? A systematic review, meta-analysis and meta-regression. Sci. Sports 2019, 34, 293–304. [Google Scholar] [CrossRef]
- Carl, D.L.; Boyne, P.; Rockwell, B.; Gerson, M.; Khoury, J.; Kissela, B.; Dunning, K. Preliminary safety analysis of high-intensity interval training (HIIT) in persons with chronic stroke. Appl. Physiol. Nutr. Metab. 2017, 42, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Crozier, J.; Roig, M.; Eng, J.J.; MacKay-Lyons, M.; Fung, J.; Ploughman, M.; Bailey, D.M.; Sweet, S.N.; Giacomantonio, N.; Thiel, A.; et al. High-Intensity Interval Training After Stroke: An Opportunity to Promote Functional Recovery, Cardiovascular Health, and Neuroplasticity. Neurorehabilit. Neural Repair 2018, 32, 543–556. [Google Scholar] [CrossRef]
- Luo, L.; Meng, H.; Wang, Z.; Zhu, S.; Yuan, S.; Wang, Y.; Wang, Q. Effect of high-intensity exercise on cardiorespiratory fitness in stroke survivors: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2019, 63, 59–68. [Google Scholar] [CrossRef]
- Askim, T.; Dahl, A.E.; Aamot, I.L.; Hokstad, A.; Helbostad, J.; Indredavik, B. High-Intensity Aerobic Interval Training for Patients 3-9 Months After Stroke. A Feasibility Study. Physiother. Res. Int. 2013, 19, 129–139. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ke, Z.; Yip, S.P.; Hu, X.-L.; Zheng, X.-X.; Tong, K.-Y. Gradually Increased Training Intensity Benefits Rehabilitation Outcome after Stroke by BDNF Upregulation and Stress Suppression. BioMed Res. Int. 2014, 2014, 925762. [Google Scholar] [CrossRef]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate infusion at rest increases BDNF blood concentration in humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef] [PubMed]
- Coco, M.; Alagona, G.; Rapisarda, G.; Costanzo, E.; Calogero, R.A.; Perciavalle, V.; Perciavalle, V. Elevated blood lactate is associated with increased motor cortex excitability. Somatosens. Mot. Res. 2010, 27, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The Effect of Acute Exercise on Serum Brain-Derived Neurotrophic Factor Levels and Cognitive Function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Górna, S.; Domaszewska, K. The Effect of Endurance Training on Serum BDNF Levels in the Chronic Post-Stroke Phase: Current Evidence and Qualitative Systematic Review. J. Clin. Med. 2022, 11, 3556. https://doi.org/10.3390/jcm11123556
Górna S, Domaszewska K. The Effect of Endurance Training on Serum BDNF Levels in the Chronic Post-Stroke Phase: Current Evidence and Qualitative Systematic Review. Journal of Clinical Medicine. 2022; 11(12):3556. https://doi.org/10.3390/jcm11123556
Chicago/Turabian StyleGórna, Sara, and Katarzyna Domaszewska. 2022. "The Effect of Endurance Training on Serum BDNF Levels in the Chronic Post-Stroke Phase: Current Evidence and Qualitative Systematic Review" Journal of Clinical Medicine 11, no. 12: 3556. https://doi.org/10.3390/jcm11123556
APA StyleGórna, S., & Domaszewska, K. (2022). The Effect of Endurance Training on Serum BDNF Levels in the Chronic Post-Stroke Phase: Current Evidence and Qualitative Systematic Review. Journal of Clinical Medicine, 11(12), 3556. https://doi.org/10.3390/jcm11123556






