Residual Disease in Patients with Axial Spondyloarthritis: A Post-Hoc Analysis of the QUASAR Study †
Abstract
1. Introduction
2. Patients and Methods
2.1. Patient Population
2.2. Disease Activity Measures
2.3. HRQoL Questionnaires
2.4. Evaluation of the Burden of Residual Disease
2.5. Statistical Analysis
3. Results
3.1. Baseline Clinical Characteristics
3.2. Characteristics of Patients in Remission (ASDAS < 1.3) or LDA (ASDAS < 2.1)
3.3. Residual Disease, Including Pain and Fatigue, in Patients Achieving Inactive Disease and LDA
3.4. Signs and Symptoms in Patients Achieving Inactive Disease and LDA
3.5. Impact of Demographic and Other Features on Residual Disease
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudwaleit, M.; van der Heijde, D.; Landewé, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The Development of Assessment of SpondyloArthritis International Society Classification Criteria for Axial Spondyloarthritis (Part II): Validation and Final Selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of Diagnostic Criteria for Ankylosing Spondylitis. A Proposal for Modification of the New York Criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Kiltz, U.; Doria, A.; Aggarwal, A.; Ramonda, R. Do We Believe in Non-Radiographic Axial Spondyloarthritis? A Debate. Autoimmun. Rev. 2021, 20, 102703. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.M.; Deodhar, A.; Gensler, L.S.; Dubreuil, M.; Yu, D.; Khan, M.A.; Haroon, N.; Borenstein, D.; Wang, R.; Biehl, A.; et al. 2019 Update of the American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2019, 71, 1599–1613. [Google Scholar] [CrossRef] [PubMed]
- van der Heijde, D.; Ramiro, S.; Landewé, R.; Baraliakos, X.; Van den Bosch, F.; Sepriano, A.; Regel, A.; Ciurea, A.; Dagfinrud, H.; Dougados, M.; et al. 2016 Update of the ASAS-EULAR Management Recommendations for Axial Spondyloarthritis. Ann. Rheum. Dis. 2017, 76, 978–991. [Google Scholar] [CrossRef]
- Manara, M.; Prevete, I.; Marchesoni, A.; D’Angelo, S.; Cauli, A.; Zanetti, A. The Italian Society for Rheumatology Recommendations for the Management of Axial Spondyloarthritis. Reumatismo 2021, 73, 71–88. [Google Scholar] [CrossRef]
- Atzeni, F.; Carriero, A.; Boccassini, L.; D’Angelo, S. Anti-IL-17 Agents in the Treatment of Axial Spondyloarthritis. ImmunoTargets Ther. 2021, 10, 141–153. [Google Scholar] [CrossRef]
- Choy, E.; Baraliakos, X.; Behrens, F.; D’Angelo, S.; de Vlam, K.; Kirkham, B.W.; Østergaard, M.; Schett, G.A.; Rissler, M.; Chaouche-Teyara, K.; et al. The Need for Comparative Data in Spondyloarthritis. Arthritis Res. Ther. 2019, 21, 32. [Google Scholar] [CrossRef]
- Kilic, G.; Kilic, E.; Nas, K.; Kamanlı, A.; Tekeoglu, İ. Residual Symptoms and Disease Burden among Patients with Psoriatic Arthritis: Is a New Disease Activity Index Required? Rheumatol. Int. 2019, 39, 73–81. [Google Scholar] [CrossRef]
- van Mens, L.J.J.; Turina, M.C.; van de Sande, M.G.H.; Nurmohamed, M.T.; van Kuijk, A.W.R.; Baeten, D.L.P. Residual Disease Activity in Psoriatic Arthritis: Discordance between the Rheumatologist’s Opinion and Minimal Disease Activity Measurement. Rheumatology 2018, 57, 283–290. [Google Scholar] [CrossRef]
- Rahman, P.; Zummer, M.; Bessette, L.; Baer, P.; Haraoui, B.; Chow, A.; Kelsall, J.; Kapur, S.; Rampakakis, E.; Psaradellis, E.; et al. Real-World Validation of the Minimal Disease Activity Index in Psoriatic Arthritis: An Analysis from a Prospective, Observational, Biological Treatment Registry. BMJ Open 2017, 7, e016619. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D.; Assessment of Spondylo Arthritis international Society. Development of an ASAS-Endorsed Disease Activity Score (ASDAS) in Patients with Ankylosing Spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.M.; Landewé, R.; van der Heijde, D.; Assessment of Spondylo Arthritis International Society (ASAS). Ankylosing Spondylitis Disease Activity Score (ASDAS): 2018 Update of the Nomenclature for Disease Activity States. Ann. Rheum. Dis. 2018, 77, 1539–1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, V.; Fong, W.; Kwan, Y.H.; Leung, Y.Y. Residual Disease Burden in Patients With Axial Spondyloarthritis and Psoriatic Arthritis Despite Low Disease Activity States in a Multiethnic Asian Population. J. Rheumatol. 2021, 48, 677–684. [Google Scholar] [CrossRef]
- D’Angelo, S.; Gilio, M.; D’Attino, R.M.; Gualberti, G.; Merolla, R.; di Luzio Paparatti, U.; Malavolta, N.; Corvaglia, S.; Marchetta, A.; Scambi, C.; et al. Observational Study on the QUality of Life of Italian Axial SpondyloARthritis Patients (QUASAR): Baseline Data. Clin. Exp. Rheumatol. 2019, 37, 748–755. [Google Scholar]
- D’Angelo, S.; Malavolta, N.; Scambi, C.; Salvarani, C.; Caso, F.; Tirri, E.; Ramonda, R.; Quarta, L.; Erre, G.L.; Riva, M.; et al. Quality of Life and Therapeutic Management of Axial Spondyloarthritis Patients in Italy: A 12-Month Prospective Observational Study. Clin. Exp. Rheumatol. 2021, 39, 961–969. [Google Scholar]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A New Approach to Defining Disease Status in Ankylosing Spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar]
- Doward, L.C.; Spoorenberg, A.; Cook, S.A.; Whalley, D.; Helliwell, P.S.; Kay, L.J.; McKenna, S.P.; Tennant, A.; van der Heijde, D.; Chamberlain, M.A. Development of the ASQoL: A Quality of Life Instrument Specific to Ankylosing Spondylitis. Ann. Rheum. Dis. 2003, 62, 20–26. [Google Scholar] [CrossRef]
- EuroQol Group. A New Facility for the Measurement of Health-Related Quality of Life. Health Policy 1990, 16, 199–208. [Google Scholar] [CrossRef]
- Overman, C.L.; Kool, M.B.; Da Silva, J.A.P.; Geenen, R. The Prevalence of Severe Fatigue in Rheumatic Diseases: An International Study. Clin. Rheumatol. 2016, 35, 409–415. [Google Scholar] [CrossRef]
- Doward, L.C.; McKenna, S.P.; Meads, D.M.; Twiss, J.; Revicki, D.; Wong, R.L.; Luo, M.P. Translation and Validation of Non-English Versions of the Ankylosing Spondylitis Quality of Life (ASQOL) Questionnaire. Health Qual. Life Outcomes 2007, 5, 7. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Herdman, M.; Fox-Rushby, J.; Rabin, R.; Badia, X.; Selai, C. Producing Other Language Versions of the EQ-5D. In The Measurement and Valuation of Health Status Using EQ-5D: A European Perspective: Evidence from the EuroQol BIOMED Research Programme; Brooks, R., Rabin, R., de Charro, F., Eds.; Springer: Dordrecht, The Netherlands, 2003; pp. 183–189. ISBN 978-94-017-0233-1. [Google Scholar]
- Scalone, L.; Ciampichini, R.; Fagiuoli, S.; Gardini, I.; Fusco, F.; Gaeta, L.; Del Prete, A.; Cesana, G.; Mantovani, L.G. Comparing the Performance of the Standard EQ-5D 3L with the New Version EQ-5D 5L in Patients with Chronic Hepatic Diseases. Qual. Life Res. 2016, 22, 1707–1716. [Google Scholar] [CrossRef] [PubMed]
- Ortolan, A.; Navarro-Compán, V.; Sepriano, A.; Landewé, R.B.M.; van der Heijde, D.; Ramiro, S. Which Disease Activity Outcome Measure Discriminates Best in Axial Spondyloarthritis? A Systematic Literature Review and Meta-Analysis. Rheumatology 2020, 59, 3990–3992. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Schöls, M.; Braun, J.; Dougados, M.; FitzGerald, O.; Gladman, D.D.; Kavanaugh, A.; Landewé, R.; Mease, P.; Sieper, J.; et al. Treating Axial Spondyloarthritis and Peripheral Spondyloarthritis, Especially Psoriatic Arthritis, to Target: 2017 Update of Recommendations by an International Task Force. Ann. Rheum. Dis. 2018, 77, 3–17. [Google Scholar] [CrossRef]
- Scriffignano, S.; Perrotta, F.M.; De Socio, A.; Lubrano, E. Role of Comorbidities in Spondyloarthritis Including Psoriatic Arthritis. Clin. Rheumatol. 2019, 38, 3–10. [Google Scholar] [CrossRef]
- Bedaiwi, M.; Sari, I.; Thavaneswaran, A.; Ayearst, R.; Haroon, N.; Inman, R.D. Fatigue in Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis: Analysis from a Longitudinal Observation Cohort. J. Rheumatol. 2015, 42, 2354–2360. [Google Scholar] [CrossRef]
- Shimabuco, A.Y.; Gonçalves, C.R.; Moraes, J.C.B.; Waisberg, M.G.; Ribeiro, A.C.d.M.; Sampaio-Barros, P.D.; Goldenstein-Schainberg, C.; Bonfa, E.; Saad, C.G.S. Factors Associated with ASDAS Remission in a Long-Term Study of Ankylosing Spondylitis Patients under Tumor Necrosis Factor Inhibitors. Adv. Rheumatol. 2018, 58, 40. [Google Scholar] [CrossRef]
- Marin, J.; Acosta Felquer, M.L.; Ferreyra Garrot, L.; Ruta, S.; Rosa, J.; Soriano, E.R. Patients with Psoriatic Arthritis Fulfilling the Minimal Disease Activity Criteria Do Not Have Swollen and Tender Joints, but Have Active Skin. J. Rheumatol. 2016, 43, 907–910. [Google Scholar] [CrossRef]
- Wervers, K.; Vis, M.; Tchetveriko, I.; Gerards, A.H.; Kok, M.R.; Appels, C.W.Y.; van der Graaff, W.L.; van Groenendael, J.H.L.M.; Korswagen, L.-A.; Veris-van Dieren, J.J.; et al. Burden of Psoriatic Arthritis According to Different Definitions of Disease Activity: Comparing Minimal Disease Activity and the Disease Activity Index for Psoriatic Arthritis. Arthritis Care Res. (Hoboken) 2018, 70, 1764–1770. [Google Scholar] [CrossRef]
- van Mens, L.J.J.; van de Sande, M.G.H.; Fluri, I.A.; Atiqi, S.; van Kuijk, A.W.R.; Baeten, D.L.P. Residual Disease Activity and Treatment Adjustments in Psoriatic Arthritis in Current Clinical Practice. Arthritis Res. Ther. 2017, 19, 226. [Google Scholar] [CrossRef]
- Tillett, W.; McHugh, N.; Orbai, A.-M.; Ogdie, A.; Leung, Y.Y.; Coates, L.C.; Mease, P.J.; Gladman, D.D.; Brooke, M.; Packham, J.; et al. Outcomes of the 2019 GRAPPA Workshop on Continuous Composite Indices for the Assessment of Psoriatic Arthritis and Membership-Recommended Next Steps. J. Rheumatol. Suppl. 2020, 96, 11–18. [Google Scholar] [CrossRef] [PubMed]
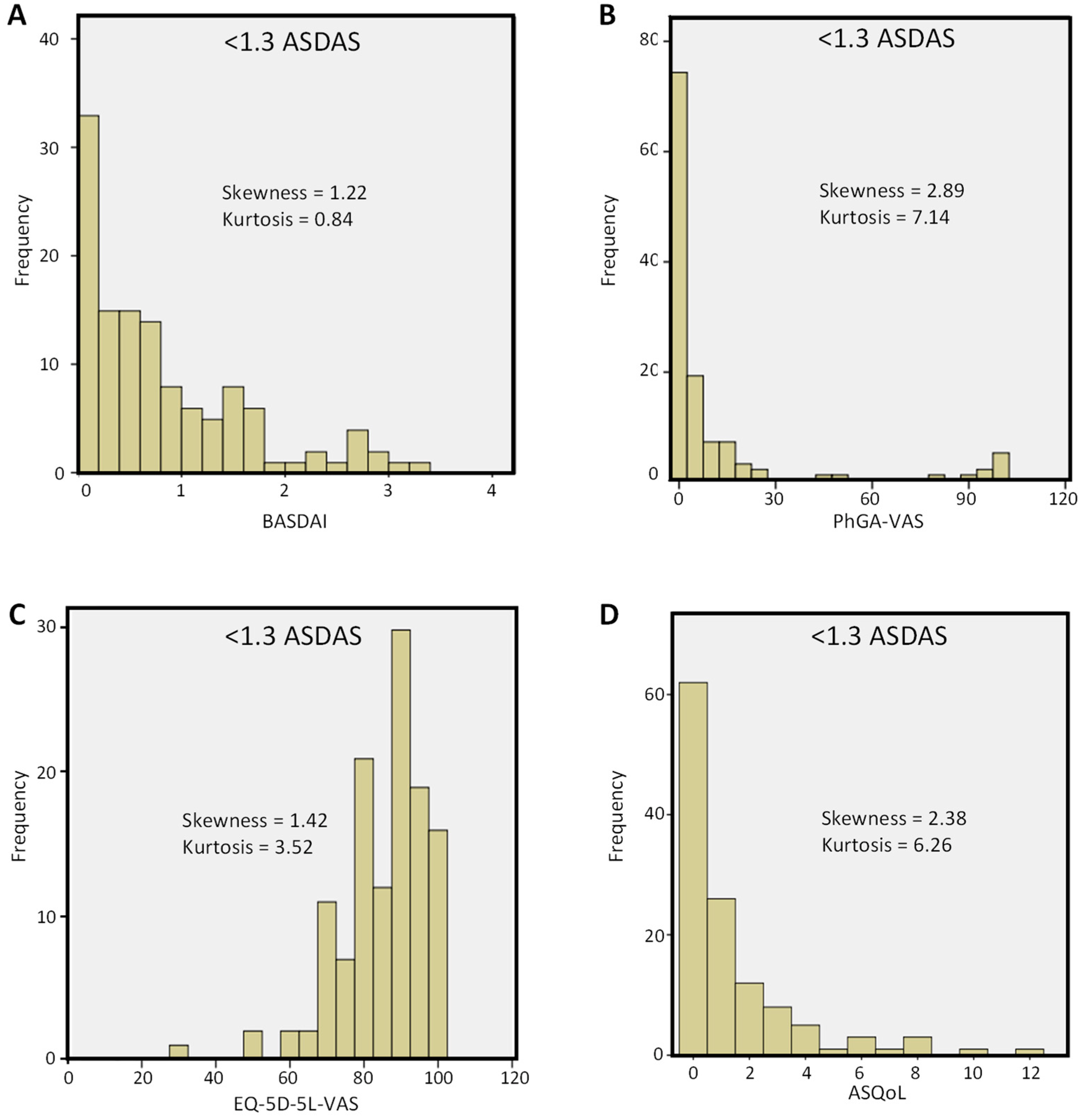
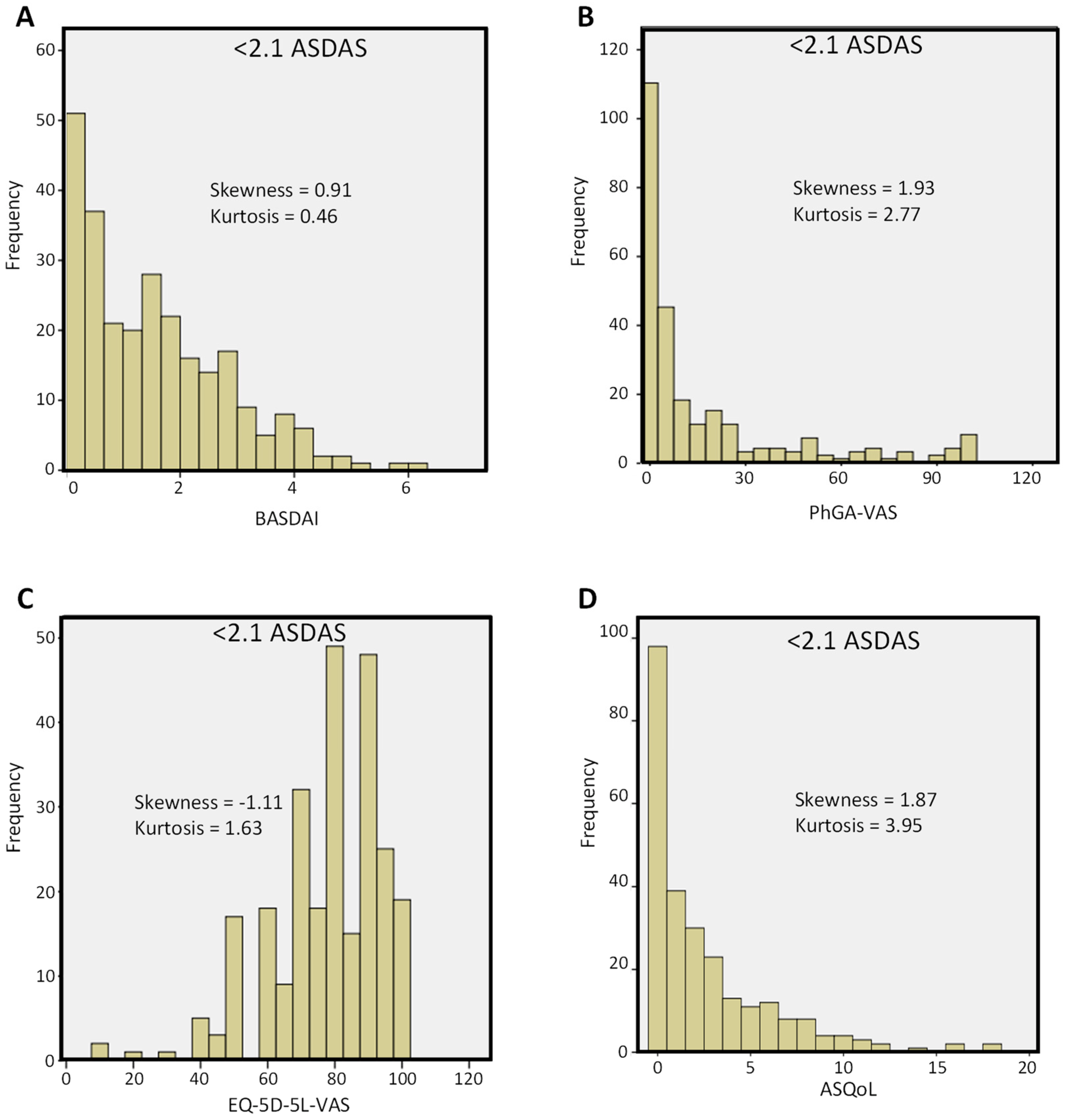
| Variable | All Patients | <1.3 ASDAS | ≥1.3 ASDAS | p-Value | <2.1 ASDAS | ≥2.1 ASDAS | p-Value |
|---|---|---|---|---|---|---|---|
| n = 480 | n = 123 (25.6%) | n = 357 (74.4%) | n = 262 (54.6%) | n = 218 (45.4%) | |||
| Age, mean ± SD | 47.5 ± 12.9 | 45.1 ± 12.3 | 48.3 ± 12.9 | 0.014 | 45.97 ± 12.7 | 49.2 ± 12.9 | 0.006 |
| Male | 307 (64) | 91 (74) | 216 (60.5) | 0.007 | 183 (69.8) | 124 (56.9) | 0.003 |
| Marital status | |||||||
| Single | 108 (22.5) | 35 (28.5) | 73 (20.4) | 67 (25.6) | 41 (18.8) | ||
| Married/living with partner | 340 (70.8) | 81 (65.9) | 259 (72.5) | 181 (69.1) | 159 (72.9) | ||
| Widow/bachelor | 7 (1.5) | 1 (0.8) | 6 (1.7) | 0.3 | 3 (1.1) | 4 (1.8) | 0.24 |
| Separated/divorced | 25 (5.2) | 6 (4.9) | 19 (5.3) | 11 (4.2) | 14 (6.4) | ||
| Smoking status | |||||||
| Never smoker | 243 (50.6) | 63 (51.2) | 180 (50.4) | 129 (49.2) | 114 (52.3) | ||
| Current smoker | 120 (25) | 26 (21.1) | 94 (26.3) | 0.42 | 62 (23.7) | 58 (26.6) | 0.30 |
| Previous smoker | 117 (24.4) | 34 (27.6) | 83 (23.2) | 71 (27.1) | 46 (21.1) | ||
| Disease duration, median (IQR) | 7 (2–12) | 9 (4–14) | 6 (2–12) | <0.001 | 8 (3–12) | 6 (1–12) | <0.001 |
| HLA-B27 | |||||||
| Positive | 244 (50.8) | 80 (65.0) | 164 (45.9) | 148 (56.5) | 96 (44.0) | ||
| Negative | 156 (32.5) | 26 (21.1) | 130 (36.4) | 0.001 | 71 (27.1) | 85 (39.0) | 0.012 |
| Not performed | 80 (16.7) | 17 (13.8) | 63 (17.6) | 43 (16.4) | 37 (17.0) | ||
| CRP (mg/L), median (IQR) | 3.4 (1.5–7) | 1.5 (0.7–3) | 4.9 (2.2–8.5) | <0.001 | 2.45 (1–5) | 5.3 (3–12.6) | <0.001 |
| ASDAS, median (IQR) | 1.9 (1.3–2.9) | 0.8 (0.6–1.1) | 2.4 (1.8–3.2) | <0.001 | 1.3 (0.8–1.7) | 3.0 (2.6–3.5) | <0.001 |
| ASQoL, median (IQR) | 4 (1–10) | 0 (0–2) | 7 (3–12) | <0.001 | 1 (0–4) | 10 (6–13) | <0.001 |
| BASDAI, median (IQR) | 2.7 (1.1–5.0) | 0.6 (0.2–1.2) | 3.9 (2.2–5.8) | <0.001 | 1.4 (0.5–2.4) | 5.3 (3.8–6.8) | <0.001 |
| PhGA-VAS, median (IQR) | 12.0 (2.0–48.2) | 2.0 (0.0–7.0) | 21.0 (5.0–54.0) | <0.001 | 4.0 (0.0–21.0) | 31.0 (9.0–63.0) | <0.001 |
| EQ-5D-5L-VAS, median (IQR) | 70.0 (50.0–85.0) | 90 (80–95) | 60 (50–80) | <0.001 | 80 (70–90) | 50 (40–70) | <0.001 |
| Extra-muscular manifestations of axSpA | 218 (45.4) | 69 (56.1) | 149 (41.7) | 0.006 | 130 (49.6) | 88 (40.4) | 0.04 |
| Concomitant disease | 206 (42.9) | 43 (35) | 163 (45.7) | 0.04 | 95 (36.3) | 111 (50.9) | 0.001 |
| Biologic treatment | 423 (82.6) | 123 (91.9) | 278 (77.9) | 0.001 | 222 (84.7) | 169 (77.5) | 0.043 |
| Question | <1.3 ASDAS n = 123 | <2.1 ASDAS n = 262 | ≥2.1 ASDAS n = 218 |
|---|---|---|---|
| Q1 (fatigue) | 0.7 (0.1–2.0) | 1.6 (0.4–3.4) | 6.1 (4.6–7.6) |
| Q2 (pain) | 0.4 (0.1–1.2) | 1.2 (0.3–3.0) | 6.5 (4.7–8.0) |
| Q3 (pain) | 0.1 (0–0.6) | 0.4 (0–1.4) | 4.5 (2.3–6.9) |
| Q4 (discomfort) | 0.2 (0–0.6) | 0.4 (0–1.8) | 4.7 (1.9–6.7) |
| Q5 (stiffness) | 0.4 (0–1.2) | 1.0 (0.1–2.5) | 6.0 (3.4–7.7) |
| Q6 (stiffness) | 0.2 (0–1.1) | 0.7 (0–2.1) | 4.5 (2.1–6.2) |
| Variable | <1.3 ASDAS | <2.1 ASDAS | ≥2.1 ASDAS |
|---|---|---|---|
| n = 123 | n = 262 | n = 218 | |
| ASQoL | |||
| Q7: I feel tired all day | 12 (9.8%) | 55 (21.0%) | 136 (62.4%) |
| Q8: I have to stop what I am doing to rest | 11 (8.9%) | 51 (19.5%) | 149 (68.3%) |
| Q12: I get tired easily | 30 (24.4%) | 100 (38.2%) | 168 (77.1%) |
| EQ-5D-5L (Q4: pain and discomfort) | |||
| 1. I have no pain or discomfort | 58 (47.2%) | 78 (29.8%) | 8 (3.7%) |
| 2. I have slight pain or discomfort | 60 (48.8%) | 146 (55.7%) | 52 (23.9%) |
| 3. I have moderate pain or discomfort | 5 (4.1%) | 35 (13.4%) | 117 (53.7%) |
| 4. I have severe pain or discomfort | 0 (0) | 3 (1.1%) | 37 (17%) |
| 5. I have extreme pain or discomfort | 0 (0) | 0 (0) | 4 (1.8%) |
| Sign or Symptom | <1.3 ASDAS | <2.1 ASDAS | ≥2.1 ASDAS |
|---|---|---|---|
| n = 123 | n = 262 | n = 218 | |
| Any symptom | 56 (45.5%) | 161 (61.5%) | 195 (89.4%) |
| Pain (any location) | 31 (25.2%) | 112 (42.7%) | 174 (79.8%) |
| Lower back pain | 19 (15.4%) | 74 (28.2%) | 135 (61.9%) |
| Upper back pain | 3 (2.4%) | 18 (6.9%) | 47 (21.6%) |
| Neck pain | 8 (6.5%) | 36 (13.7%) | 66 (30.3%) |
| Joint pain | 11 (8.9%) | 46 (17.6%) | 93 (42.7%) |
| Stiffness | 40 (32.5%) | 113 (43.1%) | 160 (73.4%) |
| Arthritis | 1 (0.8%) | 6 (2.3%) | 36 (16.5%) |
| Dactylitis | 0 (0.0%) | 2 (0.8%) | 13 (6%) |
| Enthesitis | 6 (4.9%) | 22 (8.4%) | 45 (20.6%) |
| Variable | Male | Female | Male | Female | ||
|---|---|---|---|---|---|---|
| <1.3 ASDAS | <1.3 ASDAS | p-Value | <2.1 ASDAS | <2.1 ASDAS | p-Value | |
| ASQoL | n = 91 | n = 32 | n = 183 | n = 79 | ||
| Q7: I feel tired all day | 7 (7.7%) | 5 (15.6%) | 0.3 | 27 (14.8%) | 28 (35.4%) | <0.001 |
| Q8: I have to stop what I am doing to rest | 6 (6.6%) | 5 (15.6%) | 0.15 | 27 (14.8%) | 24 (30.4%) | 0.006 |
| Q12: I get tired easily | 22 (24.2%) | 8 (25%) | 1 | 63 (34.4%) | 37 (46.8%) | 0.05 |
| EQ-5D-5L | ||||||
| 1. I have no pain or discomfort | 47 (51.6%) | 11 (34.4%) | 59 (32.2%) | 19 (24.1%) | ||
| 2. I have slight pain or discomfort | 41 (45.1%) | 19 (59.4%) | 0.1 | 102 (55.7%) | 44 (55.7%) | 0.24 |
| 3. I have moderate pain or discomfort | 3 (3.3%) | 2 (6.3%) | 20 (10.9%) | 15 (19.0%) | ||
| 4. I have severe pain or discomfort | 0 (0%) | 0 (0%) | 2 (1.1%) | 1 (1.3%) |
| Variable | Age ≤ 48 Years | Age > 48 Years | Age ≤ 48 Years | Age > 48 Years | ||
|---|---|---|---|---|---|---|
| <1.3 ASDAS | <1.3 ASDAS | p-Value | <2.1 ASDAS | <2.1 ASDAS | p-Value | |
| ASQoL | n = 77 | n = 46 | n = 153 | n = 109 | ||
| Q7: I feel tired all day | 7 (9.1) | 5 (10.9) | 0.76 | 32 (20.9) | 23 (21.1) | 1 |
| Q8: I have to stop what I am doing to rest | 6 (7.8) | 5 (10.9) | 0.75 | 23 (15.0) | 28 (25.7) | 0.04 |
| Q12: I get tired easily | 19 (24.7) | 11 (23.9) | 1 | 63 (34.4) | 37 (46.8) | 0.44 |
| EQ-5D-5L | n = 77 | n = 46 | n = 153 | n = 109 | ||
| 1. I have no pain or discomfort | 37 (48.1) | 21 (45.7) | 51 (33.3) | 27 (24.8) | ||
| 2. I have slight pain or discomfort | 38 (49.4) | 22 (47.8) | 0.85 | 86 (56.2) | 60 (55.0) | 0.17 |
| 3. I have moderate pain or discomfort | 2 (2.6) | 3 (6.5) | 16 (10.5) | 19 (17.4) | ||
| 4. I have severe pain or discomfort | 0 (0) | 0 (0) | 0 (0) | 3 (2.8) |
| Variable | ≤7 Years | >7 Years | ≤7 Years | >7 Years | ||
|---|---|---|---|---|---|---|
| <1.3 ASDAS | <1.3 ASDAS | p-Value | <2.1 ASDAS | <2.1 ASDAS | p-Value | |
| ASQoL | n = 50 | n = 73 | n = 126 | n = 136 | ||
| Q7: I feel tired all day | 3 (6.0) | 9 (12.3) | 0.36 | 29 (23.0) | 26 (19.1) | 0.55 |
| Q8: I have to stop what I am doing to rest | 3 (6.0) | 8 (11.0) | 0.52 | 25 (19.8) | 26 (19.1) | 1.00 |
| Q12: I get tired easily | 11 (22.0) | 19 (26.0) | 0.67 | 47 (37.6) | 53 (39.3) | 0.80 |
| EQ-5D-5L | n = 50 | n = 73 | n = 126 | n = 136 | ||
| 1. I have no pain or discomfort | 25 (52.0) | 32 (43.8) | 38 (30.2) | 40 (29.4) | ||
| 2. I have slight pain or discomfort | 23 (46.0) | 37 (50.7) | 0.46 | 68 (54) | 78 (57.4) | 1.00 |
| 3. I have moderate pain or discomfort | 1 (2.0) | 4 (5.5) | 19 (15.1) | 16 (11.8) | ||
| 4. I have severe pain or discomfort | 0 (0) | 0 (0) | 1 (0.8) | 2 (1.5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, S.; Salvarani, C.; Marando, F.; Gualberti, G.; Novelli, L.; Curradi, G.; Tripepi, G.; Pitino, A.; Ramonda, R.; Marchesoni, A. Residual Disease in Patients with Axial Spondyloarthritis: A Post-Hoc Analysis of the QUASAR Study. J. Clin. Med. 2022, 11, 3553. https://doi.org/10.3390/jcm11123553
D’Angelo S, Salvarani C, Marando F, Gualberti G, Novelli L, Curradi G, Tripepi G, Pitino A, Ramonda R, Marchesoni A. Residual Disease in Patients with Axial Spondyloarthritis: A Post-Hoc Analysis of the QUASAR Study. Journal of Clinical Medicine. 2022; 11(12):3553. https://doi.org/10.3390/jcm11123553
Chicago/Turabian StyleD’Angelo, Salvatore, Carlo Salvarani, Francesca Marando, Giuliana Gualberti, Lucia Novelli, Giacomo Curradi, Giovanni Tripepi, Annalisa Pitino, Roberta Ramonda, and Antonio Marchesoni. 2022. "Residual Disease in Patients with Axial Spondyloarthritis: A Post-Hoc Analysis of the QUASAR Study" Journal of Clinical Medicine 11, no. 12: 3553. https://doi.org/10.3390/jcm11123553
APA StyleD’Angelo, S., Salvarani, C., Marando, F., Gualberti, G., Novelli, L., Curradi, G., Tripepi, G., Pitino, A., Ramonda, R., & Marchesoni, A. (2022). Residual Disease in Patients with Axial Spondyloarthritis: A Post-Hoc Analysis of the QUASAR Study. Journal of Clinical Medicine, 11(12), 3553. https://doi.org/10.3390/jcm11123553






