Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach
Abstract
1. Introduction
2. Materials and Methods
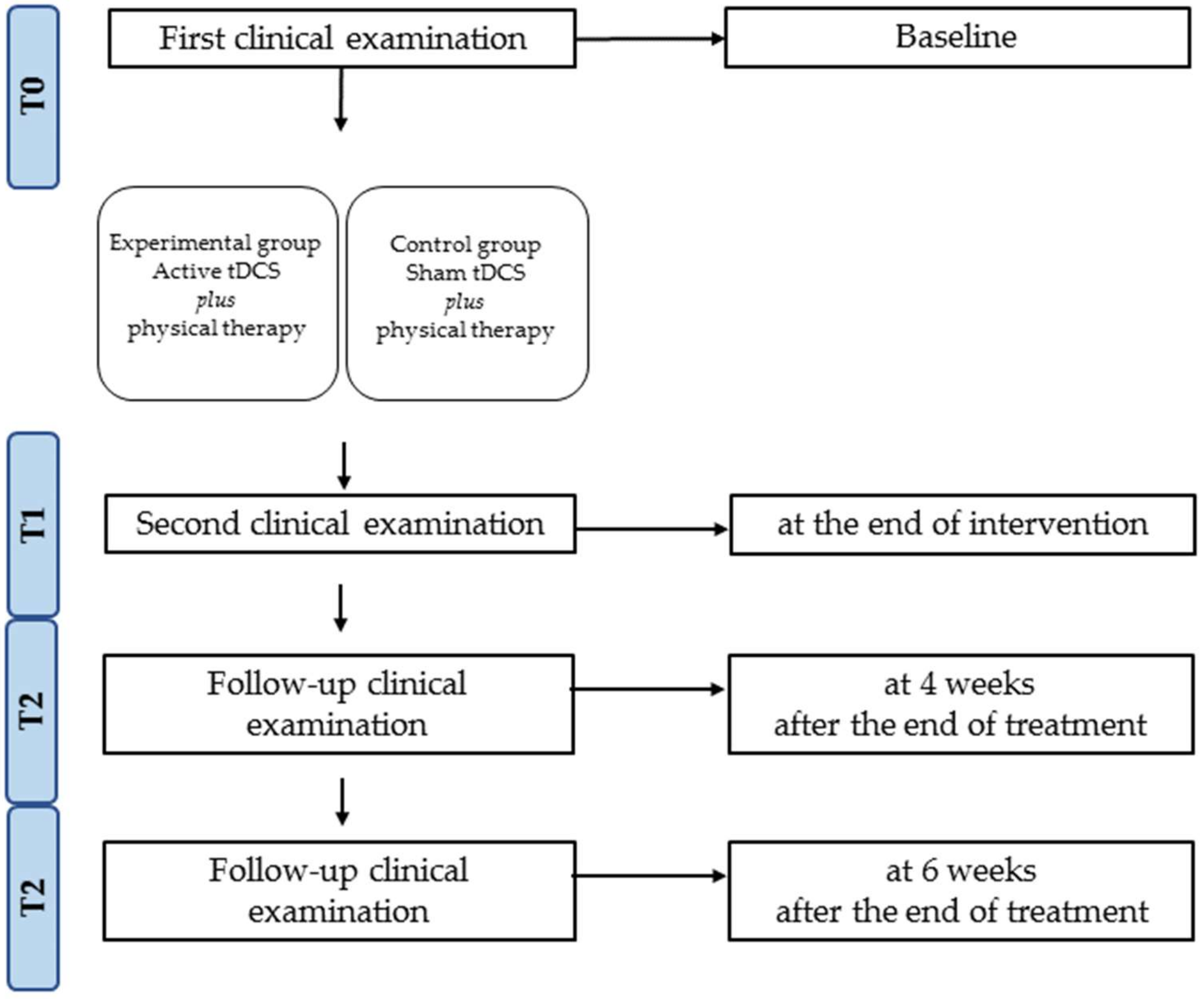
2.1. Study Design
2.2. Participants
2.3. Intervention
2.4. Outcome Measures
2.5. Statistical Analysis
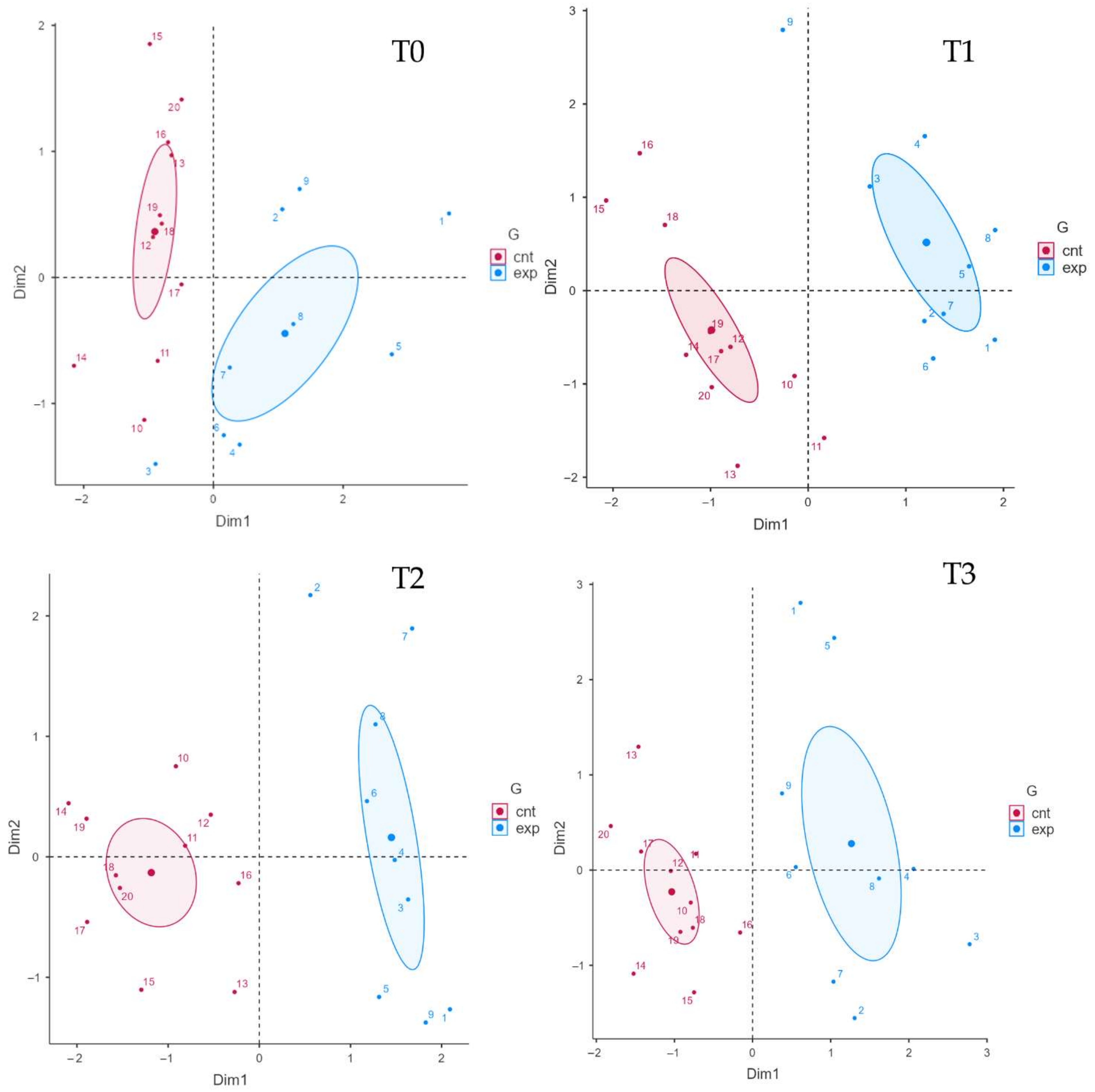
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef] [PubMed]
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Solaro, C.; de Sire, A.; Uccelli, M.M.; Mueller, M.; Bergamaschi, R.; Gasperini, C.; Restivo, D.A.; Stabile, M.R.; Patti, F. Efficacy of levetiracetam on upper limb movement in multiple sclerosis patients with cerebellar signs: A multicenter double-blind, placebo-controlled, crossover study. Eur. J. Neurol. 2020, 27, 2209–2216. [Google Scholar] [CrossRef]
- Galea, M.P.; Lizama, L.E.C.; Butzkueven, H.; Kilpatrick, T.J. Gait and balance deterioration over a 12-month period in multiple sclerosis patients with EDSS scores ≤ 3.0. Neurorehabilitation 2017, 40, 277–284. [Google Scholar] [CrossRef] [PubMed]
- LaRocca, N.G. Impact of walking impairment in multiple sclerosis: Perspectives of patients and care partners. Patient 2011, 4, 189–201. [Google Scholar] [CrossRef]
- Frechette, M.L.; Meyer, B.M.; Tulipani, L.J.; Gurchiek, R.D.; McGinnis, R.S.; Sosnoff, J.J. Next Steps in Wearable Technology and Community Ambulation in Multiple Sclerosis. Curr. Neurol. Neurosci. Rep. 2019, 19, 80. [Google Scholar] [CrossRef]
- Martin, C.L.; Phillips, B.A.; Kilpatrick, T.J.; Butzkueven, H.; Tubridy, N.; McDonald, E.; Galea, M.P. Gait and balance impairment in early multiple sclerosis in the absence of clinical disability. Mult. Scler. 2006, 12, 620–628. [Google Scholar] [CrossRef]
- Gunn, H.J.; Newell, P.; Haas, B.; Marsden, J.F.; Freeman, J.A. Identification of risk factors for falls in multiple sclerosis: A systematic review and meta-analysis. Phys. Ther. 2013, 93, 504–513. [Google Scholar] [CrossRef]
- Mazumder, R.; Murchison, C.; Bourdette, D.; Cameron, M. Falls in people with multiple sclerosis compared with falls in healthy controls. PLoS ONE 2014, 9, e107620. [Google Scholar] [CrossRef]
- Sattelmayer, K.M.; Chevalley, O.; Kool, J.; Wiskerke, E.; Denkinger, L.N.; Giacomino, K.; Opsommer, E.; Hilfiker, R. Development of an exercise programme for balance abilities in people with multiple sclerosis: A development of concept study using Rasch analysis. Arch. Physiother. 2021, 11, 29. [Google Scholar] [CrossRef]
- Comber, L.; Galvin, R.; Coote, S. Gait deficits in people with multiple sclerosis: A systematic review and meta-analysis. Gait Posture 2017, 51, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.H.; Wagner, J.M. Gait abnormalities in multiple sclerosis: Pathogenesis, evaluation, and advances in treatment. Curr. Neurol. Neurosci. Rep. 2011, 11, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Richmond, S.B.; Swanson, C.W.; Peterson, D.S.; Fling, B.W. A temporal analysis of bilateral gait coordination in people with multiple sclerosis. Mult. Scler. Relat. Disord. 2020, 45, 102445. [Google Scholar] [CrossRef] [PubMed]
- Bethoux, F. Gait disorders in multiple sclerosis. Contin. Lifelong Learn. Neurol. 2013, 19, 1007–1022. [Google Scholar] [CrossRef] [PubMed]
- Latimer-Cheung, A.E.; Pilutti, L.A.; Hicks, A.L.; Martin Ginis, K.A.; Fenuta, A.M.; MacKibbon, K.A.; Motl, R.W. Effects of exercise training on fitness, mobility, fatigue, and health-related quality of life among adults with multiple sclerosis: A systematic review to inform guideline development. Arch. Phys. Med. Rehabil. 2013, 94, 1800–1828. [Google Scholar] [CrossRef] [PubMed]
- Cameron, M.H.; Nilsagard, Y. Balance, gait, and falls in multiple sclerosis. In Handbook of Clinical Neurology; Elsevier B.V.: Amsterdam, The Netherlands, 2018; Volume 159, pp. 237–250. [Google Scholar]
- Motl, R.W.; Sandroff, B.M.; Kwakkel, G.; Dalgas, U.; Feinstein, A.; Heesen, C.; Feys, P.; Thompson, A.J. Exercise in patients with multiple sclerosis. Lancet Neurol. 2017, 16, 848–856. [Google Scholar] [CrossRef]
- Salari, N.; Hayati, A.; Kazeminia, M.; Rahmani, A.; Mohammadi, M.; Fatahian, R.; Shohaimi, S. The effect of exercise on balance in patients with stroke, Parkinson, and multiple sclerosis: A systematic review and meta-analysis of clinical trials. Neurol. Sci. 2021, 16, 848–856. [Google Scholar] [CrossRef]
- Donzé, C.; Massot, C. Rehabilitation in multiple sclerosis in 2021. La Presse Médicale 2021, 50, 104066. [Google Scholar] [CrossRef]
- Prosperini, L.; di Filippo, M. Beyond clinical changes: Rehabilitation-induced neuroplasticity in MS. Mult. Scler. J. 2019, 25, 1348–1362. [Google Scholar] [CrossRef]
- Tomassini, V.; Matthews, P.M.; Thompson, A.J.; Fuglo, D.; Geurts, J.J.; Johansen-Berg, H.; Jones, D.K.; Rocca, M.A.; Wise, R.G.; Barkhof, F.; et al. Neuroplasticity and functional recovery in multiple sclerosis. Nat. Rev. Neurol. 2012, 8, 635–646. [Google Scholar] [CrossRef]
- Tomassini, V.; Johansen-Berg, H.; Leonardi, L.; Paixão, L.; Jbabdi, S.; Palace, J.; Pozzilli, C.; Matthews, P.M. Preservation of motor skill learning in patients with multiple sclerosis. Mult. Scler. J. 2011, 17, 103–115. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Bigoni, M.; Priano, L.; Baudo, S.; Solaro, C.; Mauro, A. Constraint-Induced Movement Therapy in multiple sclerosis: Safety and three-dimensional kinematic analysis of upper limb activity. A randomized single-blind pilot study. Neurorehabilitation 2019, 45, 247–254. [Google Scholar] [CrossRef] [PubMed]
- de Sire, A.; Mauro, A.; Priano, L.; Baudo, S.; Bigoni, M.; Solaro, C. Effects of constraint-induced movement therapy on upper limb activity according to a bi-dimensional kinematic analysis in progressive multiple sclerosis patients: A randomized single-blind pilot study. Funct. Neurol. 2019, 34, 151–157. [Google Scholar] [PubMed]
- Prosperini, L.; Piattella, M.C.; Giannì, C.; Pantano, P. Functional and Structural Brain Plasticity Enhanced by Motor and Cognitive Rehabilitation in Multiple Sclerosis. Neural Plast. 2015, 2015, 481574. [Google Scholar] [CrossRef]
- Hershey, L.A. Comment: Performance improvement with computer training in Parkinson disease. Neurology 2014, 82, 1224. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Indino, A.; de Scorpio, G.; Moggio, L.; Ammendolia, A. Nintendo WiiTM versus Xbox KinectTM for functional locomotion in people with Parkinson’s disease: A systematic review and network meta-analysis. Disabil. Rehabil. 2020, 44, 331–336. [Google Scholar] [CrossRef]
- Marotta, N.; Demeco, A.; Moggio, L.; Ammendolia, A. The adjunct of transcranial direct current stimulation to Robot-assisted therapy in upper limb post-stroke treatment. J. Med. Eng. Technol. 2021, 45, 494–501. [Google Scholar] [CrossRef]
- Calafiore, D.; Invernizzi, M.; Ammendolia, A.; Marotta, N.; Fortunato, F.; Paolucci, T.; Ferraro, F.; Curci, C.; Cwirlej-Sozanska, A.; de Sire, A. Efficacy of Virtual Reality and Exergaming in Improving Balance in Patients With Multiple Sclerosis: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 773459. [Google Scholar] [CrossRef]
- Ganguly, J.; Murgai, A.; Sharma, S.; Aur, D.; Jog, M. Non-invasive Transcranial Electrical Stimulation in Movement Disorders. Front. Neurosci. 2020, 14, 522. [Google Scholar] [CrossRef]
- Iodice, R.; Manganelli, F.; Dubbioso, R. The therapeutic use of non-invasive brain stimulation in multiple sclerosis-a review. Restor. Neurol. Neurosci. 2017, 35, 497–509. [Google Scholar] [CrossRef]
- Sánchez-Kuhn, A.; Pérez-Fernández, C.; Cánovas, R.; Flores, P.; Sánchez-Santed, F. Transcranial direct current stimulation as a motor Neurorehabilitation tool: An empirical review. Biomed. Eng. Online 2017, 16, 115–136. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, G.; Di Pino, G.; Capone, F.; Ranieri, F.; Florio, L.; Todisco, V.; Tedeschi, G.; Funke, K.; Di Lazzaro, V. Neurobiological after-effects of non-invasive brain stimulation. Brain Stimul. 2017, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, G.; Choi, C.; Coghe, G.; Cocco, E.; Krupp, L.B.; Pau, M.; Charvet, L.E. Gait and Functional Mobility in Multiple Sclerosis: Immediate Effects of Transcranial Direct Current Stimulation (tDCS) Paired With Aerobic Exercise. Front. Neurol. 2020, 11, 310. [Google Scholar] [CrossRef] [PubMed]
- Hiew, S.; Nguemeni, C.; Zeller, D. Efficacy of transcranial direct current stimulation in people with multiple sclerosis: A review. Eur. J. Neurol. 2021, 29, 648–664. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, G.; Choi, C.; Shaw, M.T.; Coghe, G.; Krupp, L.; Moffat, M.; Cocco, E.; Pau, M.; Charvet, L. Walking in multiple sclerosis improves with tDCS: A randomized, double-blind, sham-controlled study. Ann. Clin. Transl. Neurol. 2020, 7, 2310–2319. [Google Scholar] [CrossRef]
- Roche, N.; Geiger, M.; Bussel, B. Mechanisms underlying transcranial direct current stimulation in rehabilitation. Ann. Phys. Rehabil. Med. 2015, 58, 214–219. [Google Scholar] [CrossRef]
- Swanson, C.W.; Proessl, F.; Stephens, J.A.; Miravalle, A.A.; Fling, B.W. Non-invasive brain stimulation to assess neurophysiologic underpinnings of lower limb motor impairment in multiple sclerosis. J. Neurosci. Methods 2021, 356, 109143. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Workman, C.D.; Kamholz, J.; Rudroff, T. Transcranial Direct Current Stimulation (tDCS) to Improve Gait in Multiple Sclerosis: A Timing Window Comparison. Front. Hum. Neurosci. 2019, 13, 420. [Google Scholar] [CrossRef]
- Oveisgharan, S.; Karimi, Z.; Abdi, S.; Sikaroodi, H. The use of brain stimulation in the rehabilitation of walking disability in patients with multiple sclerosis: A randomized double-blind clinical trial study. Curr. J. Neurol. 2019, 18, 57. [Google Scholar] [CrossRef]
- Boggio, P.S.; Nunes, A.; Rigonatti, S.P.; Nitsche, M.A.; Pascual-Leone, A.; Fregni, F. Repeated sessions of noninvasive brain DC stimulation is associated with motor function improvement in stroke patients. Restor. Neurol. Neurosci. 2007, 25, 123–129. [Google Scholar] [PubMed]
- Grecco, L.A.C.; de Almeida Carvalho Duarte, N.; Mendonça, M.E.; Cimolin, V.Ô.; Galli, M.; Fregni, F.; Oliveira, C.S. Transcranial direct current stimulation during treadmill training in children with cerebral palsy: A randomized controlled double-blind clinical trial. Res. Dev. Disabil. 2014, 35, 2840–2848. [Google Scholar] [CrossRef] [PubMed]
- Costa-Ribeiro, A.; Maux, A.; Bosford, T.; Aoki, Y.; Castro, R.; Baltar, A.; Shirahige, L.; Moura Filho, A.; Nitsche, M.A.; Monte-Silva, K. Transcranial direct current stimulation associated with gait training in Parkinson’s disease: A pilot randomized clinical trial. Dev. Neurorehabil. 2017, 20, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Scholz, M.; Haase, R.; Trentzsch, K.; Stölzer--hutsch, H.; Ziemssen, T. Improving digital patient care: Lessons learned from patient-- reported and expert--reported experience measures for the clinical practice of multidimensional walking assessment. Brain Sci. 2021, 11, 786. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Healy, B.C.; Rotstein, D.; Guttmann, C.R.G.; Bakshi, R.; Weiner, H.L.; Brodley, C.E.; Chitnis, T. Exploration of machine learning techniques in predicting multiple sclerosis disease course. PLoS ONE 2017, 12, e0174866. [Google Scholar] [CrossRef]
- Liu, H.; Guan, J.; Li, H.; Bao, Z.; Wang, Q.; Luo, X.; Xue, H. Predicting the Disease Genes of Multiple Sclerosis Based on Network Representation Learning. Front. Genet. 2020, 11, 328. [Google Scholar] [CrossRef]
- Trentzsch, K.; Schumann, P.; Śliwiński, G.; Bartscht, P.; Haase, R.; Schriefer, D.; Zink, A.; Heinke, A.; Jochim, T.; Malberg, H.; et al. Using machine learning algorithms for identifying gait parameters suitable to evaluate subtle changes in gait in people with multiple sclerosis. Brain Sci. 2021, 11, 1049. [Google Scholar] [CrossRef]
- Marzullo, A.; Kocevar, G.; Stamile, C.; Durand-Dubief, F.; Terracina, G.; Calimeri, F.; Sappey-Marinier, D. Classification of multiple sclerosis clinical profiles via graph convolutional neural networks. Front. Neurosci. 2019, 13, 594. [Google Scholar] [CrossRef]
- Martínez-Heras, E.; Solana, E.; Prados, F.; Andorrà, M.; Solanes, A.; López-Soley, E.; Montejo, C.; Pulido-Valdeolivas, I.; Alba-Arbalat, S.; Sola-Valls, N.; et al. Characterization of multiple sclerosis lesions with distinct clinical correlates through quantitative diffusion MRI. NeuroImage Clin. 2020, 28, 102411. [Google Scholar] [CrossRef]
- Piryonesi, S.M.; Rostampour, S.; Piryonesi, S.A. Predicting falls and injuries in people with multiple sclerosis using machine learning algorithms. Mult. Scler. Relat. Disord. 2021, 49, 102740. [Google Scholar] [CrossRef]
- Bécue-Bertaut, M.; Pagès, J. Multiple factor analysis and clustering of a mixture of quantitative, categorical and frequency data. Comput. Stat. Data Anal. 2008, 52, 3255–3268. [Google Scholar] [CrossRef]
- Pinto, M.; Marotta, N.; Caracò, C.; Simeone, E.; Ammendolia, A.; de Sire, A. Quality of Life Predictors in Patients With Melanoma: A Machine Learning Approach. Front. Oncol. 2022, 12, 843611. [Google Scholar] [CrossRef] [PubMed]
- Husson, F.; Le, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R; Chapman and Hall/CRC: New York, NY, USA, 2017. [Google Scholar]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Ali, A.S.; Darwish, M.H.; Shalaby, N.M.; Abbas, R.L.; Soubhy, H.Z. Efficacy of core stability versus task oriented trainings on balance in ataxic persons with multiple sclerosis. A single blinded randomized controlled trial. Mult. Scler. Relat. Disord. 2021, 50, 102866. [Google Scholar] [CrossRef]
- White, L.J.; McCoy, S.C.; Castellano, V.; Gutierrez, G.; Stevens, J.E.; Walter, G.A.; Vandenborne, K. Resistance training improves strength and functional capacity in persons with multiple sclerosis. Mult. Scler. 2004, 10, 668–674. [Google Scholar] [CrossRef]
- Cofré Lizama, L.E.; Bruijn, S.M.; Galea, M.P. Gait stability at early stages of multiple sclerosis using different data sources. Gait Posture 2020, 77, 214–217. [Google Scholar] [CrossRef]
- Sebastião, E.; Sandroff, B.M.; Learmonth, Y.C.; Motl, R.W. Validity of the Timed Up and Go Test as a Measure of Functional Mobility in Persons with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2016, 97, 1072–1077. [Google Scholar] [CrossRef]
- Storm, F.A.; Cesareo, A.; Reni, G.; Biffi, E. Wearable inertial sensors to assess gait during the 6-min walk test: A systematic review. Sensors 2020, 20, 2660. [Google Scholar] [CrossRef]
- Goldman, M.D.; Marrie, R.A.; Cohen, J.A. Evaluation of the six-minute walk in multiple sclerosis subjects and healthy controls. Mult. Scler. 2008, 14, 383–390. [Google Scholar] [CrossRef]
- Wetzel, J.L.; Fry, D.K.; Pfalzer, L.A. Six-minute walk test for persons with mild or moderate disability from multiple sclerosis: Performance and explanatory factors. Physiother. Can. 2011, 63, 166–180. [Google Scholar] [CrossRef]
- Pau, M.; Caggiari, S.; Mura, A.; Corona, F.; Leban, B.; Coghe, G.; Lorefice, L.; Marrosu, M.G.; Cocco, E. Clinical assessment of gait in individuals with multiple sclerosis using wearable inertial sensors: Comparison with patient-based measure. Mult. Scler. Relat. Disord. 2016, 10, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Ovacık, U.; Çelik, D. Minimal Clinically Important Difference of Berg Balance Scale in People with Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2019, 100, 1191. [Google Scholar] [CrossRef] [PubMed]
- Atteya, A.; Elwishy, A.; Kishk, N.; Ismail, R.S.; Badawy, R. Assessment of postural balance in multiple sclerosis patients. Egypt. J. Neurol. Psychiatry Neurosurg. 2019, 55, 7. [Google Scholar] [CrossRef]
- Prometti, P.; Olivares, A.; Gaia, G.; Bonometti, G.; Comini, L.; Scalvini, S. Biodex fall risk assessment in the elderly with ataxia: A new age-dependent derived index in rehabilitation: An observational study. Medicine 2016, 95. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J.; Valentin, D. Multiple factor analysis: Principal component analysis for multitable and multiblock data sets. Wiley Interdiscip. Rev. Comput. Stat. 2013, 5, 149–179. [Google Scholar] [CrossRef]
- Abdullah, S.S.; Rostamzadeh, N.; Sedig, K.; Garg, A.X.; McArthur, E. Visual analytics for dimension reduction and cluster analysis of high dimensional electronic health records. Informatics 2020, 7, 17. [Google Scholar] [CrossRef]
- Peres-Neto, P.R.; Jackson, D.A.; Somers, K.M. Giving meaningful interpretation to ordination axes: Assessing loading significance in principal component analysis. Ecology 2003, 84, 2347–2363. [Google Scholar] [CrossRef]
- Giacalone, D.; Ribeiro, L.M.; Frøst, M.B. Consumer-Based Product Profiling: Application of Partial Napping® for Sensory Characterization of Specialty Beers by Novices and Experts. J. Food Prod. Mark. 2013, 19, 201–218. [Google Scholar] [CrossRef]
- Beh, E.J. Exploratory multivariate analysis by example using R. J. Appl. Stat. 2012, 39, 1381–1382. [Google Scholar] [CrossRef][Green Version]
- Pagès-Hélary, S.; Dujourdy, L.; Cayot, N. Flavor compounds in blackcurrant berries: Multivariate analysis of datasets obtained with natural variability and various experimental parameters. LWT 2022, 153, 112425. [Google Scholar] [CrossRef]
- Tibshirani, R.; Walther, G.; Hastie, T. Estimating the number of clusters in a data set via the gap statistic. J. R. Stat. Soc. Ser. B Stat. Methodol. 2001, 63, 411–423. [Google Scholar] [CrossRef]
- de Sire, A.; Gallelli, L.; Marotta, N.; Lippi, L.; Fusco, N.; Calafiore, D.; Cione, E.; Muraca, L.; Maconi, A.; de Sarro, G.; et al. Vitamin D Deficiency in Women with Breast Cancer: A Correlation with Osteoporosis? A Machine Learning Approach with Multiple Factor Analysis. Nutrients 2022, 14, 1586. [Google Scholar] [CrossRef] [PubMed]
- Martino Cinnera, A.; Bisirri, A.; Leone, E.; Morone, G.; Gaeta, A. Effect of dual-task training on balance in patients with multiple sclerosis: A systematic review and meta-analysis. Clin. Rehabil. 2021, 35, 1399–1412. [Google Scholar] [CrossRef] [PubMed]
- Kalb, R.; Brown, T.R.; Coote, S.; Costello, K.; Dalgas, U.; Garmon, E.; Giesser, B.; Halper, J.; Karpatkin, H.; Keller, J.; et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult. Scler. J. 2020, 26, 1459–1469. [Google Scholar] [CrossRef]
- Learmonth, Y.C.; Ensari, I.; Motl, R.W. Physiotherapy and walking outcomes in adults with multiple sclerosis: Systematic review and meta-analysis. Phys. Ther. Rev. 2016, 21, 160–172. [Google Scholar] [CrossRef]
- Pearson, M.; Dieberg, G.; Smart, N. Exercise as a Therapy for Improvement of Walking Ability in Adults with Multiple Sclerosis: A Meta-Analysis. Arch. Phys. Med. Rehabil. 2015, 96, 1339–1348. [Google Scholar] [CrossRef]
- Snook, E.M.; Motl, R.W. Effect of exercise training on walking mobility in multiple sclerosis: A meta-analysis. Neurorehabil. Neural Repair 2009, 23, 108–116. [Google Scholar] [CrossRef]
- Wonneberger, M.; Schmidt, S. Changes of gait parameters following long-term aerobic endurance exercise in mildly disabled multiple sclerosis patients: An exploratory study. Eur. J. Phys. Rehabil. Med. 2015, 51, 755–762. [Google Scholar]
- Langeskov--Christensen, M.; Hvid, L.G.; Jensen, H.B.; Nielsen, H.H.; Petersen, T.; Stenager, E.; Dalgas, U. Efficacy of high--intensity aerobic exercise on common multiple sclerosis symptoms. Acta Neurol. Scand. 2022, 145, 229–238. [Google Scholar] [CrossRef]
- Chaves, A.R.; Devasahayam, A.J.; Kelly, L.P.; Pretty, R.W.; Ploughman, M. Exercise-Induced Brain Excitability Changes in Progressive Multiple Sclerosis: A Pilot Study. J. Neurol. Phys. Ther. 2020, 44, 132–144. [Google Scholar] [CrossRef]
- Chaves, A.R.; Devasahayam, A.J.; Riemenschneider, M.; Pretty, R.W.; Ploughman, M. Walking Training Enhances Corticospinal Excitability in Progressive Multiple Sclerosis—A Pilot Study. Front. Neurol. 2020, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Savci, S.; Inal-Ince, D.; Arikan, H.; Guclu-Gunduz, A.; Cetisli-Korkmaz, N.; Armutlu, K.; Karabudak, R. Six-minute walk distance as a measure of functional exercise capacity in multiple sclerosis. Disabil. Rehabil. 2005, 27, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Cohen, E.T.; Potter, K.; Allen, D.D.; Bennett, S.E.; Brandfass, K.G.; Widener, G.L.; Yorke, A.M. Selecting rehabilitation outcome measures for people with multiple sclerosis. Int. J. MS Care 2015, 17, 181–189. [Google Scholar] [CrossRef]
- Kalron, A.; Dolev, M.; Givon, U. Further construct validity of the Timed Up-and-Go Test as a measure of ambulation in multiple sclerosis patients. Eur. J. Phys. Rehabil. Med. 2017, 53, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Paltamaa, J.; Sjögren, T.; Peurala, S.H.; Heinonen, A. Effects of physiotherapy interventions on balance in multiple sclerosis : A systematic review and meta-analysis of randomized controlled trials. J. Rehabil. Med. 2012, 44, 811–823. [Google Scholar] [CrossRef]
- Dobbs, B.; Pawlak, N.; Biagioni, M.; Agarwal, S.; Shaw, M.; Pilloni, G.; Bikson, M.; Datta, A.; Charvet, L. Generalizing remotely supervised transcranial direct current stimulation (tDCS): Feasibility and benefit in Parkinson’s disease. J. Neuroeng. Rehabil. 2018, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, A.; Mohseni-Bandpei, M.A.; Seydi, M. The effect of tDCS on the fatigue in patients with multiple sclerosis: A systematic review of randomized controlled clinical trials. J. Clin. Neurosci. 2020, 78, 277–283. [Google Scholar] [CrossRef]
- Sharma, K.; Agarwal, S.; Mania, D.; Cucca, A.; Frucht, S.; Feigin, A.; Biagioni, M. Can Tele-Monitored transcranial Direct Current Stimulation (tDCS) Help Manage Fatigue and Cognitive Symptoms in Parkinson’s disease? Neurology 2020, 94. [Google Scholar]
- Ayache, S.S.; Chalah, M.A.; Kümpfel, T.; Padberg, F.; Lefaucheur, J.P.; Palm, U. Multiple sclerosis fatigue, its neural correlates, and its modulation with tDCS. Fortschr. Neurol. Psychiatr. 2017, 85, 260–269. [Google Scholar] [CrossRef]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Mhalla, A.; Abdellaoui, M.; Créange, A.; Lefaucheur, J.P.; Ayache, S.S. Effects of left DLPFC versus right PPC tDCS on multiple sclerosis fatigue. J. Neurol. Sci. 2017, 372, 131–137. [Google Scholar] [CrossRef]
- Ferrucci, R.; Vergari, M.; Cogiamanian, F.; Bocci, T.; Ciocca, M.; Tomasini, E.; de Riz, M.; Scarpini, E.; Priori, A. Transcranial direct current stimulation (tDCS) for fatigue in multiple sclerosis. Neurorehabilitation 2014, 34, 121–127. [Google Scholar] [CrossRef] [PubMed]



| Experimental Group (n = 9) | Control Group (n = 8) | p Value | |
|---|---|---|---|
| Male/Female | 3/6 | 2/6 | p = 0.213 |
| Age (years) | 43.22 ± 10.46 | 39.75 ± 8.39 | p = 0.081 |
| EDSS | 3 [1] | 3 [0] | p = 0.154 |
| 6MWT (meters) | 300.1 ± 18.8 | 294.5 ± 13.39 | p = 0.134 |
| Gait velocity (m/s) | 0.87 ± 0.26 | 0.92 ± 0.24 | p = 0.070 |
| Gait cycle (meters) | 1.01 ± 0.17 | 0.96 ± 0.46 | p = 0.076 |
| TUG (seconds) | 14.11 ± 3.5 | 12.8 ± 1.1 | p = 0.072 |
| BBS | 48.33 ± 7.4 | 50.4 ± 1.4 | p = 0.356 |
| FRI | 1.31 ± 0.3 | 1.25 ± 0.3 | p = 0.069 |
| T0 | T1 | ∆T0-T1 p-Value | T2 | ∆T1-T2 p-Value | T3 | ∆T2-T3 p-Value | ||
|---|---|---|---|---|---|---|---|---|
| 6MWT (meters) | experimental | 300.1 ± 18.8 | 322.0 ± 17.0 | 0.006 | 317.3 ± 19.1 | 0.007 | 303.7 ± 16.9 | 0.006 |
| control | 294.5 ± 13.4 | 299.5 ± 14.5 | 0.009 | 284.2 ± 14.9 | 0.006 | 281.5 ± 13.7 | 0.084 | |
| Gait velocity (m/s) | experimental | 0.9 ± 0.3 | 0.8 ± 0.3 | 0.331 | 0.8 ± 0.5 | 0.105 | 0.8 ± 0.5 | 0.312 |
| control | 0.9 ± 0.2 | 0.9 ± 0.4 | 0.227 | 0.8 ± 1.2 | 0.109 | 0.7 ± 0.4 | 0.059 | |
| Gait cycle (meters) | experimental | 1.0 ± 0.2 | 0.8 ± 0.4 | 0.451 | 0.8 ± 0.41 | 0.245 | 0.8 ± 0.2 | 0.677 |
| control | 1.0 ± 0.5 | 1.1 ± 0.4 | 0.423 | 1.0 ± 0.52 | 0.311 | 0.9 ± 0.5 | 0.546 | |
| TUG (seconds) | experimental | 14.1 ± 3.5 | 12.8 ± 5.0 | 0.031 | 13.0 ± 3.7 | 0.154 | 12.0 ± 3.9 | 0.042 |
| control | 12.8 ± 1.1 | 11.3 ± 3.9 | 0.043 | 10.3 ± 2.6 | 0.06 | 11.3 ± 2.1 | 0.044 | |
| BBS | experimental | 48.3 ± 7.4 | 53.3 ± 3.9 | 0.023 | 52.2 ± 5.1 | 0.095 | 50.3 ± 6.8 | 0.083 |
| control | 50.4 ± 1.4 | 51.4 ± 4.4 | 0.162 | 52.4 ± 3.3 | 0.094 | 49.8 ± 2.0 | 0.079 | |
| FRI | experimental | 1.3 ± 0.3 | 1.0 ± 0.8 | 0.099 | 1.0 ± 0.6 | 0.876 | 1.0 ± 0.5 | 0.765 |
| control | 1.3 ± 0.3 | 1.2 ± 0.5 | 0.203 | 1.2 ± 0.6 | 0.785 | 0.1 ± 0.6 | 0.672 |
| Chi-Squared | p-Value | Kendall’s W | ||
|---|---|---|---|---|
| 6MWT | experimental | 9.14 | 0.03 | 0.34 |
| control | 5.35 | 0.15 | 0.20 | |
| between-group | 9.24 | 0.03 | 0.23 | |
| Gait velocity | experimental | 2.00 | 0.57 | 0.07 |
| control | 20.92 | 0.01 | 0.77 | |
| between-group | 2.43 | 0.49 | 0.34 | |
| Gait cycle | experimental | 8.43 | 0.04 | 0.31 |
| control | 1.25 | 0.74 | 0.05 | |
| between-group | 0.60 | 0.90 | 0.05 | |
| TUG | experimental | 9.93 | 0.02 | 0.37 |
| control | 11.4 | 0.01 | 0.42 | |
| between-group | 5.64 | 0.13 | 0.35 | |
| BBS | experimental | 4.48 | 0.21 | 0.17 |
| control | 3.32 | 0.34 | 0.12 | |
| between-group | 3.74 | 0.29 | 010 | |
| FRI | experimental | 4.74 | 0.19 | 0.18 |
| control | 4.75 | 0.19 | 0.18 | |
| between-group | 3.24 | 0.36 | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marotta, N.; de Sire, A.; Marinaro, C.; Moggio, L.; Inzitari, M.T.; Russo, I.; Tasselli, A.; Paolucci, T.; Valentino, P.; Ammendolia, A. Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach. J. Clin. Med. 2022, 11, 3505. https://doi.org/10.3390/jcm11123505
Marotta N, de Sire A, Marinaro C, Moggio L, Inzitari MT, Russo I, Tasselli A, Paolucci T, Valentino P, Ammendolia A. Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach. Journal of Clinical Medicine. 2022; 11(12):3505. https://doi.org/10.3390/jcm11123505
Chicago/Turabian StyleMarotta, Nicola, Alessandro de Sire, Cinzia Marinaro, Lucrezia Moggio, Maria Teresa Inzitari, Ilaria Russo, Anna Tasselli, Teresa Paolucci, Paola Valentino, and Antonio Ammendolia. 2022. "Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach" Journal of Clinical Medicine 11, no. 12: 3505. https://doi.org/10.3390/jcm11123505
APA StyleMarotta, N., de Sire, A., Marinaro, C., Moggio, L., Inzitari, M. T., Russo, I., Tasselli, A., Paolucci, T., Valentino, P., & Ammendolia, A. (2022). Efficacy of Transcranial Direct Current Stimulation (tDCS) on Balance and Gait in Multiple Sclerosis Patients: A Machine Learning Approach. Journal of Clinical Medicine, 11(12), 3505. https://doi.org/10.3390/jcm11123505










