Free Skin Grafting to Reconstruct Donor Sites after Radial Forearm Flap Harvesting: A Prospective Study with Platelet-Rich Fibrin (PRF)
Abstract
:1. Introduction
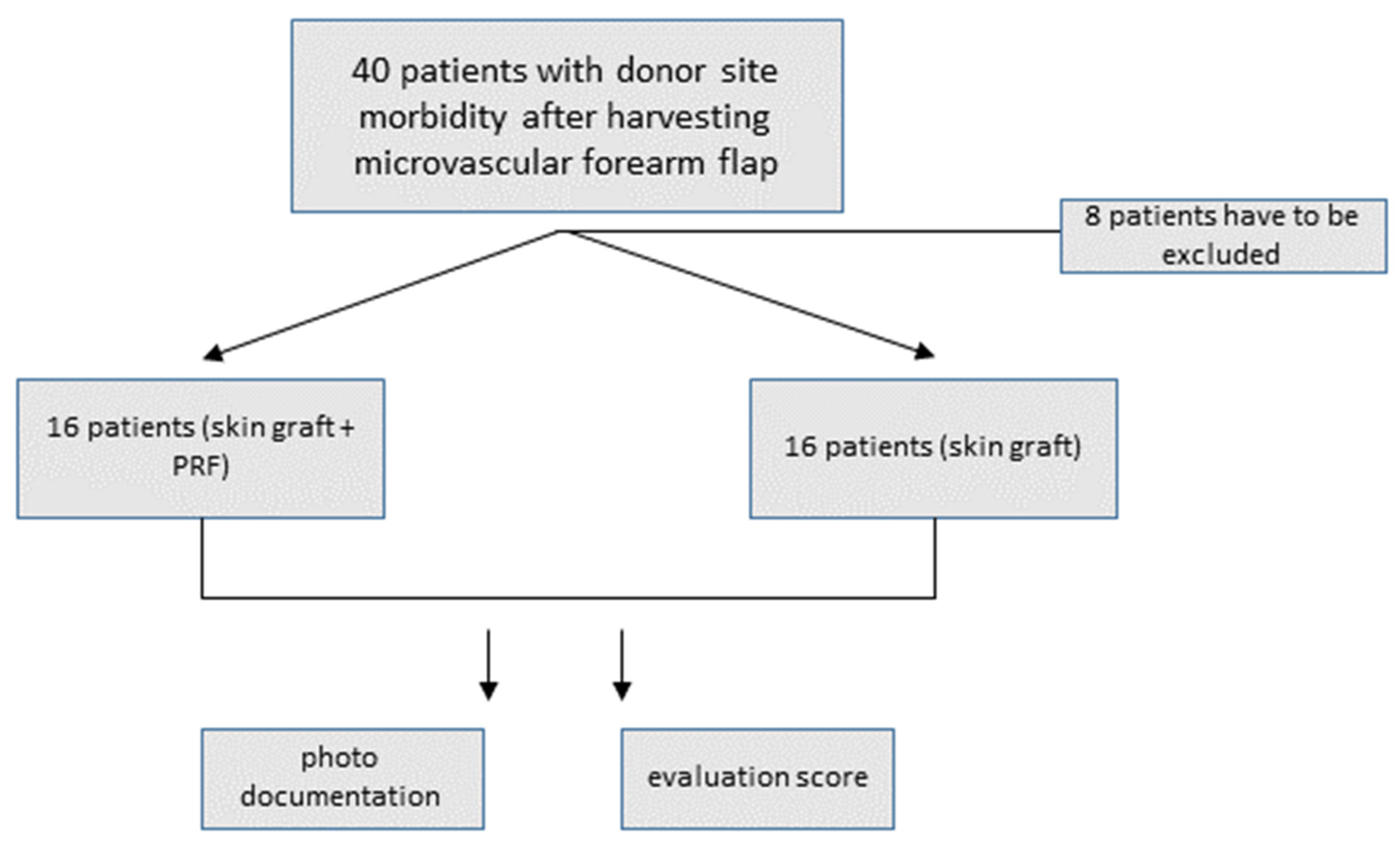
2. Materials and Methods
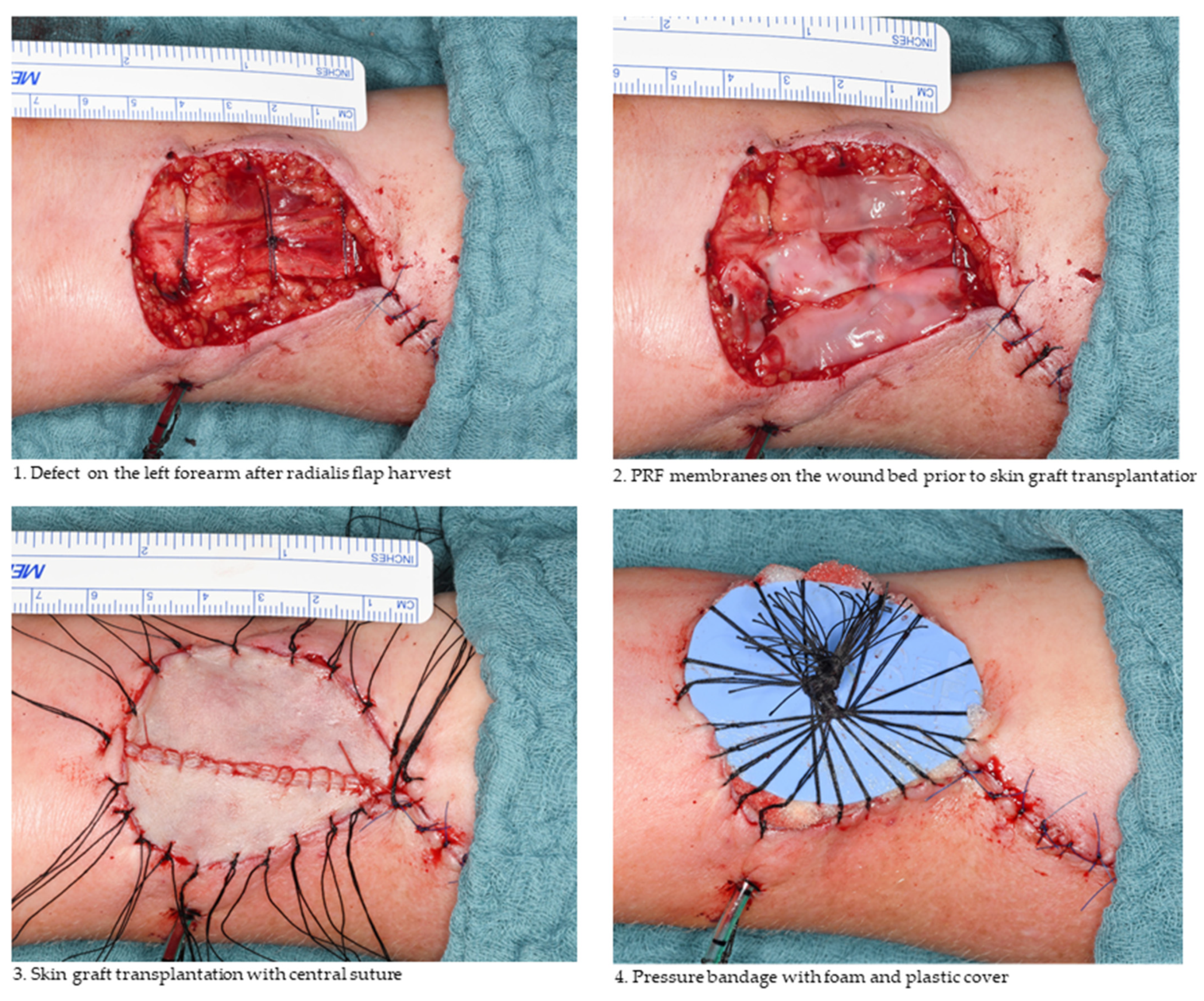
2.1. Free Skin Graft Harvest
2.2. Platelet-Rich Fibrin Preparation
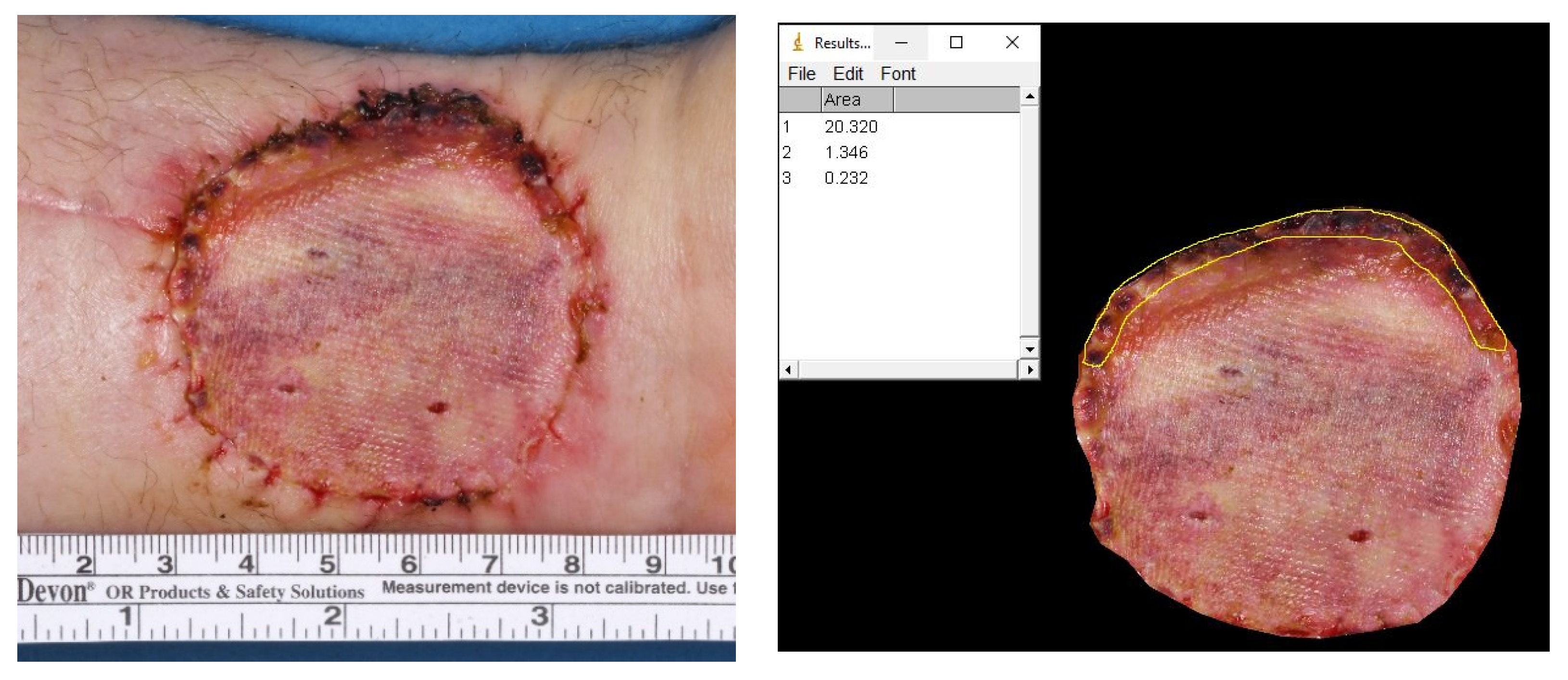
2.3. Photographic Coverage Rate Analysis
2.4. Evaluation Score
2.5. Statistical Evaluation
3. Results
3.1. Descriptive Statistics
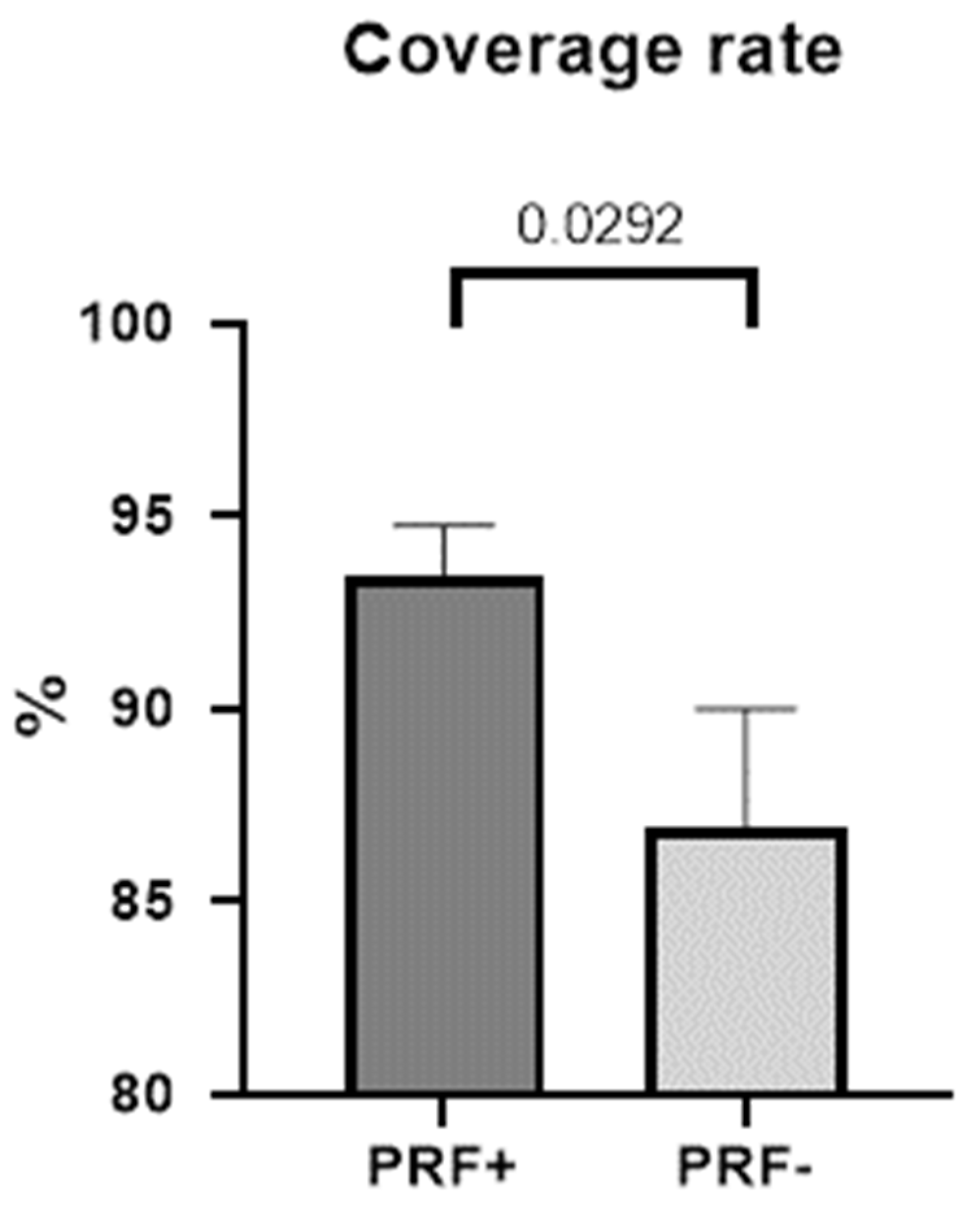
3.2. Coverage Rate and Extent of Dehiscence
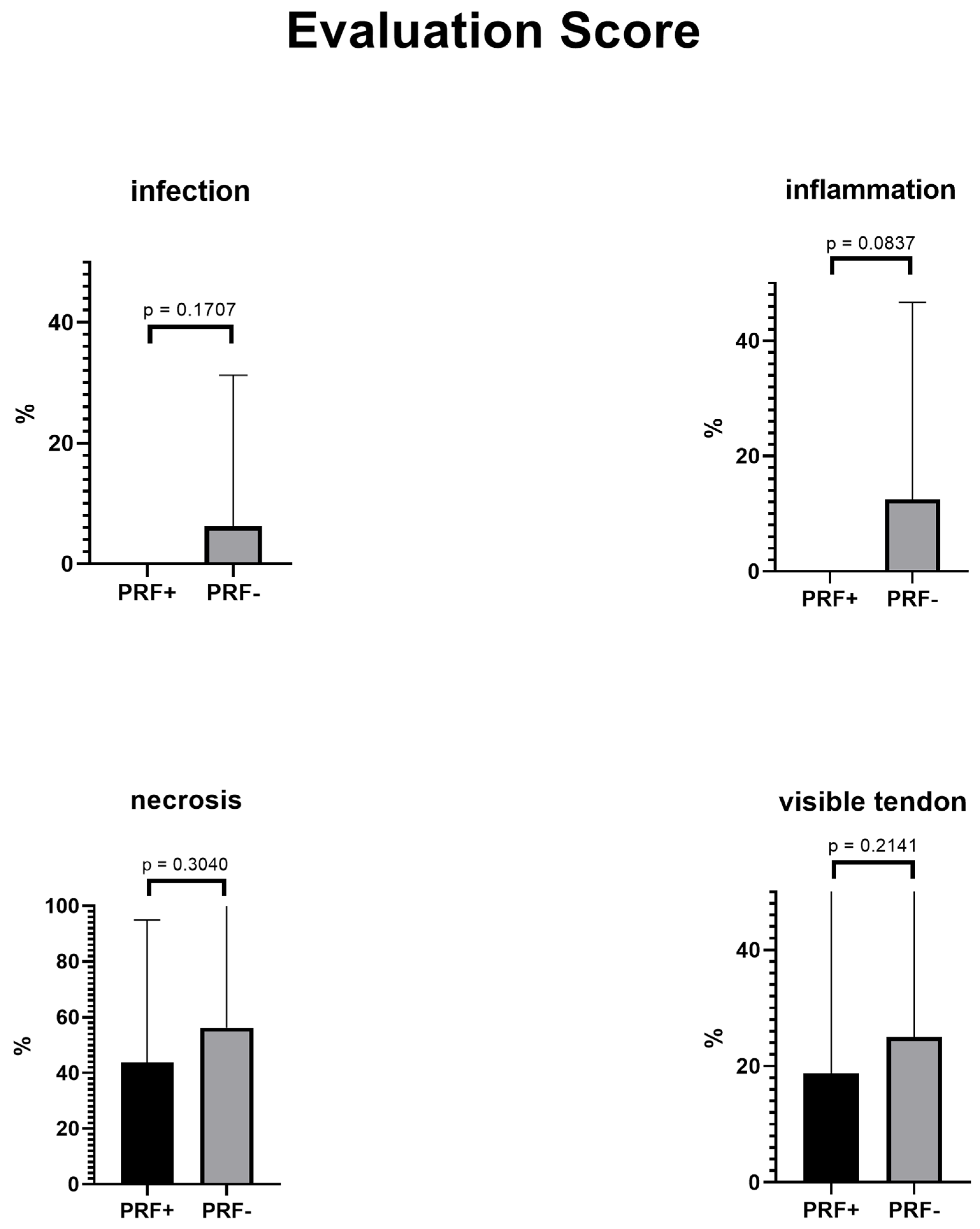
3.3. Evaluation Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pohlenz, P.; Klatt, J.; Schön, G.; Blessmann, M.; Li, L.; Schmelzle, R. Microvascular free flaps in head and neck surgery: Complications and outcome of 1000 flaps. Int. J. Oral Maxillofac. Surg. 2012, 41, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Mahy, P.; Reychler, H. Closure of radial forearm free flap donor site defect with a local meshed full-thickness skin graft: A retrospective study of an original technique. J. Cranio-Maxillofac. Surg. 2007, 35, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.M.; Rychlik, S.; Harris, J.; Seikaly, H.; Biron, V.L.; O’Connell, D.A. Donor site morbidity following radial forearm free flap reconstruction with split thickness skin grafts using negative pressure wound therapy. J. Otolaryngol.-Head Neck Surg. 2019, 48, 21. [Google Scholar] [CrossRef] [PubMed]
- Richardson, D.; Fisher, S.E.; Vaughan, D.E.; Brown, J.S. Radial Forearm Flap Donor-Site Complications and Morbidity: A Prospective Study. Plast. Reconstr. Surg. 1997, 99, 109–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis III, W.J.; Wu, C.; Sieber, D.; Vandevender, D.K. A comparison of full and split thickness skin grafts in radial forearm donor sites. J. Hand Microsurg. 2011, 3, 18–24. [Google Scholar] [CrossRef] [Green Version]
- Braza, M.E.; Fahrenkopf, M.P. Split-Thickness Skin Grafts. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Moreno-Sánchez, M.; González-García, R.; Ruiz-Laza, L.; de Zaldívar, D.M.S.; Moreno-García, C.; Monje, F. Closure of the Radial Forearm Free Flap Donor Site Using the Combined Local Triangular Full-Thickness Skin Graft. J. Oral Maxillofac. Surg. 2016, 74, 204–211. [Google Scholar] [CrossRef]
- Chang, E.I.; Liu, J. Prospective Comparison of Donor-Site Morbidity following Radial Forearm and Ulnar Artery Perforator Flap Harvest. Plast. Reconstr. Surg. 2020, 145, 1267–1274. [Google Scholar] [CrossRef]
- Ghanaati, S.; Booms, P.; Orlowska, A.; Kubesch, A.; Lorenz, J.; Rutkowski, J.; Landes, C.; Sader, R.; Kirkpatrick, C.; Choukroun, J. Advanced Platelet-Rich Fibrin: A New Concept for Cell-Based Tissue Engineering by Means of Inflammatory Cells. J. Oral Implant. 2014, 40, 679–689. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part II: Platelet-related biologic features. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e45–e50. [Google Scholar] [CrossRef]
- Ekström, K.; Omar, O.; Granéli, C.; Wang, X.; Vazirisani, F.; Thomsen, P. Monocyte Exosomes Stimulate the Osteogenic Gene Expression of Mesenchymal Stem Cells. PLoS ONE 2013, 8, e75227. [Google Scholar] [CrossRef]
- Maciel, J.; Oliveira, M.I.; Colton, E.; McNally, A.K.; Oliveira, C.; Anderson, J.M.; Barbosa, M.A. Adsorbed Fibrinogen Enhances Production of Bone- and Angiogenic-Related Factors by Monocytes/Macrophages. Tissue Eng. Part A 2014, 20, 250–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pirraco, R.; Reis, R.L.; Marques, A.P. Effect of monocytes/macrophages on the early osteogenic differentiation of hBMSCs. J. Tissue Eng. Regen. Med. 2012, 7, 392–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Bagdadi, K.; Kubesch, A.; Yu, X.; Al-Maawi, S.; Orlowska, A.; Dias, A.; Booms, P.; Dohle, E.; Sader, R.; Kirkpatrick, C.J.; et al. Reduction of relative centrifugal forces increases growth factor release within solid platelet-rich-fibrin (PRF)-based matrices: A proof of concept of LSCC (low speed centrifugation concept). Eur. J. Trauma Emerg. Surg. 2017, 45, 467–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wend, S.; Kubesch, A.; Orlowska, A.; Al-Maawi, S.; Zender, N.; Dias, A.; Miron, R.J.; Sader, R.; Booms, P.; Kirkpatrick, C.J.; et al. Reduction of the relative centrifugal force influences cell number and growth factor release within injectable PRF-based matrices. J. Mater. Sci. Mater. Med. 2017, 28, 188. [Google Scholar] [CrossRef]
- Miron, R.J.; Zucchelli, G.; Pikos, M.A.; Salama, M.; Lee, S.; Guillemette, V.; Fujioka-Kobayashi, M.; Bishara, M.; Zhang, Y.; Wang, H.-L.; et al. Use of platelet-rich fibrin in regenerative dentistry: A systematic review. Clin. Oral Investig. 2017, 21, 1913–1927. [Google Scholar] [CrossRef]
- Riecke, B.; Assaf, A.; Heiland, M.; Al-Dam, A.; Gröbe, A.; Blessmann, M.; Wikner, J. Local full-thickness skin graft of the donor arm—A novel technique for the reduction of donor site morbidity in radial forearm free flap. Int. J. Oral Maxillofac. Surg. 2015, 44, 937–941. [Google Scholar] [CrossRef]
- Di Giuli, R.; Dicorato, P.; Kaciulyte, J.; Marinari, N.; Conversi, F.; Mazzocchi, M. Donor Site Wound Healing in Radial Forearm Flap: A Comparative Study between Dermal Substitute and Split-Thickness Skin Graft Versus Full-Thickness Skin Graft Primary Coverage. Ann. Plast Surg. 2021, 86, 655–660. [Google Scholar] [CrossRef]
- Andrews, B.T.; Smith, R.B.; Chang, K.E.; Scharpf, J.; Goldstein, D.P.; Funk, G.F. Management of the Radial Forearm Free Flap Donor Site with the Vacuum-Assisted Closure (VAC) System. Laryngoscope 2006, 116, 1918–1922. [Google Scholar] [CrossRef]
- Vidrine, D.M.; Kaler, S.; Rosenthal, E.L. A Comparison of Negative-Pressure Dressings Versus Bolster and Splinting of the Radial Forearm Donor Site. Otolaryngol. Neck Surg. 2005, 133, 403–406. [Google Scholar] [CrossRef]
- Mourão, C.F.D.A.B.; Calasans-Maia, M.D.; Del Fabbro, M.; Vieira, F.L.D.; Machado, R.C.D.M.; Capella, R.; Miron, R.J.; Alves, G.G. The use of Platelet-rich Fibrin in the management of medication-related osteonecrosis of the jaw: A case series. J. Stomatol. Oral Maxillofac. Surg. 2019, 121, 84–89. [Google Scholar] [CrossRef]
- Dohan, D.M.; Choukroun, J.; Diss, A.; Dohan, S.L.; Dohan, A.J.; Mouhyi, J.; Gogly, B. Platelet-rich fibrin (PRF): A second-generation platelet concentrate. Part I: Technological concepts and evolution. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 101, e37–e44. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wan, Y.; Lin, Y.; Jiang, H. The application of platelet-rich plasma for skin graft enrichment: A meta-analysis. Int. Wound J. 2020, 17, 1650–1658. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, A.; Gupta, A.; Iii, V.I.M.; Romo, M.; Garg, I.; Tapia, D.; Gudino, P.; Lam, S. Efficacy of Platelet-Rich Plasma in Reduction of Post-Operative Split-Thickness Skin Graft Loss and Hematoma Formation: A Meta-Analysis. Cureus 2021, 13, e15160. [Google Scholar] [CrossRef] [PubMed]
- Nica, O.; Popa, D.G.; Grecu, A.F.; Ciucă, E.M.; Ciurea, M.E. Histological aspects of full-thickness skin grafts augmented with platelet-rich fibrin in rat model. Rom. J. Morphol. Embryol. Rev. Roum. De Morphol. Et Embryol. 2019, 60, 581–588. [Google Scholar]
- Wu, P.I.; Diaz, R.; Borg-Stein, J. Platelet-Rich Plasma. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 825–853. [Google Scholar] [CrossRef]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A comparative study of platelet-rich fibrin (PRF) and platelet-rich plasma (PRP) on the effect of proliferation and differentiation of rat osteoblasts in vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of platelet concentrates: From pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Huang, F.-M.; Yang, S.-F.; Zhao, J.-H.; Chang, Y.-C. Platelet-rich Fibrin Increases Proliferation and Differentiation of Human Dental Pulp Cells. J. Endod. 2010, 36, 1628–1632. [Google Scholar] [CrossRef]
- Xia, C.-Y.; Yu, A.-X.; Qi, B.; Zhou, M.; Li, Z.-H.; Wang, W.-Y. Analysis of blood flow and local expression of angiogenesis-associated growth factors in infected wounds treated with negative pressure wound therapy. Mol. Med. Rep. 2014, 9, 1749–1754. [Google Scholar] [CrossRef] [Green Version]
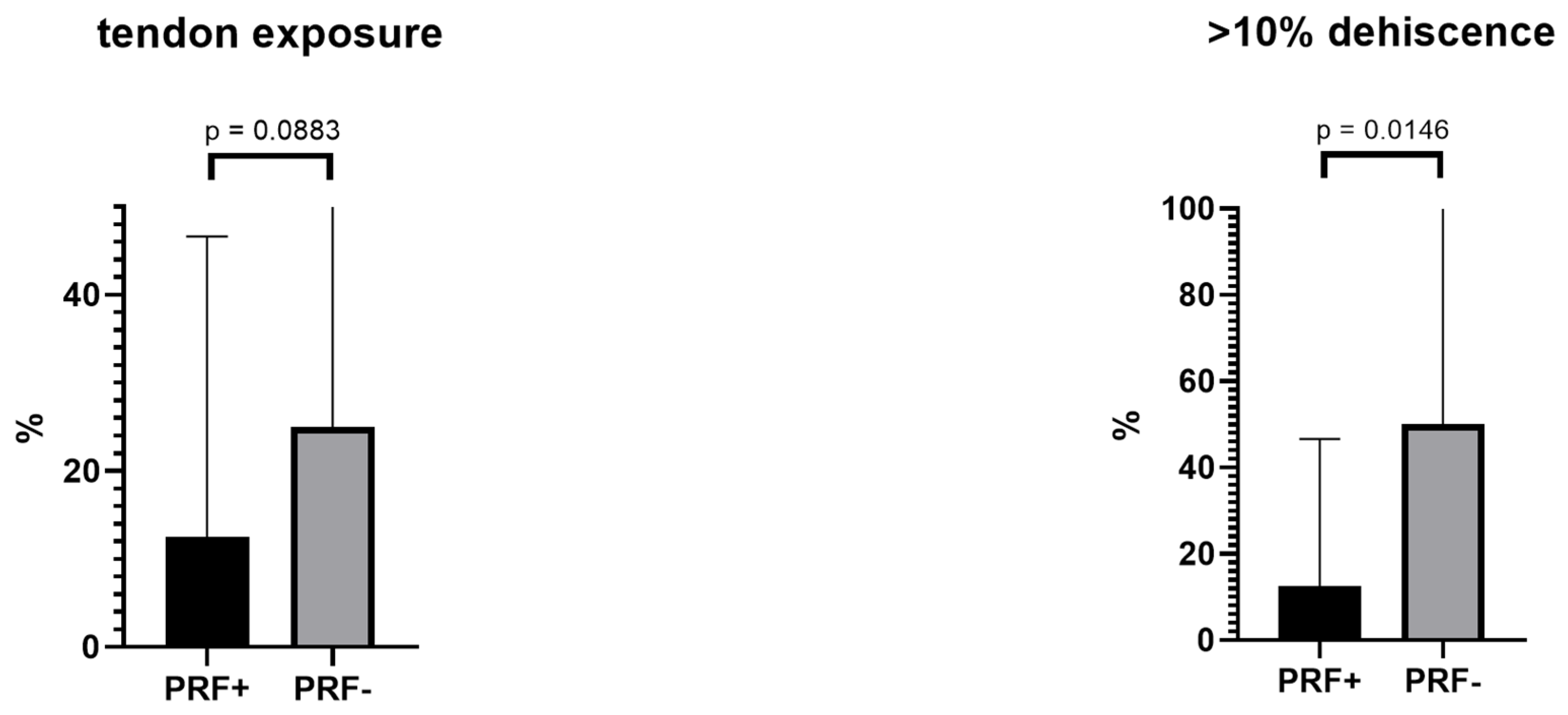
| Yes | No | ||
|---|---|---|---|
| Total score | 0–6 | ||
| Infection | 1 | 0 | |
| Inflammation | 1 | 0 | |
| Necrosis | 1 | 0 | |
| Visible tendon * | 1 | 0 | |
| Tendon exposure * | 1 | 0 | |
| Transplant loss > 10% | 1 | 0 | |
| Total transplant loss ** | 6 | 0 |
| PRF+ | PRF− | |
|---|---|---|
| Age (±SD) | 63.50 ± 18.04 | 62.38 ± 20.49 |
| Participants (n) | 16 | 16 |
| Gender | ||
| Women | 5 | 6 |
| Men | 11 | 10 |
| Diagnosis | ||
| Oral cancer | 12 | 16 |
| Skin cancer | 4 | 0 |
| Graft size (cm2; ±SD) | 19.5 ± 9.14 | 18.4 ± 5.95 |
| Skin graft (n) | ||
| Full-thickness | 6 | 12 |
| Split-thickness | 10 | 4 |
| Reconstruction | ||
| Primary | 4 | 13 |
| Secondary | 12 | 3 |
| Intravenous antibiotics | ||
| No antibiotic therapy | 5 | 2 |
| Ampicillin/sulbactam | 11 | 13 |
| Clindamycin | 0 | 1 |
| Patients | Dehiscence in % | Infections | p (Dehiscence) | |
|---|---|---|---|---|
| Intravenous antibiosis | 0.3888 * | |||
| None | 7 | 7.16 | 0 | |
| Ampicillin/sulbactam | 25 | 10.77 | 1 | |
| or clindamycin |
| M (±SD) in % | Md | Range | p * | 95% CI | |
|---|---|---|---|---|---|
| Coverage rate | |||||
| PRF+ | 93.44 ± 5.212 | 94.73 | 80.92–100 | ||
| PRF− | 86.96 ± 12.10 | 89.26 | 54.81–100 | ||
| Difference | 6.483 ± 3294 | 0.0292 | −0.2450–13.21 |
| PRF | Non-PRF | Difference | p-Value | |
|---|---|---|---|---|
| Mean total score | 1.5 | 2.688 | 1.188 | 0.0458 |
| Infection | 0 | 1 | 1 | 0.1627 |
| Inflammation | 0 | 2 | 2 | 0.0768 |
| Necrosis | 7 | 9 | 2 | 0.2477 |
| Visible tendon | 3 | 4 | 1 | 0.3405 |
| Tendon exposure | 2 | 4 | 2 | 0.1907 |
| >10% dehiscence | 2 | 8 | 6 | 0.0108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Straub, A.; Brands, R.; Borgmann, A.; Vollmer, A.; Hohm, J.; Linz, C.; Müller-Richter, U.; Kübler, A.C.; Hartmann, S. Free Skin Grafting to Reconstruct Donor Sites after Radial Forearm Flap Harvesting: A Prospective Study with Platelet-Rich Fibrin (PRF). J. Clin. Med. 2022, 11, 3506. https://doi.org/10.3390/jcm11123506
Straub A, Brands R, Borgmann A, Vollmer A, Hohm J, Linz C, Müller-Richter U, Kübler AC, Hartmann S. Free Skin Grafting to Reconstruct Donor Sites after Radial Forearm Flap Harvesting: A Prospective Study with Platelet-Rich Fibrin (PRF). Journal of Clinical Medicine. 2022; 11(12):3506. https://doi.org/10.3390/jcm11123506
Chicago/Turabian StyleStraub, Anton, Roman Brands, Anna Borgmann, Andreas Vollmer, Julian Hohm, Christian Linz, Urs Müller-Richter, Alexander C. Kübler, and Stefan Hartmann. 2022. "Free Skin Grafting to Reconstruct Donor Sites after Radial Forearm Flap Harvesting: A Prospective Study with Platelet-Rich Fibrin (PRF)" Journal of Clinical Medicine 11, no. 12: 3506. https://doi.org/10.3390/jcm11123506
APA StyleStraub, A., Brands, R., Borgmann, A., Vollmer, A., Hohm, J., Linz, C., Müller-Richter, U., Kübler, A. C., & Hartmann, S. (2022). Free Skin Grafting to Reconstruct Donor Sites after Radial Forearm Flap Harvesting: A Prospective Study with Platelet-Rich Fibrin (PRF). Journal of Clinical Medicine, 11(12), 3506. https://doi.org/10.3390/jcm11123506








