Differences in Ectopic Pregnancy Rates between Fresh and Frozen Embryo Transfer after In Vitro Fertilization: A Large Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
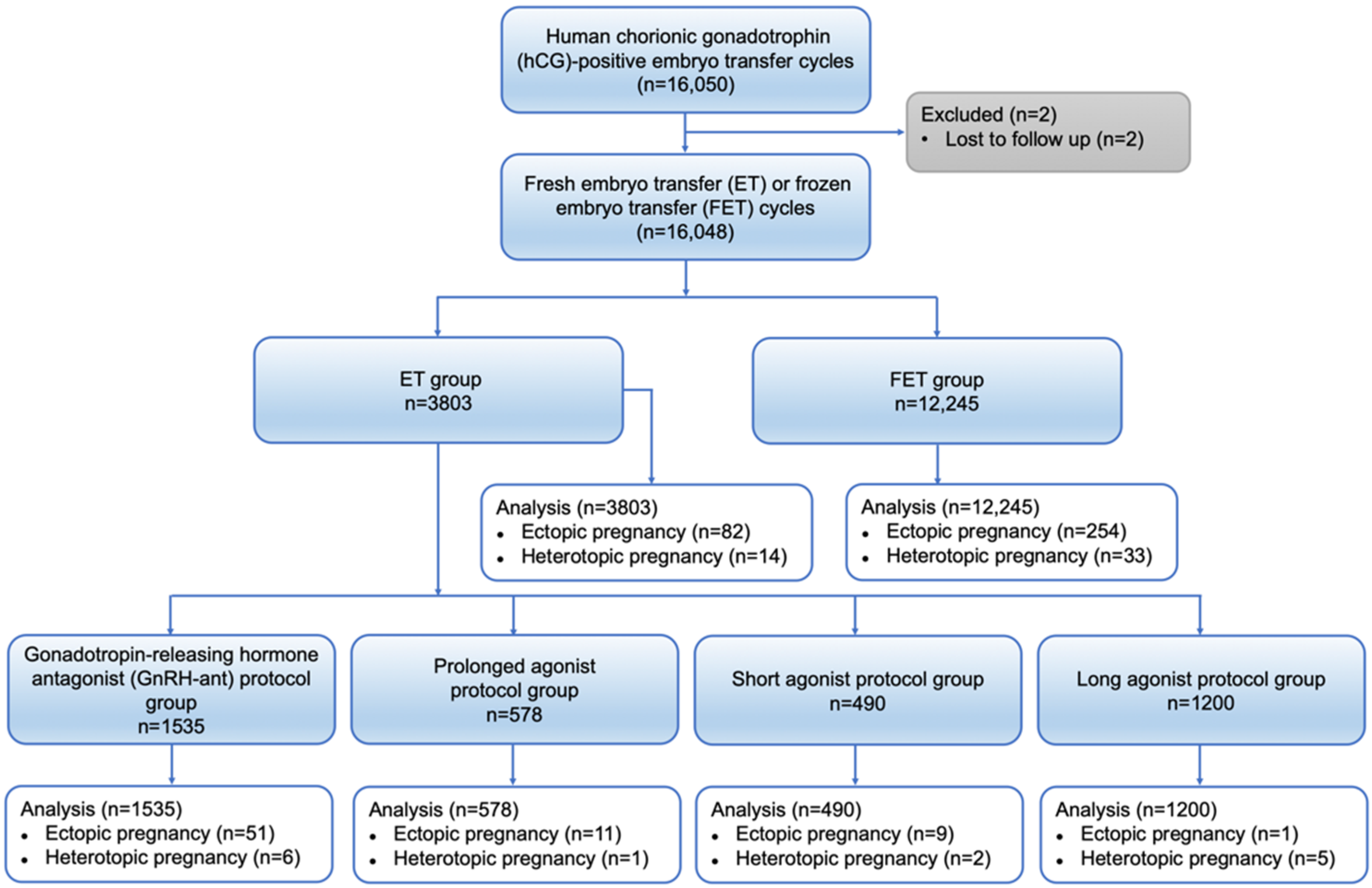
2.1. Study Design and Participants
2.2. Ovarian Stimulation, Monitoring and Oocyte Retrieval Operation
2.3. Insemination, and Embryo Culture
2.4. Fresh Embryo Transfer
2.5. Endometrial Preparation and Frozen Embryo Transfer
2.6. Outcome Measures
2.7. Statistical Analysis
3. Results
3.1. Patient and Cycle Characteristics
3.2. Ectopic Pregnancy Outcome between FET and ET Group
3.3. Ectopic Pregnancy Outcome between Different Ovarian Stimulation Groups in ET Cycles
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marcus, S.F.; Brinsden, P.R. Analysis of the incidence and risk factors associated with ectopic pregnancy following in-vitro fertilization and embryo transfer. Hum. Reprod. 1995, 10, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Clayton, H.B.; Schieve, L.A.; Peterson, H.B.; Jamieson, D.J.; Reynolds, M.A.; Wright, V.C. Ectopic pregnancy risk with assisted reproductive technology procedures. Obstet. Gynecol. 2006, 107, 595–604. [Google Scholar] [CrossRef] [PubMed]
- Pisarska, M.D.; Carson, S.A.; Buster, J.E. Ectopic pregnancy. Lancet 1998, 351, 1115–1120. [Google Scholar] [CrossRef]
- Chang, H.J.; Suh, C.S. Ectopic pregnancy after assisted reproductive technology: What are the risk factors? Curr. Opin. Obstet. Gynecol. 2010, 22, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Refaat, B.; Dalton, E.; Ledger, W.L. Ectopic pregnancy secondary to in vitro fertilisation-embryo transfer: Pathogenic mechanisms and management strategies. Reprod. Biol. Endocrinol. 2015, 13, 30. [Google Scholar] [CrossRef] [PubMed]
- Killick, S.R. Ultrasound and the receptivity of the endometrium. Reprod. Biomed. Online 2007, 15, 63–67. [Google Scholar] [CrossRef]
- Lesny, P.; Killick, S.R. The junctional zone of the uterus and its contractions. BJOG 2004, 111, 1182–1189. [Google Scholar] [CrossRef]
- Paltieli, Y.; Eibschitz, I.; Ziskind, G.; Ohel, G.; Silbermann, M.; Weichselbaum, A. High progesterone levels and ciliary dysfunction—A possible cause of ectopic pregnancy. J. Assist. Reprod. Genet. 2000, 17, 103–106. [Google Scholar] [CrossRef]
- Fanchin, R.; Ayoubi, J.M.; Olivennes, F.; Righini, C.; de Ziegler, D.; Frydman, R. Hormonal influence on the uterine contractility during ovarian stimulation. Hum. Reprod. 2000, 15, 90–100. [Google Scholar] [CrossRef]
- Martinez-Conejero, J.A.; Simon, C.; Pellicer, A.; Horcajadas, J.A. Is ovarian stimulation detrimental to the endometrium? Reprod. Biomed. Online 2007, 15, 45–50. [Google Scholar] [CrossRef]
- Ishihara, O.; Kuwahara, A.; Saitoh, H. Frozen-thawed blastocyst transfer reduces ectopic pregnancy risk: An analysis of single embryo transfer cycles in Japan. Fertil. Steril. 2011, 95, 1966–1969. [Google Scholar] [CrossRef] [PubMed]
- Huang, B.; Hu, D.; Qian, K.; Ai, J.H.; Li, Y.F.; Jin, L.; Zhu, G.J.; Zhang, H.W. Is frozen embryo transfer cycle associated with a significantly lower incidence of ectopic pregnancy? An analysis of more than 30,000 cycles. Fertil. Steril. 2014, 102, 1345–1349. [Google Scholar] [CrossRef] [PubMed]
- Londra, L.; Moreau, C.; Strobino, D.; Garcia, J.; Zacur, H.; Zhao, Y.L. Ectopic pregnancy after in vitro fertilization: Differences between fresh and frozen-thawed cycles. Fertil. Steril. 2015, 104, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Pyrgiotis, E.; Sultan, K.M.; Neal, G.S.; Liu, H.C.; Grifo, J.A.; Rosenwaks, Z. Ectopic Pregnancies after in-Vitro Fertilization and Embryo-Transfer. J. Assist. Reprod. Gen. 1994, 11, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Trimarchi, J.; Keefe, D.; Frankfurter, D. High incidence of ectopic pregnancy following frozen embryo transfer. Fertil. Steril. 2003, 80, S178. [Google Scholar] [CrossRef]
- Bu, Z.; Xiong, Y.; Wang, K.; Sun, Y. Risk factors for ectopic pregnancy in assisted reproductive technology: A 6-year, single-center study. Fertil. Steril. 2016, 106, 90–94. [Google Scholar] [CrossRef]
- Jun, S.H.; Milki, A.A. Ectopic pregnancy rates with frozen compared with fresh blastocyst transfer. Fertil. Steril. 2007, 88, 629–631. [Google Scholar] [CrossRef]
- Decleer, W.; Osmanagaoglu, K.; Meganck, G.; Devroey, P. Slightly lower incidence of ectopic pregnancies in frozen embryo transfer cycles versus fresh in vitro fertilization-embryo transfer cycles: A retrospective cohort study. Fertil. Steril. 2014, 101, 162–165. [Google Scholar] [CrossRef]
- Cummins, J.M.; Breen, T.M.; Harrison, K.L.; Shaw, J.M.; Wilson, L.M.; Hennessey, J.F. A formula for scoring human embryo growth rates in in vitro fertilization: Its value in predicting pregnancy and in comparison with visual estimates of embryo quality. J. Vitr. Fertil. Embryo Transf. 1986, 3, 284–295. [Google Scholar] [CrossRef]
- Du, T.; Chen, H.; Fu, R.; Chen, Q.J.; Wang, Y.; Mol, B.W.; Kuang, Y.P.; Lyu, Q.F. Comparison of ectopic pregnancy risk among transfers of embryos vitrified on day 3, day 5, and day 6. Fertil. Steril. 2017, 108, 108–116.e1. [Google Scholar] [CrossRef]
- Steptoe, P.C.; Edwards, R.G. Reimplantation of a human embryo with subsequent tubal pregnancy. Lancet 1976, 1, 880–882. [Google Scholar] [CrossRef]
- Society for Assisted Reproductive Technology; American Society for Reproductive Medicine. Assisted reproductive technology in the United States: 2001 results generated from the American Society for Reproductive Medicine/Society for Assisted Reproductive Technology registry. Fertil. Steril. 2007, 87, 1253–1266. [Google Scholar] [CrossRef] [PubMed]
- Wiser, A.; Gilbert, A.; Nahum, R.; Orvieto, R.; Haas, J.; Hourvitz, A.; Weissman, A.; Younes, G.; Dirnfeld, M.; Hershko, A.; et al. Effects of treatment of ectopic pregnancy with methotrexate or salpingectomy in the subsequent IVF cycle. Reprod. Biomed. Online 2013, 26, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Weigert, M.; Gruber, D.; Pernicka, E.; Bauer, P.; Feichtinger, W. Previous tubal ectopic pregnancy raises the incidence of repeated ectopic pregnancies in in vitro fertilization-embryo transfer patients. J. Assist. Reprod. Genet. 2009, 26, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Santos-Ribeiro, S.; Tournaye, H.; Polyzos, N.P. Trends in ectopic pregnancy rates following assisted reproductive technologies in the UK: A 12-year nationwide analysis including 160,000 pregnancies. Hum. Reprod. 2016, 31, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Perkins, K.M.; Boulet, S.L.; Kissin, D.M.; Jamieson, D.J.; National ART Surveillance (NASS) Group. Risk of ectopic pregnancy associated with assisted reproductive technology in the United States, 2001–2011. Obstet. Gynecol. 2015, 125, 70–78. [Google Scholar] [CrossRef]
- Wu, J.; Li, D.; Liu, X.; Li, Q.; He, X.; Wei, J.; Li, X.; Li, M.; Rehman, A.U.; Xia, Y.; et al. IDDB: A comprehensive resource featuring genes, variants and characteristics associated with infertility. Nucleic Acids Res. 2021, 49, D1218–D1224. [Google Scholar] [CrossRef]
- Lesny, P.; Killick, S.R.; Robinson, J.; Maguiness, S.D. Transcervical embryo transfer as a risk factor for ectopic pregnancy. Fertil. Steril. 1999, 72, 305–309. [Google Scholar] [CrossRef]
- Kolibianakis, E.; Bourgain, C.; Albano, C.; Osmanagaoglu, K.; Smitz, J.; Van Steirteghem, A.; Devroey, P. Effect of ovarian stimulation with recombinant follicle-stimulating hormone, gonadotropin releasing hormone antagonists, and human chorionic gonadotropin on endometrial maturation on the day of oocyte pick-up. Fertil. Steril. 2002, 78, 1025–1029. [Google Scholar] [CrossRef]
- Shapiro, B.S.; Daneshmand, S.T.; Garner, F.C.; Aguirre, M.; Thomas, S. Large blastocyst diameter, early blastulation, and low preovulatory serum progesterone are dominant predictors of clinical pregnancy in fresh autologous cycles. Fertil. Steril. 2008, 90, 302–309. [Google Scholar] [CrossRef]
- Shao, R.; Egecioglu, E.; Weijdegard, B.; Kopchick, J.J.; Fernandez-Rodriguez, J.; Andersson, N.; Billig, H. Dynamic regulation of estrogen receptor-alpha isoform expression in the mouse fallopian tube: Mechanistic insight into estrogen-dependent production and secretion of insulin-like growth factors. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E1430–E1442. [Google Scholar] [CrossRef] [PubMed]
- Nakahari, T.; Nishimura, A.; Shimamoto, C.; Sakai, A.; Kuwabara, H.; Nakano, T.; Tanaka, S.; Kohda, Y.; Matsumura, H.; Mori, H. The regulation of ciliary beat frequency by ovarian steroids in the guinea pig Fallopian tube: Interactions between oestradiol and progesterone. Biomed. Res. 2011, 32, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Knutzen, V.; Stratton, C.J.; Sher, G.; McNamee, P.I.; Huang, T.T.; Soto-Albors, C. Mock embryo transfer in early luteal phase, the cycle before in vitro fertilization and embryo transfer: A descriptive study. Fertil. Steril. 1992, 57, 156–162. [Google Scholar] [CrossRef]
- Londra, L.; Moreau, C.; Strobino, D.; Bhasin, A.; Zhao, Y. Is the type of gonadotropin-releasing hormone suppression protocol for ovarian hyperstimulation associated with ectopic pregnancy in fresh autologous cycles for in vitro fertilization? Fertil. Steril. 2016, 106, 666–672. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weiss, A.; Beck-Fruchter, R.; Golan, J.; Lavee, M.; Geslevich, Y.; Shalev, E. Ectopic pregnancy risk factors for ART patients undergoing the GnRH antagonist protocol: A retrospective study. Reprod. Biol. Endocrinol. 2016, 14, 12. [Google Scholar] [CrossRef][Green Version]
| Characteristics | ET Group (n = 3803) | FET Group (n = 12,245) | p Value |
|---|---|---|---|
| Age of women (year) | 39.45 ± 5.999 | 35.19 ± 4.531 | <0.001 |
| BMI of women | 21.74 ± 3.057 | 22.27 ± 3.367 | <0.001 |
| Infertility duration(year) | 3.60 ± 2.996 | 3.07 ± 2.696 | <0.001 |
| Infertility causes, n (%) | |||
| Tubal factor | 2364 (62.16%) | 7058 (57.64%) | <0.001 |
| Male factor | 551 (14.49%) | 1587 (12.95%) | <0.05 |
| Endometriosis | 155 (4.08%) | 412 (3.36%) | <0.05 |
| PCOS | 107 (2.81%) | 849 (6.93%) | <0.001 |
| Uterine factor | 51 (1.34%) | 206 (1.68%) | 0.14 |
| DOR | 48 (1.26%) | 193 (1.58%) | 0.16 |
| Others | 527 (13.86%) | 1940 (15.84%) | <0.01 |
| History of ectopic pregnancy, n (%) | 277 (7.28%) | 1818 (14.85%) | <0.001 |
| Nature cycle, n (%) | |||
| Yes | 0 (0.00%) | 1281 (10.46%) | <0.001 |
| No | 3803 (100.00%) | 10,964 (89.54%) | <0.001 |
| No. of embryos transferred, n (%) | |||
| 1 | 694 (18.25%) | 4111 (33.57%) | <0.001 |
| 2 | 2883 (75.81%) | 8132 (66.41%) | <0.001 |
| 3 | 224 (5.89%) | 2 (0.02%) | <0.001 |
| 4 | 2 (0.05%) | 0 (0.00%) | <0.05 |
| Stage of embryos transferred, n (%) | |||
| Blastocyst | 27 (0.71%) | 3593 (29.34%) | <0.001 |
| Non-blastocyst | 3776 (99.29%) | 8741 (71.38%) | <0.001 |
| Endometrial thickness (mm) | 11.54 ± 2.720 | 10.83 ± 2.434 | <0.001 |
| ET Group (n = 3803) | FET Group (n = 12,245) | p Value | |
|---|---|---|---|
| Ectopic pregnancy, n (%) | 82 (2.16%) | 254 (2.07%) | 0.76 |
| Heterotopic pregnancy, n (%) | 14 (0.37%) | 33 (0.27%) | 0.33 |
| Ectopic Pregnancy | Heterotopic Pregnancy | |||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| ET group | Reference | Reference | Reference | Reference |
| FET group | 0.96 (0.75–1.24) | 0.93 (0.71–1.22) | 0.73 (0.39–1.37) | 0.72 (0.34–1.50) |
| GnRH-ant Protocol (n = 1535) | Prolonged Agonist Protocol (n = 578) | Short Agonist Protocol (n = 490) | Long Agonist Protocol (n = 1200) | |
|---|---|---|---|---|
| Ectopic pregnancy, n (%) | 51 (3.32%) | 11 (1.90%) | 9 (1.84%) | 11 (0.92%) a |
| Heterotopic pregnancy, n (%) | 6 (0.39%) | 1 (0.17%) | 2 (0.41%) | 5 (0.42%) |
| Ectopic Pregnancy | Heterotopic Pregnancy | |||
|---|---|---|---|---|
| Crude OR (95% CI) | Adjusted OR (95% CI) | Crude OR (95% CI) | Adjusted OR (95% CI) | |
| GnRH-ant protocol | Reference | Reference | Reference | Reference |
| prolonged agonist protocol | 0.57 (0.29–1.09) | 0.59 (0.30–1.16) | 0.44 (0.05–3.68) | 0.43 (0.05–3.58) |
| short agonist protocol | 0.54 (0.27–1.11) | 0.81 (0.38–1.71) | 1.04 (0.21–5.19) | 1.05 (0.19–5.84) |
| long agonist protocol | 0.27 (0.14–0.52) *** | 0.45 (0.22–0.93) * | 1.07 (0.33–3.50) | 1.00 (0.26–3.92) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Z.; Li, D.; Chen, Q.; Chai, W.; Lyu, Q.; Cai, R.; Kuang, Y.; Lu, X. Differences in Ectopic Pregnancy Rates between Fresh and Frozen Embryo Transfer after In Vitro Fertilization: A Large Retrospective Study. J. Clin. Med. 2022, 11, 3386. https://doi.org/10.3390/jcm11123386
Hu Z, Li D, Chen Q, Chai W, Lyu Q, Cai R, Kuang Y, Lu X. Differences in Ectopic Pregnancy Rates between Fresh and Frozen Embryo Transfer after In Vitro Fertilization: A Large Retrospective Study. Journal of Clinical Medicine. 2022; 11(12):3386. https://doi.org/10.3390/jcm11123386
Chicago/Turabian StyleHu, Zhijie, Danjun Li, Qiuju Chen, Weiran Chai, Qifeng Lyu, Renfei Cai, Yanping Kuang, and Xuefeng Lu. 2022. "Differences in Ectopic Pregnancy Rates between Fresh and Frozen Embryo Transfer after In Vitro Fertilization: A Large Retrospective Study" Journal of Clinical Medicine 11, no. 12: 3386. https://doi.org/10.3390/jcm11123386
APA StyleHu, Z., Li, D., Chen, Q., Chai, W., Lyu, Q., Cai, R., Kuang, Y., & Lu, X. (2022). Differences in Ectopic Pregnancy Rates between Fresh and Frozen Embryo Transfer after In Vitro Fertilization: A Large Retrospective Study. Journal of Clinical Medicine, 11(12), 3386. https://doi.org/10.3390/jcm11123386








