Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case
Abstract
1. Introduction
2. Materials and Methods
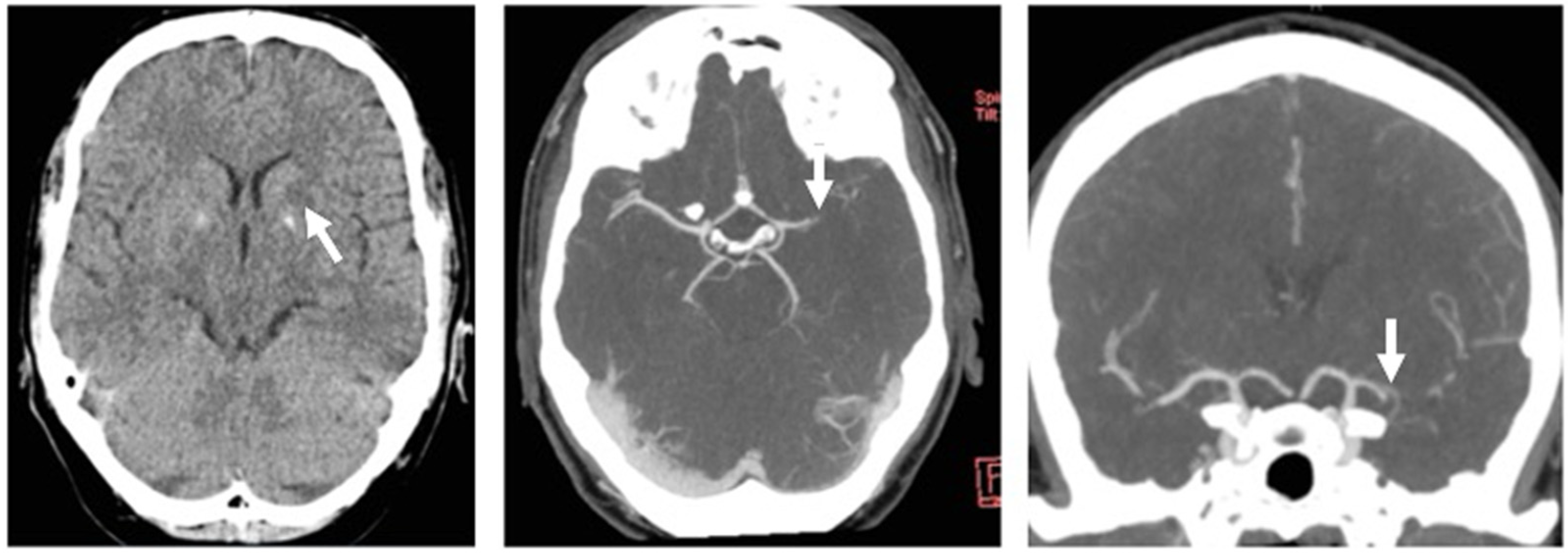
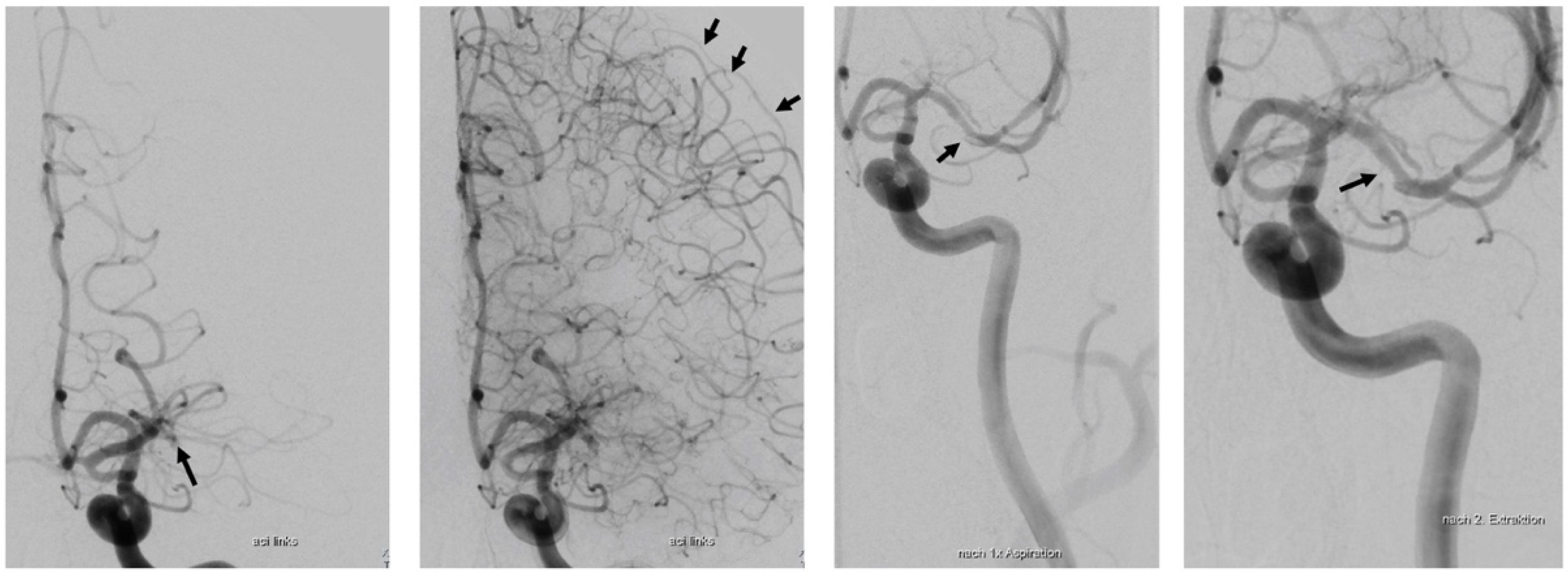
2.1. Case Report
2.2. Transcranial Color-Coded Sonography (TCCS)
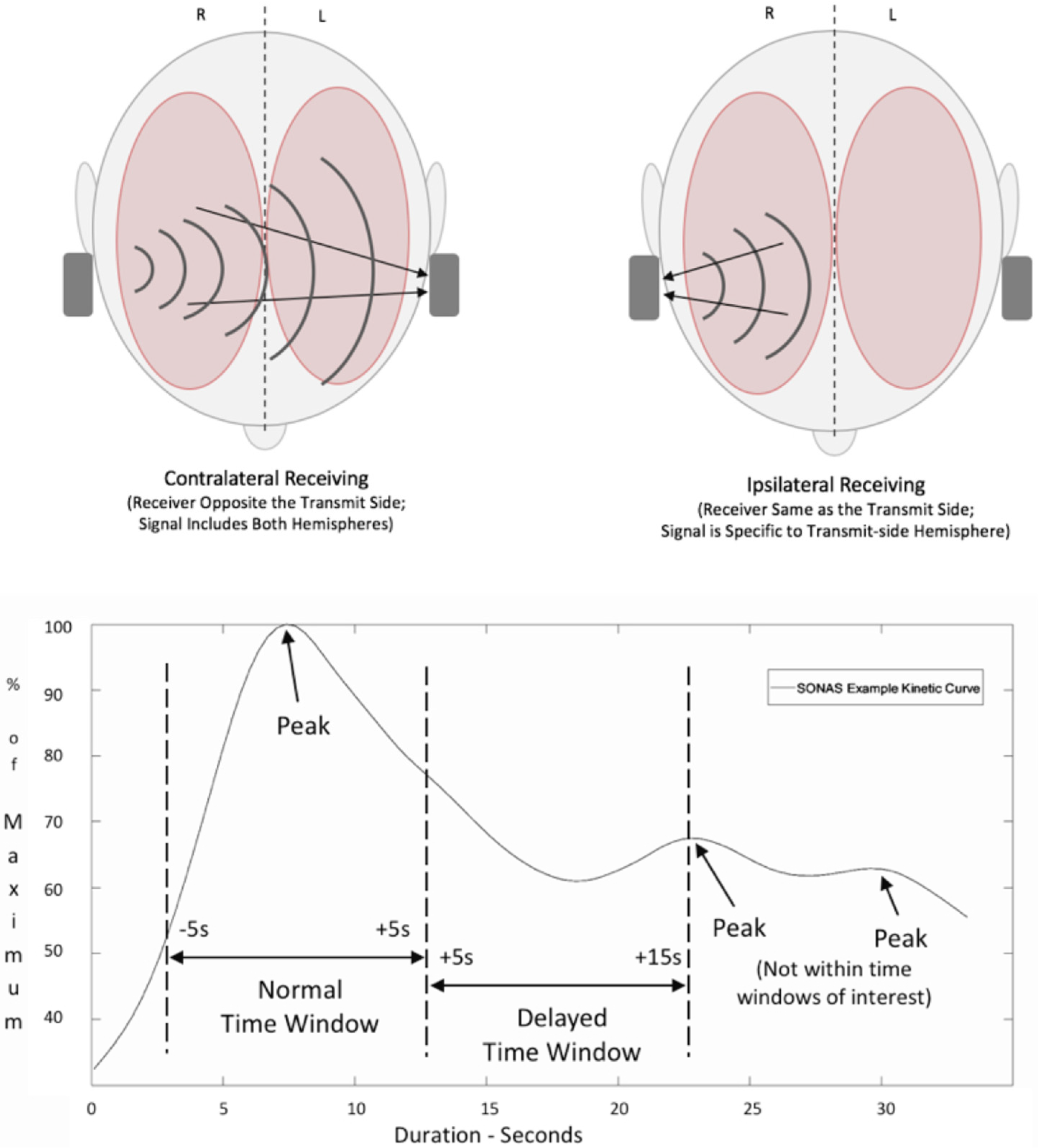
2.3. Brain Perfusion Ultrasound (BPU) Using SONAS®
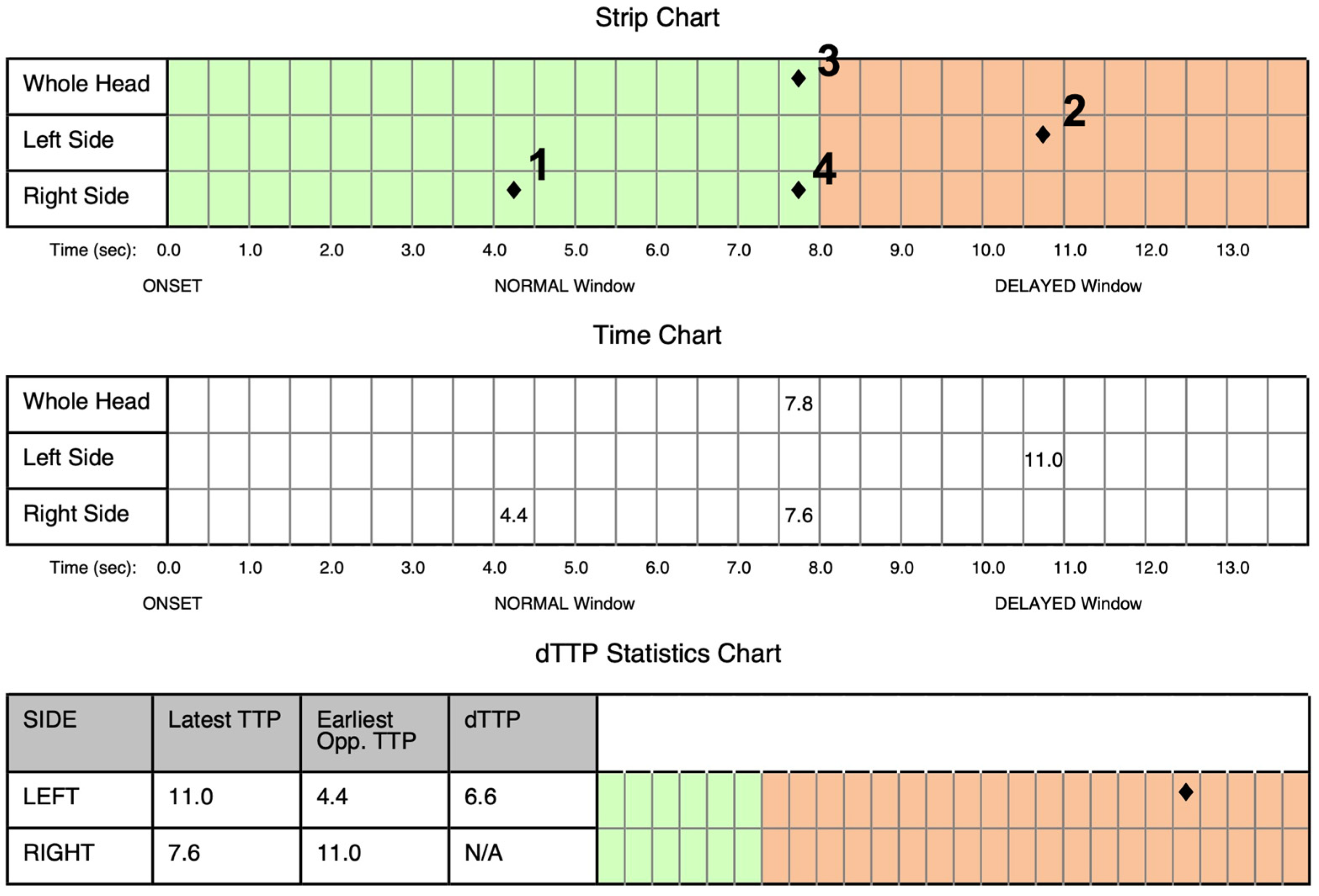
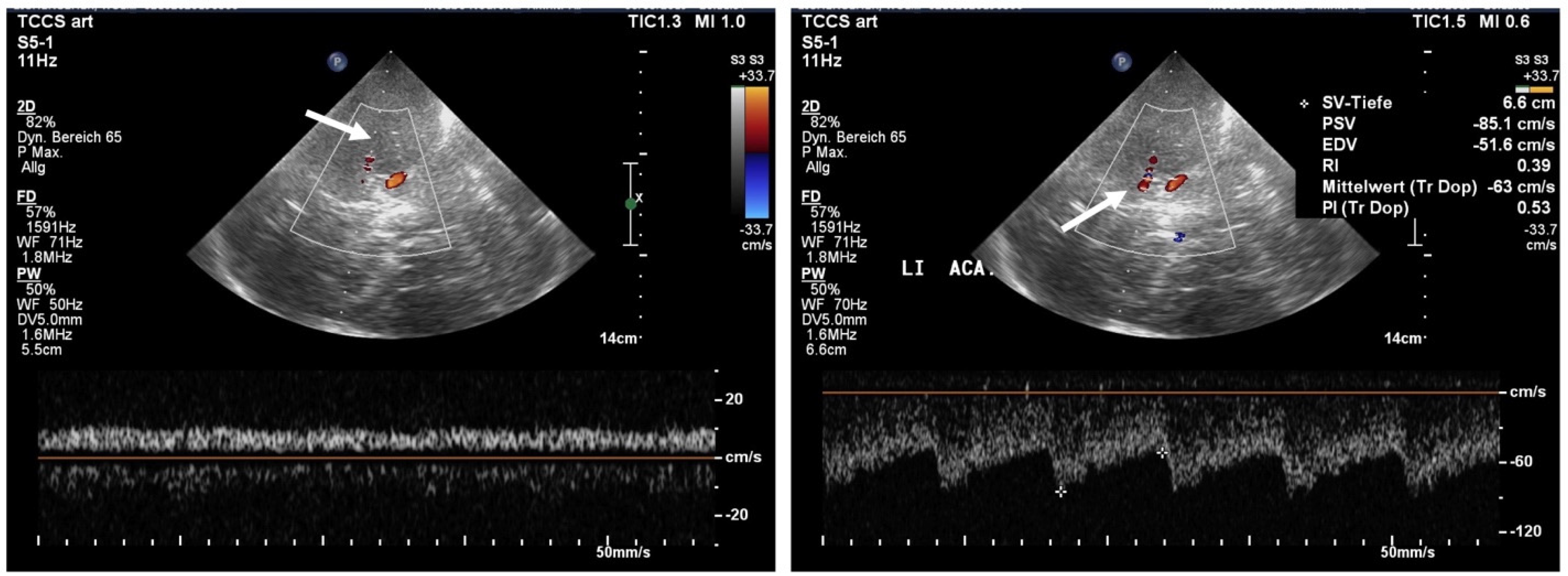
3. Results
Case Report Continued
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goyal, M.; Menon, B.K.; van Zwam, W.H.; Dippel, D.W.; Mitchell, P.J.; Demchuk, A.M.; Davalos, A.; Majoie, C.B.; van der Lugt, A.; de Miquel, M.A.; et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet 2016, 387, 1723–1731. [Google Scholar] [CrossRef]
- Jovin, T.G.; Nogueira, R.G.; Lansberg, M.G.; Demchuk, A.M.; Martins, S.O.; Mocco, J.; Ribo, M.; Jadhav, A.P.; Ortega-Gutierrez, S.; Hill, M.D.; et al. Thrombectomy for anterior circulation stroke beyond 6 h from time last known well (AURORA): A systematic review and individual patient data meta-analysis. Lancet 2022, 399, 249–258. [Google Scholar] [CrossRef]
- Holodinsky, J.K.; Patel, A.B.; Thornton, J.; Kamal, N.; Jewett, L.R.; Kelly, P.J.; Murphy, S.; Collins, R.; Walsh, T.; Cronin, S.; et al. Drip and ship versus direct to endovascular thrombectomy: The impact of treatment times on transport decision-making. Eur. Stroke J. 2018, 3, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Eyding, J.; Kitzrow, M.; Bartig, D.; Weimar, C.; Hacke, W.; Krogias, C. Distribution and evolution of acute interventional ischemic stroke treatment in Germany from 2010 to 2016. Neurol. Res. Pract. 2019, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Jauch, E.C.; Schwamm, L.H.; Panagos, P.D.; Barbazzeni, J.; Dickson, R.; Dunne, R.; Foley, J.; Fraser, J.F.; Lassers, G.; Martin-Gill, C.; et al. Recommendations for Regional Stroke Destination Plans in Rural, Suburban, and Urban Communities from the Prehospital Stroke System of Care Consensus Conference: A Consensus Statement from the American Academy of Neurology, American Heart Association/American Stroke Association, American Society of Neuroradiology, National Association of EMS Physicians, National Association of State EMS Officials, Society of NeuroInterventional Surgery, and Society of Vascular and Interventional Neurology: Endorsed by the Neurocritical Care Society. Stroke 2021, 52, e133–e152. [Google Scholar]
- Schlachetzki, F.; Herzberg, M.; Holscher, T.; Ertl, M.; Zimmermann, M.; Ittner, K.P.; Pels, H.; Bogdahn, U.; Boy, S. Transcranial ultrasound from diagnosis to early stroke treatment: Part 2: Prehospital neurosonography in patients with acute stroke: The Regensburg stroke mobile project. Cerebrovasc. Dis. 2012, 33, 262–271. [Google Scholar] [CrossRef] [PubMed]
- Herzberg, M.; Boy, S.; Holscher, T.; Ertl, M.; Zimmermann, M.; Ittner, K.P.; Pemmerl, J.; Pels, H.; Bogdahn, U.; Schlachetzki, F. Prehospital stroke diagnostics based on neurological examination and transcranial ultrasound. Crit. Ultrasound J. 2014, 6, 3. [Google Scholar] [CrossRef]
- Audebert, H.; Fassbender, K.; Hussain, M.S.; Ebinger, M.; Turc, G.; Uchino, K.; Davis, S.; Alexandrov, A.; Grotta, J.; Group, P. The PRE-hospital Stroke Treatment Organization. Int. J. Stroke 2017, 12, 932–940. [Google Scholar] [CrossRef]
- Walter, S.; Zhao, H.; Easton, D.; Bil, C.; Sauer, J.; Liu, Y.; Lesmeister, M.; Grunwald, I.Q.; Donnan, G.A.; Davis, S.M.; et al. Air-Mobile Stroke Unit for access to stroke treatment in rural regions. Int. J. Stroke 2018, 13, 568–575. [Google Scholar] [CrossRef]
- Kilic, M.; Pflug, K.; Theiss, S.; Webert, M.; Hirschmann, N.; Wagner, A.; Boy, S.; Ertl, M.; Linker, R.A.; Schlachetzki, F.; et al. Prehospital Identification of Middle Cerebral Artery Occlusion—A Stroke Education Program and Transcranial Ultrasound for Paramedics. Austin. J. Clin. Neurol. 2020, 7, 1142. [Google Scholar]
- Kilic, M.; Scalzo, F.; Lyle, C.; Baldaranov, D.; Dirnbacher, M.; Honda, T.; Liebeskind, D.S.; Schlachetzki, F. A mobile battery-powered brain perfusion ultrasound (BPU) device designed for prehospital stroke diagnosis: Correlation to perfusion MRI in healthy volunteers. Neurol. Res. Pract. 2022, 4, 13. [Google Scholar] [CrossRef]
- Gerriets, T.; Postert, T.; Goertler, M.; Stolz, E.; Schlachetzki, F.; Sliwka, U.; Seidel, G.; Weber, S.; Kaps, M. DIAS I: Duplex-sonographic assessment of the cerebrovascular status in acute stroke. A useful tool for future stroke trials. Stroke 2000, 31, 2342–2345. [Google Scholar] [CrossRef]
- Bogdahn, U.; Holscher, T.; Rosin, L.; Gotz, B.; Schlachetzki, F. Contrast-Enhanced Transcranial and Extracranial Duplex Sonography: Preliminary Results of a Multicenter Phase II/III Study with SonoVuetrade mark. Echocardiography 1999, 16 Pt 2, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; Marko, M. Optimising prehospital stroke triage in a changing landscape. Lancet Neurol. 2021, 20, 166–168. [Google Scholar] [CrossRef]
- Duvekot, M.H.C.; Venema, E.; Rozeman, A.D.; Moudrous, W.; Vermeij, F.H.; Biekart, M.; Lingsma, H.F.; Maasland, L.; Wijnhoud, A.D.; Mulder, L.; et al. Comparison of eight prehospital stroke scales to detect intracranial large-vessel occlusion in suspected stroke (PRESTO): A prospective observational study. Lancet Neurol. 2021, 20, 213–221. [Google Scholar] [CrossRef]
- Duloquin, G.; Graber, M.; Garnier, L.; Mohr, S.; Giroud, M.; Vergely, C.; Bejot, Y. Assessment of Clinical Scales for Detection of Large Vessel Occlusion in Ischemic Stroke Patients from the Dijon Stroke Registry. J. Clin. Med. 2021, 10, 5893. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, S.; Demchuk, A. Clinical and Technological Approaches to the Prehospital Diagnosis of Large Vessel Occlusion. Stroke 2018, 49, 1036–1043. [Google Scholar] [CrossRef]
- Haight, T.; Tabaac, B.; Patrice, K.A.; Phipps, M.S.; Butler, J.; Johnson, B.; Aycock, A.; Toral, L.; Yarbrough, K.L.; Schrier, C.; et al. The Maryland Acute Stroke Emergency Medical Services Routing Pilot: Expediting Access to Thrombectomy for Stroke. Front. Neurol. 2021, 12, 663472. [Google Scholar] [CrossRef]
- Yperzeele, L.; Van Hooff, R.J.; De Smedt, A.; Valenzuela Espinoza, A.; Van de Casseye, R.; Hubloue, I.; De Keyser, J.; Brouns, R. Prehospital stroke care: Limitations of current interventions and focus on new developments. Cerebrovasc. Dis. 2014, 38, 1–9. [Google Scholar] [CrossRef]
- Antipova, D.; Eadie, L.; Macaden, A.; Wilson, P. Diagnostic accuracy of clinical tools for assessment of acute stroke: A systematic review. BMC Emerg. Med. 2019, 19, 49. [Google Scholar] [CrossRef]
- Ramos-Pachon, A.; Lopez-Cancio, E.; Bustamante, A.; de la Ossa, N.P.; Millan, M.; Hernandez-Perez, M.; Garcia-Berrocoso, T.; Cardona, P.; Rubiera, M.; Serena, J.; et al. D-Dimer as Predictor of Large Vessel Occlusion in Acute Ischemic Stroke. Stroke 2021, 52, 852–858. [Google Scholar] [CrossRef] [PubMed]
- Luger, S.; Jaeger, H.S.; Dixon, J.; Bohmann, F.O.; Schaefer, J.; Richieri, S.P.; Larsen, K.; Hov, M.R.; Bache, K.G.; Foerch, C.; et al. Diagnostic Accuracy of Glial Fibrillary Acidic Protein and Ubiquitin Carboxy-Terminal Hydrolase-L1 Serum Concentrations for Differentiating Acute Intracerebral Hemorrhage from Ischemic Stroke. Neurocrit. Care 2020, 33, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Gomez, J.L.; Mayo, P.H.; Koenig, S.J. Point-of-Care Ultrasonography. N. Engl. J. Med. 2021, 385, 1593–1602. [Google Scholar] [CrossRef] [PubMed]
- Valaikiene, J.; Schlachetzki, F.; Azevedo, E.; Kaps, M.; Lochner, P.; Katsanos, A.H.; Walter, U.; Baracchini, C.; Bartels, E.; Skoloudik, D. Point-of-Care Ultrasound in Neurology—Report of the EAN SPN/ESNCH/ERcNsono Neuro-POCUS Working Group. Ultraschall Med. 2022. [CrossRef] [PubMed]
- Meairs, S. Contrast-enhanced ultrasound perfusion imaging in acute stroke patients. Eur. Neurol. 2008, 59 (Suppl. S1), 17–26. [Google Scholar] [CrossRef]
- Rim, S.J.; Leong-Poi, H.; Lindner, J.R.; Couture, D.; Ellegala, D.; Mason, H.; Durieux, M.; Kassel, N.F.; Kaul, S. Quantification of cerebral perfusion with "Real-Time" contrast-enhanced ultrasound. Circulation 2001, 104, 2582–2587. [Google Scholar] [CrossRef]
- Antipova, D.; Eadie, L.; Makin, S.; Shannon, H.; Wilson, P.; Macaden, A. The use of transcranial ultrasound and clinical assessment to diagnose ischaemic stroke due to large vessel occlusion in remote and rural areas. PLoS ONE 2020, 15, e0239653. [Google Scholar] [CrossRef]
- Mort, A.; Eadie, L.; Regan, L.; Macaden, A.; Heaney, D.; Bouamrane, M.M.; Rushworth, G.; Wilson, P. Combining transcranial ultrasound with intelligent communication methods to enhance the remote assessment and management of stroke patients: Framework for a technology demonstrator. Health Inform. J. 2016, 22, 691–701. [Google Scholar] [CrossRef]
| Brain Perfusion Ultrasound | Transcranial Color-Coded Sonography | |
|---|---|---|
| Information | Hemispheric brain perfusion | Ipsilateral intracranial arteries |
| Operator qualification | Low due to automated analysis | High, requires anatomical knowledge for interpretation |
| Controls | Single button, sequential operation | Variable keyboard complexity, pro–grammable presets |
| Bone penetration | High | Low to high, can be increased using echo-enhancing agents |
| Echo-enhancing agents | Required | Optional |
| Presentation | Generation of time-intensity curves on-site | Real-time depiction of vessel occlusion |
| Potential additional information | Raised intracranial pressure | Intracerebral hemorrhage Brain parenchymal shift (i.e., midline displacement, intracerebral hemorrhage, hydrocephalus) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kilic, M.; Wendl, C.; Wilfling, S.; Olmes, D.; Linker, R.A.; Schlachetzki, F. Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case. J. Clin. Med. 2022, 11, 3384. https://doi.org/10.3390/jcm11123384
Kilic M, Wendl C, Wilfling S, Olmes D, Linker RA, Schlachetzki F. Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case. Journal of Clinical Medicine. 2022; 11(12):3384. https://doi.org/10.3390/jcm11123384
Chicago/Turabian StyleKilic, Mustafa, Christina Wendl, Sibylle Wilfling, David Olmes, Ralf Andreas Linker, and Felix Schlachetzki. 2022. "Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case" Journal of Clinical Medicine 11, no. 12: 3384. https://doi.org/10.3390/jcm11123384
APA StyleKilic, M., Wendl, C., Wilfling, S., Olmes, D., Linker, R. A., & Schlachetzki, F. (2022). Acute Middle Cerebral Artery Occlusion Detection Using Mobile Non-Imaging Brain Perfusion Ultrasound—First Case. Journal of Clinical Medicine, 11(12), 3384. https://doi.org/10.3390/jcm11123384






