Primary Orbital Reconstruction with Selective Laser Melting (SLM) of Patient-Specific Implants (PSIs): An Overview of 96 Surgically Treated Patients
Abstract
:1. Introduction
2. Materials and Methods
- Fractures of the medial orbital wall;
- Fractures of the posterior third of the orbital floor;
- Complex comminuted orbital fractures;
- Orbital wall fractures, including the transition zone between medial wall and orbital floor.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Manolidis, S.; Weeks, B.H.; Kirby, M.; Scarlett, M.; Hollier, L. Classification and surgical management of orbital fractures: Experience with 111 orbital reconstructions. J. Craniofac. Surg. 2002, 13, 726–737; discussion 738. [Google Scholar] [CrossRef] [PubMed]
- Jank, S.; Schuchter, B.; Emshoff, R.; Strobl, H.; Koehler, J.; Nicasi, A.; Norer, B.; Baldissera, I. Clinical signs of orbital wall fractures as a function of anatomic location. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2003, 96, 149–153. [Google Scholar] [CrossRef]
- Tong, L.; Bauer, R.J.; Buchman, S.R. A current 10-year retrospective survey of 199 surgically treated orbital floor fractures in a nonurban tertiary care center. Plast. Reconstr. Surg. 2001, 108, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Shere, J.L.; Boole, J.R.; Holtel, M.R.; Amoroso, P.J. An analysis of 3599 midfacial and 1141 orbital blowout fractures among 4426 United States Army Soldiers, 1980–2000. Otolaryngol.—Head Neck Surg. Off. J. Am. Acad. Otolaryngol.-Head Neck Surg. 2004, 130, 164–170. [Google Scholar] [CrossRef]
- Chi, M.J.; Ku, M.; Shin, K.H.; Baek, S. An analysis of 733 surgically treated blowout fractures. Ophthalmologica 2010, 224, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Gosau, M.; Schöneich, M.; Draenert, F.G.; Ettl, T.; Driemel, O.; Reichert, T.E. Retrospective analysis of orbital floor fractures—Complications, outcome, and review of literature. Clin. Oral Investig. 2011, 15, 305–313. [Google Scholar] [CrossRef]
- Antoun, J.S.; Lee, K.H. Sports-related maxillofacial fractures over an 11-year period. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2008, 66, 504–508. [Google Scholar] [CrossRef]
- Burnstine, M.A. Clinical recommendations for repair of isolated orbital floor fractures. Ophthalmology 2002, 109, 1207–1210. [Google Scholar] [CrossRef]
- Gellrich, N.-C.; Schramm, A.; Hammer, B.; Rojas, S.; Cufi, D.; Lagrèze, W.; Schmelzeisen, R. Computer-assisted secondary reconstruction of unilateral posttraumatic orbital deformity. Plast. Reconstr. Surg. 2002, 110, 1417–1429. [Google Scholar]
- Rana, M.; Gellrich, M.-M.; Gellrich, N.-C. Customised reconstruction of the orbital wall and engineering of selective laser melting (SLM) core implants. Br. J. Oral Maxillofac. Surg. 2015, 53, 208–209. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Szymor, P. Comparison of pre-bent titanium mesh versus polyethylene implants in patient specific orbital reconstructions. Head Face Med. 2013, 9, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huempfner-Hierl, H.; Doerfler, H.-M.; Kruber, D.; Hierl, T. Morphologic comparison of preformed orbital meshes. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2015, 73, 1119–1123. [Google Scholar] [CrossRef] [PubMed]
- Rana, M.; Essig, H.; Rücker, M.; Ruecker, M.; Gellrich, N.-C. Development and demonstration of a novel computer planning solution for predefined correction of enophthalmos in anophthalmic patients using prebended 3D titanium-meshes—A technical note. J. Oral Maxillofac. Surg. 2012, 70, e631-8. [Google Scholar] [CrossRef] [PubMed]
- Zizelmann, C.; Gellrich, N.C.; Metzger, M.C.; Schoen, R.; Schmelzeisen, R.; Schramm, A. Computer-assisted reconstruction of orbital floor based on cone beam tomography. Br. J. Oral Maxillofac. Surg. 2007, 45, 79–80. [Google Scholar] [CrossRef]
- Wan, K.H.; Chong, K.K.L.; Young, A.L. The Role of Computer-Assisted Technology in Post-Traumatic Orbital Reconstruction: A PRISMA-driven Systematic Review. Sci. Rep. 2015, 5, 17914. [Google Scholar] [CrossRef] [Green Version]
- Schmelzeisen, R.; Gellrich, N.C.; Schoen, R.; Gutwald, R.; Zizelmann, C.; Schramm, A. Navigation-aided reconstruction of medial orbital wall and floor contour in cranio-maxillofacial reconstruction. Injury 2004, 35, 955–962. [Google Scholar] [CrossRef]
- Rana, M.; Chui, C.H.K.; Wagner, M.; Zimmerer, R.; Rana, M.; Gellrich, N.-C. Increasing the accuracy of orbital reconstruction with selective laser-melted patient-specific implants combined with intraoperative navigation. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2015, 73, 1113–1118. [Google Scholar] [CrossRef] [Green Version]
- Sidebottom, A.J.; Sissons, G. Radiographic screening for midfacial fracture in A&E. Br. J. Radiol. 1999, 72, 523–524. [Google Scholar]
- Schuknecht, B.; Graetz, K. Radiologic assessment of maxillofacial, mandibular, and skull base trauma. Eur. Radiol. 2005, 15, 560–568. [Google Scholar] [CrossRef] [Green Version]
- Dempf, R.; Hausamen, J.E. Gesichtsschädelfrakturen. Unfallchirurg 2000, 103, 301–313. [Google Scholar] [CrossRef]
- Girotto, J.A.; MacKenzie, E.; Fowler, C.; Redett, R.; Robertson, B.; Manson, P.N. Long-term physical impairment and functional outcomes after complex facial fractures. Plast. Reconstr. Surg. 2001, 108, 312–327. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.R.; Dierks, E.J.; Bell, R.B. Does intraoperative navigation restore orbital dimensions in traumatic and post-ablative defects? J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2012, 40, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.B.; Markiewicz, M.R. Computer-assisted planning, stereolithographic modeling, and intraoperative navigation for complex orbital reconstruction: A descriptive study in a preliminary cohort. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2009, 67, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.; Schreurs, R.; Dubois, L.; Maal, T.J.J.; Gooris, P.J.J.; Becking, A.G. The advantages of advanced computer-assisted diagnostics and three-dimensional preoperative planning on implant position in orbital reconstruction. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2018, 46, 715–721. [Google Scholar] [CrossRef]
- Sigron, G.R.; Barba, M.; Chammartin, F.; Msallem, B.; Berg, B.-I.; Thieringer, F.M. Functional and Cosmetic Outcome after Reconstruction of Isolated, Unilateral Orbital Floor Fractures (Blow-Out Fractures) with and without the Support of 3D-Printed Orbital Anatomical Models. J. Clin. Med. 2021, 10, 3509. [Google Scholar] [CrossRef]
- Gonzalez, L.V.; Arango, A.; López, J.P.; Gnecco, J.P. Technological Integration of Virtual Surgical Planning, Surgical Navigation, Endoscopic Support and Patient-Specific Implant in Orbital Trauma. J. Maxillofac. Oral Surg. 2021, 20, 459–463. [Google Scholar] [CrossRef]
- Hsieh, T.-Y.; Said, M.; Dedhia, R.D.; Timbang, M.R.; Steele, T.O.; Strong, E.B. Assessment of the Learning Curve for Virtual Surgical Planning in Orbital Fractures. Craniomaxillofac. Trauma Reconstr. 2020, 13, 186–191. [Google Scholar] [CrossRef]
- García-Cano, E.; Malagón-Hidalgo, H.O.; Gónzalez-Magaña, F.; Monroy-Cedillo, J.L. Assesing Intraoperative Virtual Navigation on My Craniofacial Surgery Fellowship for Orbital Fractures Repair: Is it Useful? J. Craniofac. Surg. 2021, 32, 238–241. [Google Scholar] [CrossRef]
- Schramm, A.; Suarez-Cunqueiro, M.M.; Barth, E.L.; Essig, H.; Bormann, K.-H.; Kokemueller, H.; Rücker, M.; Gellrich, N.-C. Computer-assisted navigation in craniomaxillofacial tumors. J. Craniofac. Surg. 2008, 19, 1067–1074. [Google Scholar] [CrossRef]
- Mazurek-Popczyk, J.; Palka, L.; Arkusz, K.; Dalewski, B.; Baldy-Chudzik, K. Personalized, 3D- printed fracture fixation plates versus commonly used orthopedic implant materials—Biomaterials characteristics and bacterial biofilm formation. Injury 2022, 53, 938–946. [Google Scholar] [CrossRef]
- Palka, L.; Mazurek-Popczyk, J.; Arkusz, K.; Baldy-Chudzik, K. Susceptibility to biofilm formation on 3D-printed titanium fixation plates used in the mandible: A preliminary study. J. Oral Microbiol. 2020, 12, 1838164. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, H.; Eren, H.; Sander, K.; Kübler, N.R.; Handschel, J. Orbital floor fractures—Short- and intermediate-term complications depending on treatment procedures. Head Face Med. 2016, 12, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gass, M.; Füßinger, M.A.; Metzger, M.C.; Schwarz, S.; Bähr, J.D.; Brandenburg, L.; Weingart, J.; Schlager, S. Virtual reconstruction of orbital floor defects using a statistical shape model. J. Anat. 2022, 240, 323–329. [Google Scholar] [CrossRef] [PubMed]
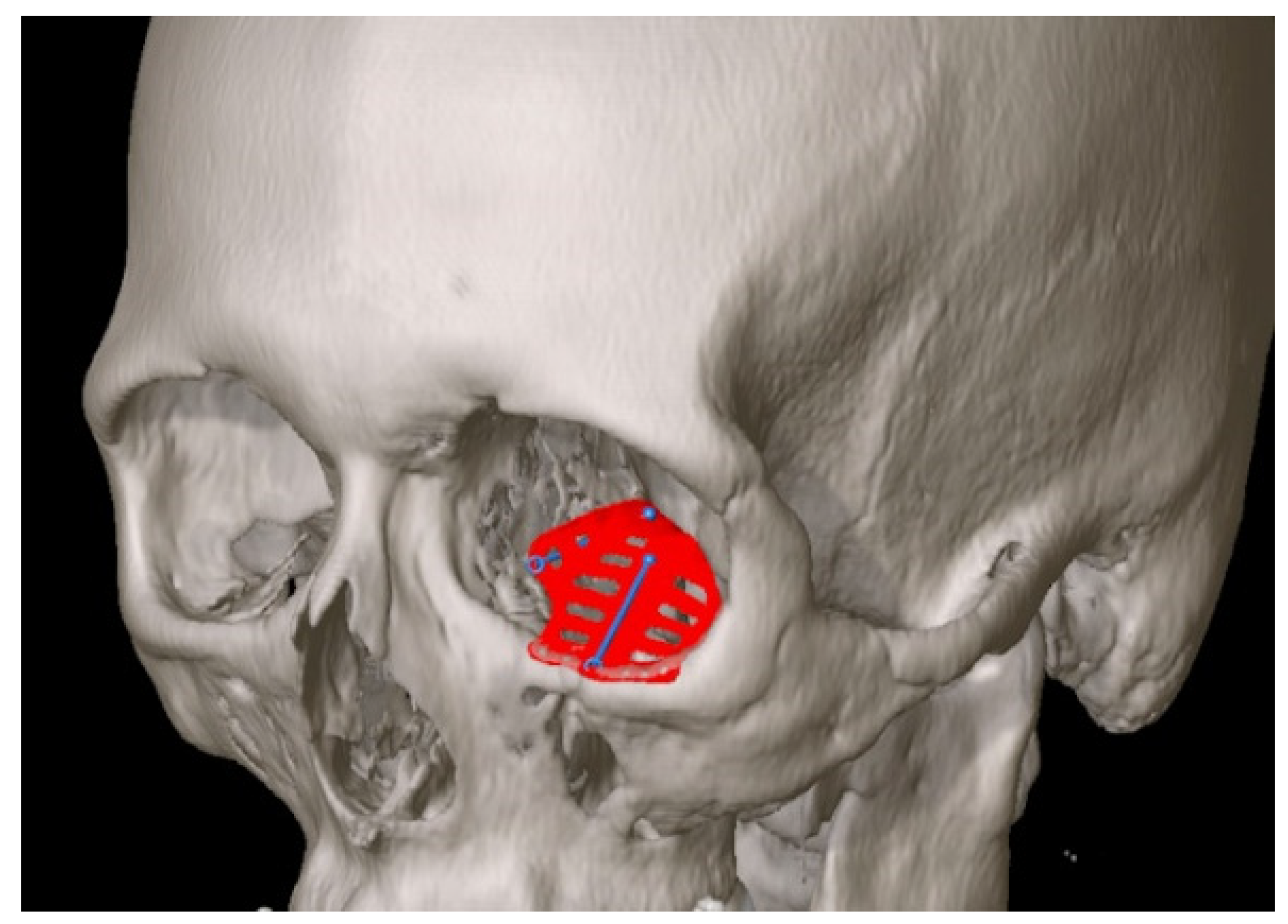
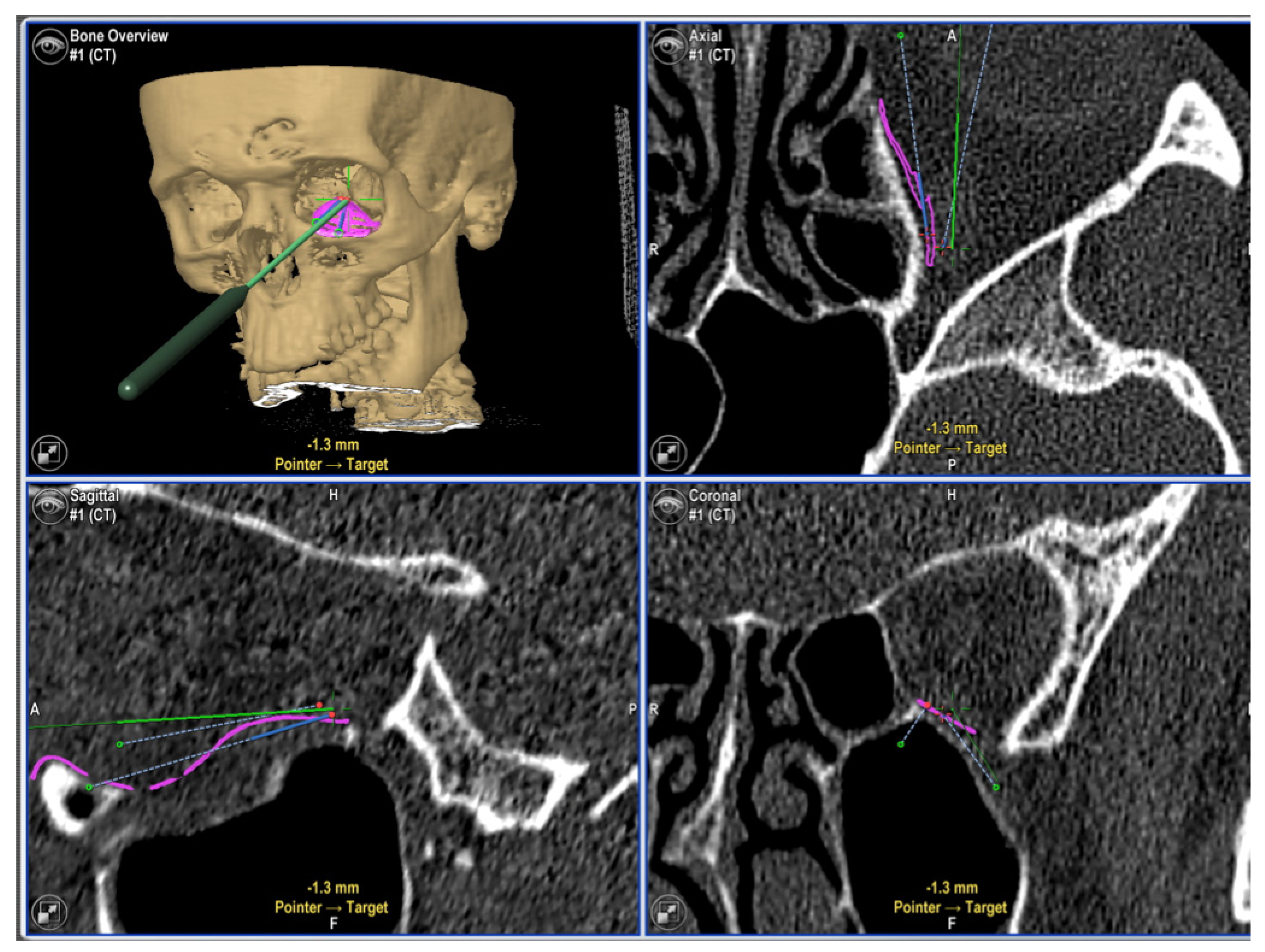
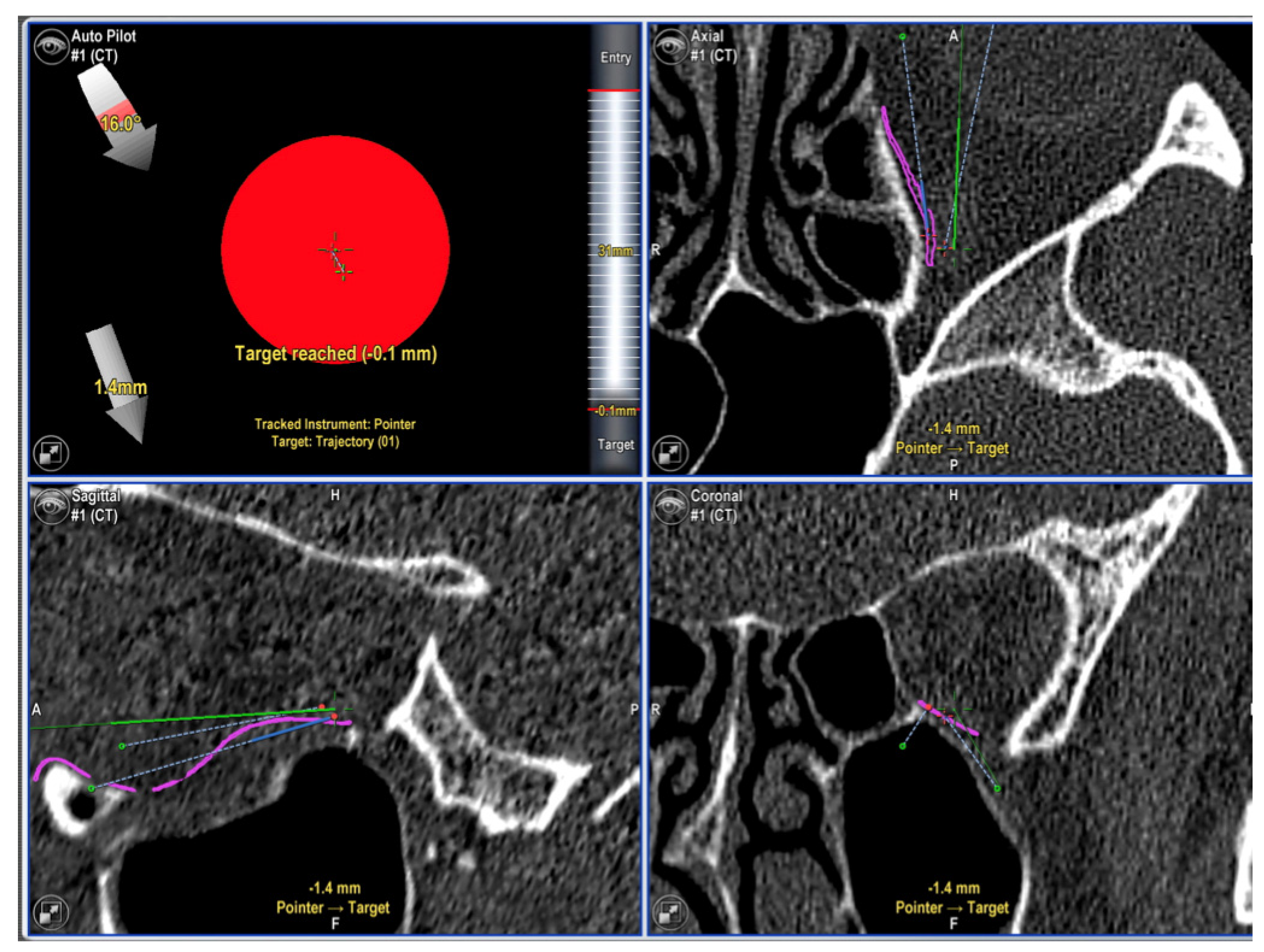
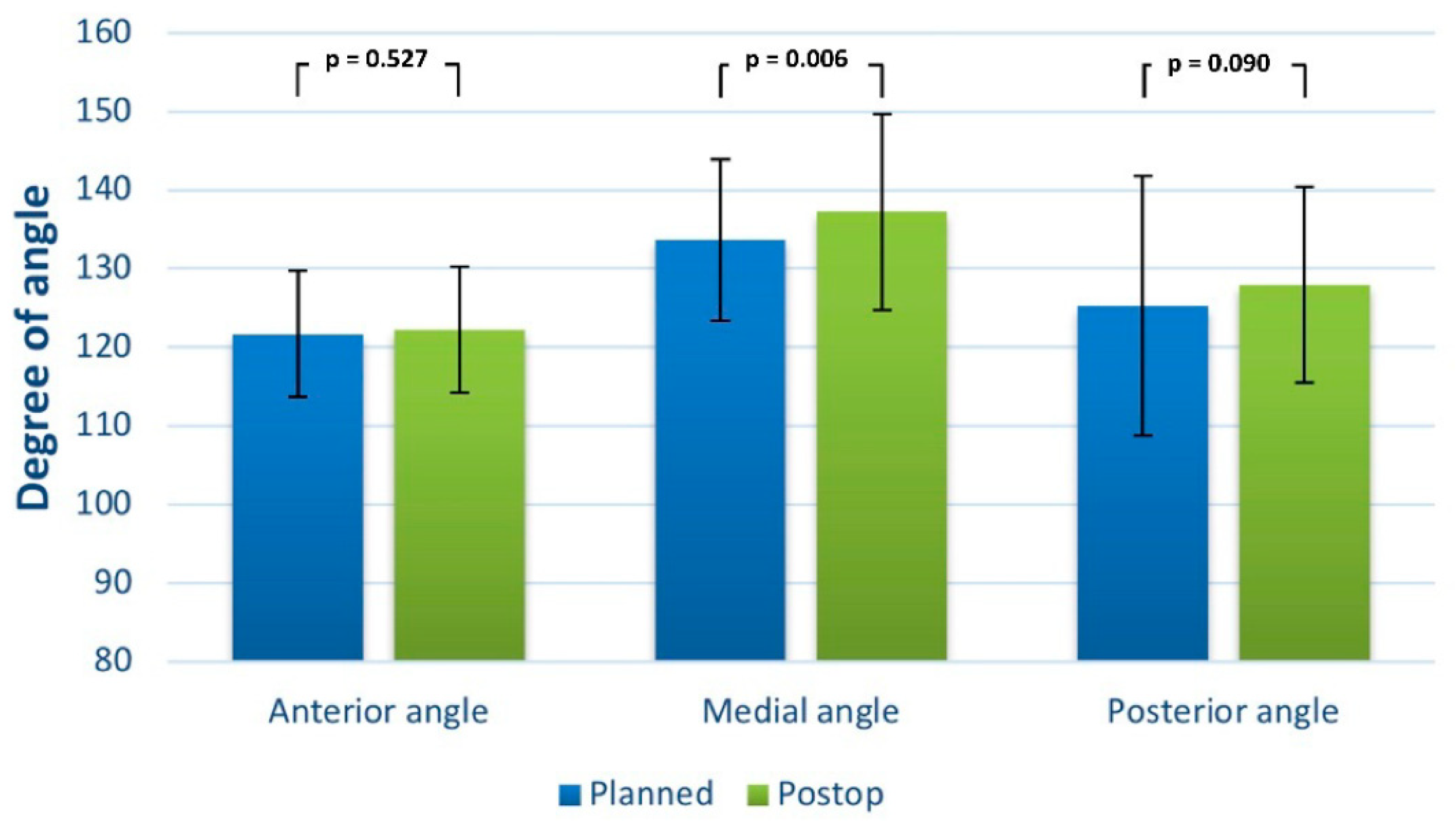
| Variables | Number of Patients |
|---|---|
| Self-reported sex | |
| Female | 34 |
| Male | 62 |
| Etiology of defects | |
| Traffic accident | 14 |
| Assault or Violence | 22 |
| Horse-associated accident | 7 |
| Golf ball hit | 1 |
| Bike spill | 18 |
| Stumble spill | 18 |
| Other cause | 16 |
| Type of traumatic injury | |
| Isolated orbital fracture | 69 |
| Zygomaticomaxillary complex, naso-orbital-ethmoidal, panfacial | 27 |
| Number of stages for surgery | |
| One stage procedure | 47 |
| Two stage procedure | 49 |
| Wall types for reconstruction | |
| Single wall | 20 |
| Two wall | 76 |
| Indication for surgery * | |
| Double vision initially | 15 |
| Enophthalmos | 53 |
| Hypoglobus | 7 |
| Exopthalmos | 13 |
| Hypaesthesia | 1 |
| Defect size and degree of dislocation | 63 |
| Surgical access | |
| Transconjunctival, retroseptal | All (96) |
| Navigation tools | |
| Calvarian screws | 8 |
| Dental splint | 88 |
| Average defect size in mm | mean (SD) |
| Coronal | 23.96 (6.52) |
| Sagittal | 25.91 (4.49) |
| Mean | SD | Min | Max | |
|---|---|---|---|---|
| One-wall fracture | 110 | 61.20 | 42 | 270 |
| Multi-wall fracture | 118 | 83.77 | 47 | 480 |
| Combination of panfacial and orbital restoration | 164 | 139.86 | 50 | 600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rana, M.; Moellmann, H.L.; Schorn, L.; Lommen, J.; Rana, M.; Wilkat, M.; Hufendiek, K. Primary Orbital Reconstruction with Selective Laser Melting (SLM) of Patient-Specific Implants (PSIs): An Overview of 96 Surgically Treated Patients. J. Clin. Med. 2022, 11, 3361. https://doi.org/10.3390/jcm11123361
Rana M, Moellmann HL, Schorn L, Lommen J, Rana M, Wilkat M, Hufendiek K. Primary Orbital Reconstruction with Selective Laser Melting (SLM) of Patient-Specific Implants (PSIs): An Overview of 96 Surgically Treated Patients. Journal of Clinical Medicine. 2022; 11(12):3361. https://doi.org/10.3390/jcm11123361
Chicago/Turabian StyleRana, Majeed, Henriette L. Moellmann, Lara Schorn, Julian Lommen, Madiha Rana, Max Wilkat, and Karsten Hufendiek. 2022. "Primary Orbital Reconstruction with Selective Laser Melting (SLM) of Patient-Specific Implants (PSIs): An Overview of 96 Surgically Treated Patients" Journal of Clinical Medicine 11, no. 12: 3361. https://doi.org/10.3390/jcm11123361
APA StyleRana, M., Moellmann, H. L., Schorn, L., Lommen, J., Rana, M., Wilkat, M., & Hufendiek, K. (2022). Primary Orbital Reconstruction with Selective Laser Melting (SLM) of Patient-Specific Implants (PSIs): An Overview of 96 Surgically Treated Patients. Journal of Clinical Medicine, 11(12), 3361. https://doi.org/10.3390/jcm11123361








