Aromatase Inhibitors and Risk of Metabolic and Cardiovascular Adverse Effects in Breast Cancer Patients—A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Search
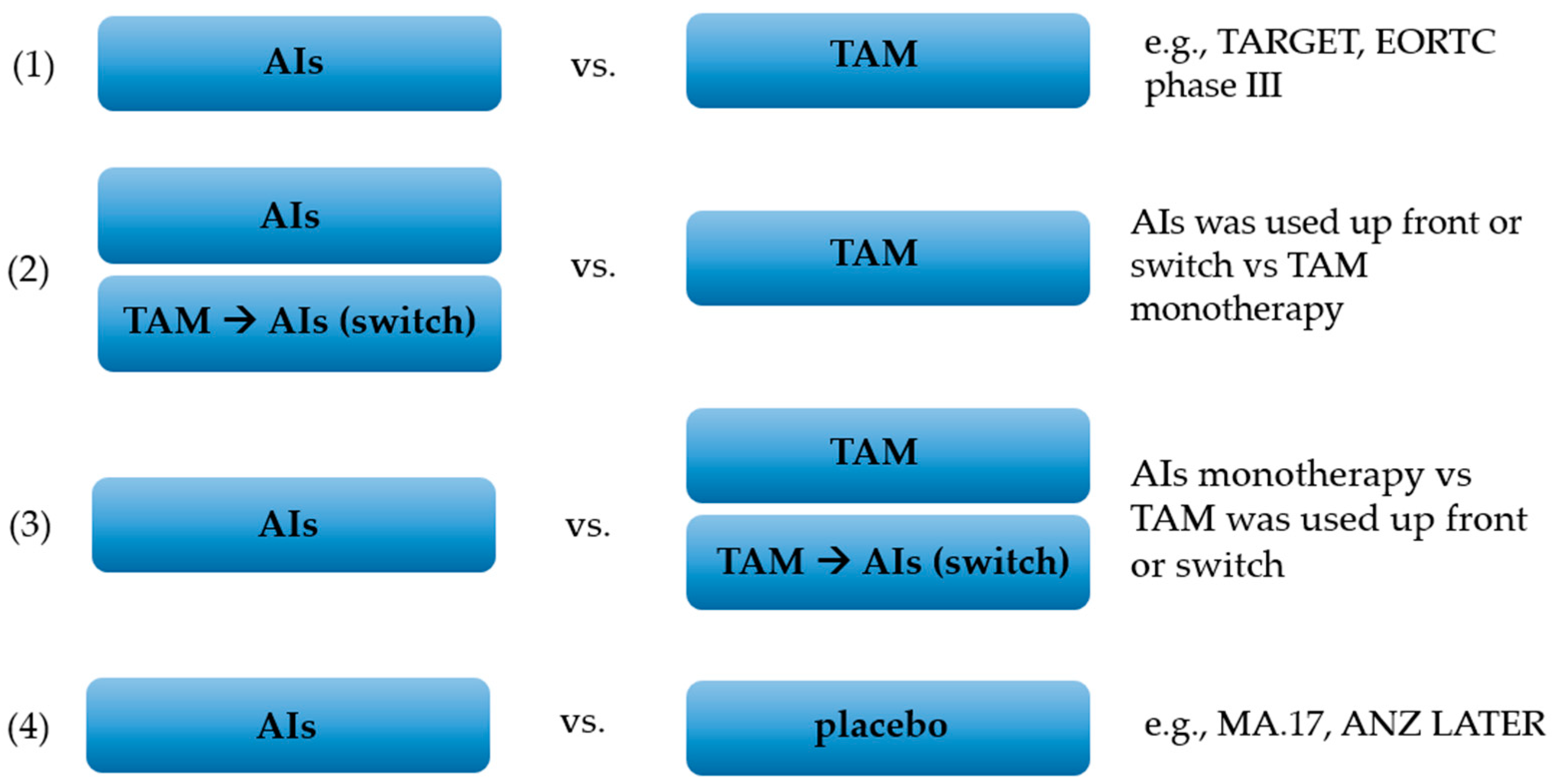
2.2. Study Selection and Data Collection Process
2.3. Quality Assessment
2.4. Statistical Analysis
3. Results
3.1. Quality Assessment
3.2. Cardiovascular Events
3.3. Arterial Hypertension
3.4. Body Weight Gain
3.5. Dyslipidemia
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boszkiewicz, K.; Sawicka, E.; Piwowar, A. The impact of xenoestrogens on effectiveness of treatment for hormone-dependent breast cancer–current state of knowledge and perspectives for research. Ann. Agric. Environ. Med. 2020, 27, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Eve, L.; Fervers, B.; Le Romancer, M.; Etienne-Selloum, N. Exposure to Endocrine Disrupting Chemicals and Risk of Breast Cancer. Int. J. Mol. Sci. 2020, 21, 9139. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast cancer treatment. A review. JAMA 2019, 321, 288–300. [Google Scholar] [CrossRef]
- Harbeck, N.; Penault-Llorca, F.; Cortes, J.; Gnant, M.; Houssami, N.; Poortmans, P.; Ruddy, K.; Tsang, J.; Cardoso, F. Breast cancer. Oncol. Clin. Pract. 2020, 16, 207–260. [Google Scholar] [CrossRef]
- Peters, A.; Tadi, P. Aromatase Inhibitors; StatPearls Publishing: Treasure Island, FL, USA. Available online: www.ncbi.nlm.nih.gov/books/NBK557856/ (accessed on 23 March 2022).
- Tenti, S.; Correale, P.; Cheleschi, S.; Fioravanti, A.; Pirtoli, L. Aromatase Inhibitors—Induced Musculoskeletal Disorders: Current Knowledge on Clinical and Molecular Aspects. Int. J. Mol. Sci. 2020, 21, 5625. [Google Scholar] [CrossRef] [PubMed]
- Khosrow-Khavar, F.; Filion, K.B.; Al-Qurashi, S.; Torabi, N.; Bouganim, N.; Suissa, S.; Azoulay, L. Cardiotoxicity of aromatase inhibitors and tamoxifen in postmeno-pausal women with breast cancer: A systematic review and meta-analysis of randomized controlled trials. Ann. Oncol. 2017, 28, 487–496. [Google Scholar] [CrossRef]
- Gibb, F.W.; Dixon, J.M.; Clarke, C.; Homer, N.Z.; Faqehi, A.M.M.; Andrew, R.; Walker, B.R. Higher insulin resistance and adiposity in postmenopausal women with breast cancer treated with aromatase inhibitors. J. Clin. Endocrinol. Metab. 2019, 104, 3670–3678. [Google Scholar] [CrossRef] [PubMed]
- Hamood, R.; Hamood, H.; Merhasin, I.; Keinan-Boker, L. Diabetes After Hormone Therapy in Breast Cancer Survivors: A Case-Cohort Study. J. Clin. Oncol. 2018, 36, 2061–2069. [Google Scholar] [CrossRef] [Green Version]
- Foglietta, J.; Inno, A.; de Iuliis, F.; Sini, V.; Duranti, S.; Turazza, M.; Tarantini, L.; Gori, S. Cardiotoxicity of Aromatase Inhibitors in Breast Cancer Patients. Clin. Breast Cancer 2016, 17, 11–17. [Google Scholar] [CrossRef]
- Khosrow-Khavar, F.; Filion, K.B.; Bouganim, N.; Suissa, S.; Azoulay, L. Aromatase inhibitors and the risk of cadiovascular outcomes in wom-en with breast cancer. Circulation 2020, 141, 549–559. [Google Scholar] [CrossRef]
- Seong-Hee, K.; Hyun-Sook, K. Menopause-associated lipid metabolic disorders and foods beneficial for postmenopausal women. Nutrients 2020, 12, 202. [Google Scholar]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef]
- Schochter, F.; Rack, B.; Tzschaschel, M.; Polasik, A.; Andergassen, U.; Trapp, E.; Alunni-Fabbroni, M.; Schneeweiss, A.; Müller, V.; Pantel, K.; et al. Endocrine Treatment with 2 Years of Tamoxifen versus 2 Years of Exemestane in Postmenopausal Patients with High-Risk Early Breast Cancer and Persisting Circulating Tumor Cells—First Results of the SUCCESS C Endocrine Treatment Sub-Study. Oncol. Res. Treat. 2018, 41, 93–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, I.; Yardley, D.; Burris, H.A.; De Boer, R.; Amadori, D.; McIntyre, K.; Ejlertsen, B.; Gnant, M.; Jonat, W.; Pritchard, K.I.; et al. Comparative Efficacy and Safety of Adjuvant Letrozole Versus Anastrozole in Postmenopausal Patients With Hormone Receptor–Positive, Node-Positive Early Breast Cancer: Final Results of the Randomized Phase III Femara Versus Anastrozole Clinical Evaluation (FACE) Trial. J. Clin. Oncol. 2017, 35, 1041–1048. [Google Scholar] [CrossRef] [Green Version]
- Iwata, H.; Masuda, N.; Ohno, S.; Rai, Y.; Sato, Y.; Ohsumi, S.; Hashigaki, S.; Nishizawa, Y.; Hiraoka, M.; Morimoto, T.; et al. A randomized, double-blind, controlled study of exemestane versus anastrozole for the first-line treatment of postmenopausal Japanese women with hormone-receptor-positive advanced breast cancer. Breast Cancer Res. Treat. 2013, 139, 441–451. [Google Scholar] [CrossRef] [Green Version]
- Goss, P.E.; Ingle, J.N.; Pritchard, K.I.; Ellis, M.I.; Sledge, G.W.; Budd, G.T.; Rabaglio, M.; Ansari, R.H.; Johnson, D.B.; Tozer, T.; et al. Exemestane versus anastrozole in postmenopausal women with early breast cancer: NCIC CTG MA.27-a randomized controlled phase III trial. J. Clin. Oncol. 2013, 31, 1398–1404. [Google Scholar] [CrossRef]
- Fujiwara, Y.; Takatsuka, Y.; Imoto, S.; Inaji, H.; Ikeda, T.; Akiyama, F.; Tamura, M.; Miyoshi, K.; Iwata, H.; Mitsuyama, S.; et al. Outcomes of Japanese breast cancer patients treated with pre-operative and post-operative anastrozole or tamoxifen. Cancer Sci. 2011, 103, 491–496. [Google Scholar] [CrossRef]
- Takei, H.; Ohsumi, S.; Shimozuma, K.; Takehara, M.; Suemasu, K.; Ohashi, Y.; Hozumi, Y. Health-related quality of life, psychological distress, and adverse events in postmenopausal women with breast cancer who receive tamoxifen, exemestane, or anastrozole as adjuvant endocrine therapy: National Surgical Adjuvant Study of Breast Cancer 04 (N-SAS BC 04). Breast Cancer Res. Treat. 2012, 133, 227–236. [Google Scholar] [CrossRef]
- Van de Velde, C.J.; Rea, D.; Seynaeve, C.; Putter, H.; Hasenburg, A.; Vannetzel, J.M.; Paridaens, R.; Markopoulos, C.; Hozumi, Y.; Hille, E.T.; et al. Adjuvant tamoxifen and exemestane in early breast cancer (TEAM): A ran-domised phase 3 trial. Lancet 2011, 377, 321–331. [Google Scholar] [CrossRef]
- Aihara, T.; Takatsuka, Y.; Ohsumi, S.; Aogi, K.; Hozumi, Y.; Imoto, S.; Mukai, H.; Iwata, H.; Watanabe, T.; Shimizu, C.; et al. Phase III randomized adjuvant study of tamoxifen alone versus sequential ta-moxifen and anastrozole in Japanese postmenopausal women with hormone-responsive breast cancer: N-SAS BC03 study. Breast Cancer Res. Treat. 2010, 121, 379–387. [Google Scholar] [CrossRef]
- Paridaens, R.J.; Dirix, L.Y.; Beex, L.V.; Nooij, M.; Cameron, D.A.; Cufer, T.; Piccart, M.J.; Bogaerts, J.; Therasse, P. Phase III Study Comparing Exemestane With Tamoxifen As First-Line Hormonal Treatment of Metastatic Breast Cancer in Postmenopausal Women: The European Organisation for Research and Treatment of Cancer Breast Cancer Cooperative Group. J. Clin. Oncol. 2008, 26, 4883–4890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufmann, M.; Jonat, W.; Hilfrich, J.; Eidtmann, H.; Gademann, G.; Zuna, I.; Von Minckwitz, G. Improved Overall Survival in Postmenopausal Women With Early Breast Cancer After Anastrozole Initiated After Treatment With Tamoxifen Compared With Continued Tamoxifen: The ARNO 95 Study. J. Clin. Oncol. 2007, 25, 2664–2670. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Kilburn, L.S.; Snowdon, C.F.; Paridaens, R.; Coleman, R.E.; Jones, S.E.; Jassem, J.; Van de Velde, C.J.H.; Delozier, T.; Alvarez, I.; et al. Survival and safety of exemestane versus tamoxifen after 2–3 years’ ta-moxifen treatment (Intergroup Exemestane Study): A randomised controlled trial. Lancet 2007, 369, 559–570. [Google Scholar] [CrossRef]
- Boccardo, F.; Rubagotti, A.; Guglielmini, P.; Fini, A.; Paladini, G.; Mesiti, M.; Rinaldini, M.; Scali, S.; Porpiglia, M.; Benedetto, C.; et al. Switching to anastrozole versus continued tamoxifen treatment of early breast cancer. Updated results of the Italian tamoxifen anastrozole (ITA) trial. Ann. Oncol. 2006, 17, vii10–vii14. [Google Scholar] [CrossRef]
- Thürlimann, B.; Keshaviah, A.; Coates, A.S.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Castiglione-Gertsch, M.; Gelber, R.D.; Rabaglio, M.; et al. A Comparison of Letrozole and Tamoxifen in Postmenopausal Women with Early Breast Cancer. N. Engl. J. Med. 2005, 353, 2747–2757. [Google Scholar] [CrossRef] [Green Version]
- Goss, P.E.; Ingle, J.N.; Martino, S.; Robert, N.J.; Muss, H.B.; Piccart, M.J.; Castiglione, M.; Tu, D.; Shepherd, L.E.; Pritchard, K.I.; et al. Randomized Trial of Letrozole Following Tamoxifen as Extended Adjuvant Therapy in Receptor-Positive Breast Cancer: Updated Findings from NCIC CTG MA.17. JNCI J. Natl. Cancer Inst. 2005, 97, 1262–1271. [Google Scholar] [CrossRef]
- Paridaens, R.; Dirix, L.; Lohrisch, C.; Beex, L.; Nooij, M.; Cameron, D.; Biganzoli, L.; Cufer, T.; Duchateau, L.; Hamilton, A.; et al. Mature results of a randomized phase II multicenter study of exemestane versus tamoxifen as first-line hormone therapy for postmenopausal women with metastatic breast cancer. Ann. Oncol. 2003, 14, 1391–1398. [Google Scholar] [CrossRef]
- Baum, M.; Budzar, A.U.; Cuzick, J.; Forbes, J.; Houghton, J.H.; Klijn, J.G.M.; Sahmoud, T.; ATAC Trialists’ Group. Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: First results of the ATAC randomised trial. Lancet 2002, 359, 2131–2139. [Google Scholar] [CrossRef]
- Bonneterre, J.; Thürlimann, B.; Robertson, J.R.; Krzakowski, M.; Mauriac, L.; Koralewski, P.; Vergote, I.; Webster, A.; Steinberg, M.; Von Euler, M. Anastrozole Versus Tamoxifen as First-Line Therapy for Advanced Breast Cancer in 668 Postmenopausal Women: Results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability Study. J. Clin. Oncol. 2000, 18, 3748–3757. [Google Scholar] [CrossRef] [PubMed]
- Nabholtz, J.; Buzdar, A.; Pollak, M.; Harwin, W.; Burton, G.; Mangalik, A.; Steinberg, M.; Webster, A.; Von Euler, M. Anastrozole Is Superior to Tamoxifen as First-Line Therapy for Advanced Breast Cancer in Postmenopausal Women: Results of a North American Multicenter Randomized Trial. J. Clin. Oncol. 2000, 18, 3758–3767. [Google Scholar] [CrossRef] [PubMed]
- Mamounas, E.P.; Bandos, H.; Lembersky, B.C.; Jeong, J.H.; Geyer, C.E., Jr.; Rastogi, P.; Fehrenbacher, L.; Graham, M.L.; Chia, S.K.; Brufsky, A.M.; et al. Use of letrozole after aromatase inhibitor-based therapy in postmeno-pausal breast cancer (NRG Oncology/NSABP B-42): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019, 20, 88–99. [Google Scholar] [CrossRef]
- Zdenkowski, N.; Forbes, J.F.; Boyle, F.M.; Kannourakis, G.; Gill, P.G.; Bayliss, E.; Saunders, C.; Della-Fiorentina, S.; Kling, N.; Campbell, I.; et al. Observation versus late reintroduction of letrozole as adjuvant endocrine therapy for hormone receptor-positive breast cancer (ANZ0501 LATER): An open-label randomised, controlled trial. Ann. Oncol. 2016, 27, 806–812. [Google Scholar] [CrossRef] [PubMed]
- Sund, M.; Garcia-Argibay, M.; Garmo, H.; Ahlgren, J.; Wennstig, A.-K.; Fredriksson, I.; Lindman, H.; Valachis, A. Aromatase inhibitors use and risk for cardiovascular disease in breast cancer patients: A population-based cohort study. Breast 2021, 59, 157–164. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, L.; Li, K.; Zou, H. Comparative study on individual aromatase inhibitors on cardiovascular safety profile: A net-work meta-analysis. OncoTargets Ther. 2015, 8, 2721–2730. [Google Scholar]
- He, Y.; Zhang, J.; Shen, G.; Lu, X.; Yang, H. Aromatase inhibitors and risk of cardiovascular events in breast cancer patients: A system-atic review and meta-analysis. BMC Pharmacol. Toxicol. 2019, 20, 62. [Google Scholar] [CrossRef] [Green Version]
- Blaes, A.; Beckwith, H.; Florea, N.; Hebbel, R.; Solovey, A.; Potter, D.; Yee, D.; Vogel, R.; Luepker, R.; Duprez, D. Vascular function in breast cancer survivors on aromatase inhibitors: A pilot study. Breast Cancer Res. Treat. 2017, 166, 541–547. [Google Scholar] [CrossRef]
- Sestak, I.; Harvie, M.; Howell, A.; Forbes, J.F.; Dowsett, M.; Cuzick, J. Weight change associated with anastrozole and tamoxifen treatment in postmeno-pausal women with or at high risk of developing breast cancer. Breast Cancer Res. Treat. 2012, 134, 727–734. [Google Scholar] [CrossRef]
- Nyrop, K.A.; Deal, A.M.; Shachar, S.S.; Park, J.; Choi, S.K.; Lee, J.T.; O’Hare, E.A.; Wheless, A.; Carey, L.A.; Muss, H.B. Weight trajectories in women receiving systemic adjuvant therapy for breast cancer. Breast Cancer Res. Treat. 2019, 179, 709–720. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, A.; Wang, J.; Ma, F.; Liu, J.; Fan, Y.; Luo, Y.; Zhang, P.; Li, Q.; Xu, B.; et al. Steroidal aromatase inhibitors have a more favorable effect on lipid profiles than non-steroidal aromatase inhibitors in postmenopausal women with early breast cancer: A prospective cohort study. Ther. Adv. Med. Oncol. 2020, 12, 1758835920956880. [Google Scholar] [CrossRef] [PubMed]
- Ye, F.; Wen, J.; Yang, A.; Li, N.; Yu, P.; Wei, W.; Tang, J. The Influence of Hormone Therapy on secondary diabetes mellitus in Breast Cancer: A Me-ta-analysis. Clin. Breast Cancer 2022, 22, e48–e58. [Google Scholar] [CrossRef] [PubMed]





| TRIAL | TRIAL ARM (n Included in Safety Analysis) | TREATMENT | TRIAL DESIGN | AGE (Mean) | CANCER STAGE | PRIMARY TREATMENT | ||
|---|---|---|---|---|---|---|---|---|
| Surgery (%) | Radiotherapy (%) | Systemic Therapy (%) | ||||||
| FATA-GIM3 [15] | switch group = 1761 upfront group = 1766 | adjuvant | upfront strategy vs. switch strategy; six treatment groups: ANA 1 mg, EXE 25 mg, LET 2,5 mg for 5 years; TAM 20 mg for 2 years followed by administration ANA or EXE or LET for 3 years | 64 | early | 100% | 1247 (67%) | 712 (39%) |
| upfront group = 1766 | 100% | 1253 (68%) | 703 (38%) | |||||
| ANA = 1175 | 100% | 801 (65%) | 469 (39%) | |||||
| EXE = 1177 | 100% | 854 (69%) | 474 (38%) | |||||
| LET = 1175 | 100% | 845 (69%) | 472 (39%) | |||||
| SUCCESS C [16] | EXE = 54 | adjuvant | 5 years EXE vs. 2 years TAM + 3 years EXE | EXE-63 | early | ND | ND | 100% |
| TAM-EXE = 54 | TAM-EXE -60.5 | ND | ND | 100% | ||||
| FACE [17] | LET = 2049 | adjuvant | LET (2.5 mg) vs. ANA (1 mg) for 5 years | 62 | early | ND | 652 (31.6%) | 1294 (62.7%) |
| ANA = 2062 | ND | 621 (29.9%) | 1267 (61.1%) | |||||
| Iwata et al, 2013 [18] | EXE = 149 | first-line | EXE 25 mg vs. ANA 1 mg continued until disease progression, intolerable adverse event or death | EXE-63.4 | advanced | ND | 35 (23.5%) | 103 (69.1%) |
| ANA = 149 | ANA-64 | ND | 28 (18.8%) | 100 (67.1%) | ||||
| MA.27 [19] | EXE = 3761 | adjuvant | EXE 25 mg vs. ANA 1 mg for 5 years | EXE-63.9 | early | 3789 (100%) | ND | 1163 (31%) |
| ANA = 3759 | ANA-64.3 | 3787 (100%) | ND | 1164 (31%) | ||||
| PROACT [20] | ANA = 48 | neoadjuvant and adjuvant | pre-operative (3 months) and post-operative (5 years or until recurrence, withdrawal) treatment TAM (20 mg) vs. ANA (1 mg) | ANA-61.5 | locally advanced | 48 (100%) | 18 (41.9%) | 10 (23.3%) |
| TAM = 48 | TAM-61.6 | 49 (100%) | 17 (39.5%) | 20 (46.5%) | ||||
| N-SAS BC04 [21] | EXE = 55 | adjuvant | EXE for 5 years vs. 2.5–3 years TAM followed by EXE to a total of 5 years vs. ANA for 5 years | EXE-63.2 | early | 15 (27.3%) | 35 (63.6%) | 21 (38.2%) |
| TAM = 56 | TAM-63.0 | 18 (32.1%) | 36 (64.3%) | 23 (41.1%) | ||||
| ANA = 55 | ANA-62.9 | 18 (32.7%) | 34 (61.8%) | 21 (38.2%) | ||||
| TEAM [22] | TAM = 4814 | adjuvant | 25 mg EXE vs. TAM (20 mg) --> EXE for 5 years (EXE after 2.5–3 years TAM) | TAM ≥ 50–97% | early | 4868 (100%) | 3320 (68%) | 1740 (36%) |
| EXE = 4852 | EXE ≥ 50–97% | 4898 (100%) | 3377 (69%) | 1773 (36%) | ||||
| N-SAS BC03 [23] | TAM = 349 | adjuvant | TAM for 5 years vs. TAM (20 mg) for 1–4 years --> ANA (1 mg) to complete 5 years of hormone therapy | TAM-60.2 | early | 349 (100%) | ND | 186 (53.3%) |
| ANA = 347 | ANA-59.5 | 347 (100%) | ND | 187 (53.9%) | ||||
| EORTC phase III, 2008 [24] | EXE = 182 | first-line | TAM 20 mg vs. EXE 25 mg until disease progression or unacceptable toxicity occurred | EXE-63 | metastatic | ND | 75 (41.2%) | 76 (41.7%) |
| TAM = 189 | TAM-62 | ND | 79 (41.8%) | 79 (41.8%) | ||||
| ARNO-95 [25] | ANA = 445 | adjuvant | TAM for 5 years vs. TAM for 2 years --> ANA for 3 years | ANA-60.9 | early | 489 (100%) | 326 (66.7%) | ND |
| TAM = 452 | TAM-60.5 | 490 (100%) | 332 (67.8%) | ND | ||||
| IES [26] | EXE = 2320 | adjuvant | TAM 20 mg for 5 years vs. TAM 20 mg for 2 or 3 years, then switch to EXE 25 mg to complete a total of five years of adjuvant endocrine treatment | EXE-64.3 | early | 2349 (99.9%) | ND | 766 (32.4%) chemoth.; 567 (24.0%) hormone-th. |
| TAM = 2338 | TAM-64.2 | 2365 (99.7%) | ND | 765 (32.1%) chemoth.; 557 (23.4%) hormone-th. | ||||
| ITA [27] | TAM = 225 | adjuvant | TAM 20 mg (2–3 years) --> ANA 1 mg to complete 5-years treatment vs. TAM 20 mg for 5 years | 63 | early | 225 (100%) | 110 (49%) | 150 (67%) |
| ANA = 223 | 223 (100%) | 120 (54%) | 149 (67%) | |||||
| BIG-98 [28] | LET (LET for 5 years; LET --> TAM) = 3975 | adjuvant | LET (2.5 mg) vs. TAM (20 mg) vs. LET (2 years) --> TAM (3 years) vs. TAM (2 years) --> LET (3 years) for 5 years (this analysis compares the two groups assigned to receive LET initially with the two groups assigned to receive TAM initially) | 61 | early | 4003 (100%) | 2867 (71.6%) | 1012 (25.3%) |
| TAM (TAM for 5 years; TAM --> LET) = 3988 | 4007 (100%) | 2870 (71.6%) | 1012 (25.3%) | |||||
| MA.17 [29] | LET = 2572 | extended adjuvant | LET (2.5 mg) vs. placebo for 5 years | LET-62.0 | early | 1286 (50%) | 1550 (60%) | 1175 (46%) |
| PBO = 2577 | PBO-62.0 | 1301 (50%) | 1528 (59%) | 1177 (46%) | ||||
| EORTC phase II trial [30] | EXE = 61 | first-line | TAM 20 mg vs. EXE 25 mg; treatment was continued until progression of disease, unacceptable toxity, patient refusal or start of any new anti-cancer therapy | EXE-62 | metastatic | ND | 59% | 42% |
| TAM = 59 | TAM-63 | ND | 59% | 43% | ||||
| ATAC [31] | ANA = 3092 | adjuvant | ANA 1 mg + TAM placebo vs. ANA placebo + TAM 20 mg vs. ANA 1 mg + TAM 20 mg for 5 years | ANA-64.1 | early | 1494 (47.8%) | 1978 (63.3%) | 698 (22.3%) |
| TAM = 3094 | TAM-64.1 | 1474 (47.3%) | 1946 (62.5%) | 647 (20.8%) | ||||
| comb = 3097 | comb-64.3 | 1502 (48.1%) | 1936 (62.0%) | 651 (20.8%) | ||||
| TARGET [32] | ANA = 336 | first-line | ANA 1 mg vs. TAM 20 mg; trial treatment was continued until disease progression | ANA-67 | advanced | ND | ND | 105 (30.8%) |
| TAM = 329 | TAM-66 | ND | ND | 97 (29.6%) | ||||
| Nabholtz et al., 2000 [33] | ANA = 170 | first-line | ANA 1 mg vs. TAM 20 mg; trial treatment was continued until disease progression | ANA-68 | advanced | ND | ND | 68 (39.8%) |
| TAM = 182 | TAM-67 | ND | ND | 70 (38.4%) | ||||
| NRG Oncology/NSABP B-42 [34] | PBO = 1933 | extended adjuvant | LET 2.5 mg vs. placebo for 5 years | ND | early | 775 (39.1%) | ND | ND |
| LET = 1941 | ND | 782 (39.4%) | ND | ND | ||||
| ANZ 0501 LATER [35] | observ = 181 | extended adjuvant | LET 2.5 mg for 5 years vs. observation | observ-64 | early | 67 (37.4%) | 126 (70.4%) | 86 (48.0%) |
| LET = 176 | LET- 65 | 64 (35.4%) | 130 (71.8%) | 75 (41.4%) | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boszkiewicz, K.; Piwowar, A.; Petryszyn, P. Aromatase Inhibitors and Risk of Metabolic and Cardiovascular Adverse Effects in Breast Cancer Patients—A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 3133. https://doi.org/10.3390/jcm11113133
Boszkiewicz K, Piwowar A, Petryszyn P. Aromatase Inhibitors and Risk of Metabolic and Cardiovascular Adverse Effects in Breast Cancer Patients—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(11):3133. https://doi.org/10.3390/jcm11113133
Chicago/Turabian StyleBoszkiewicz, Kamila, Agnieszka Piwowar, and Paweł Petryszyn. 2022. "Aromatase Inhibitors and Risk of Metabolic and Cardiovascular Adverse Effects in Breast Cancer Patients—A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 11: 3133. https://doi.org/10.3390/jcm11113133
APA StyleBoszkiewicz, K., Piwowar, A., & Petryszyn, P. (2022). Aromatase Inhibitors and Risk of Metabolic and Cardiovascular Adverse Effects in Breast Cancer Patients—A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(11), 3133. https://doi.org/10.3390/jcm11113133






