An sEMG-Controlled Forearm Bracelet for Assessing and Training Manual Dexterity in Rehabilitation: A Systematic Review
Abstract
:1. Introduction
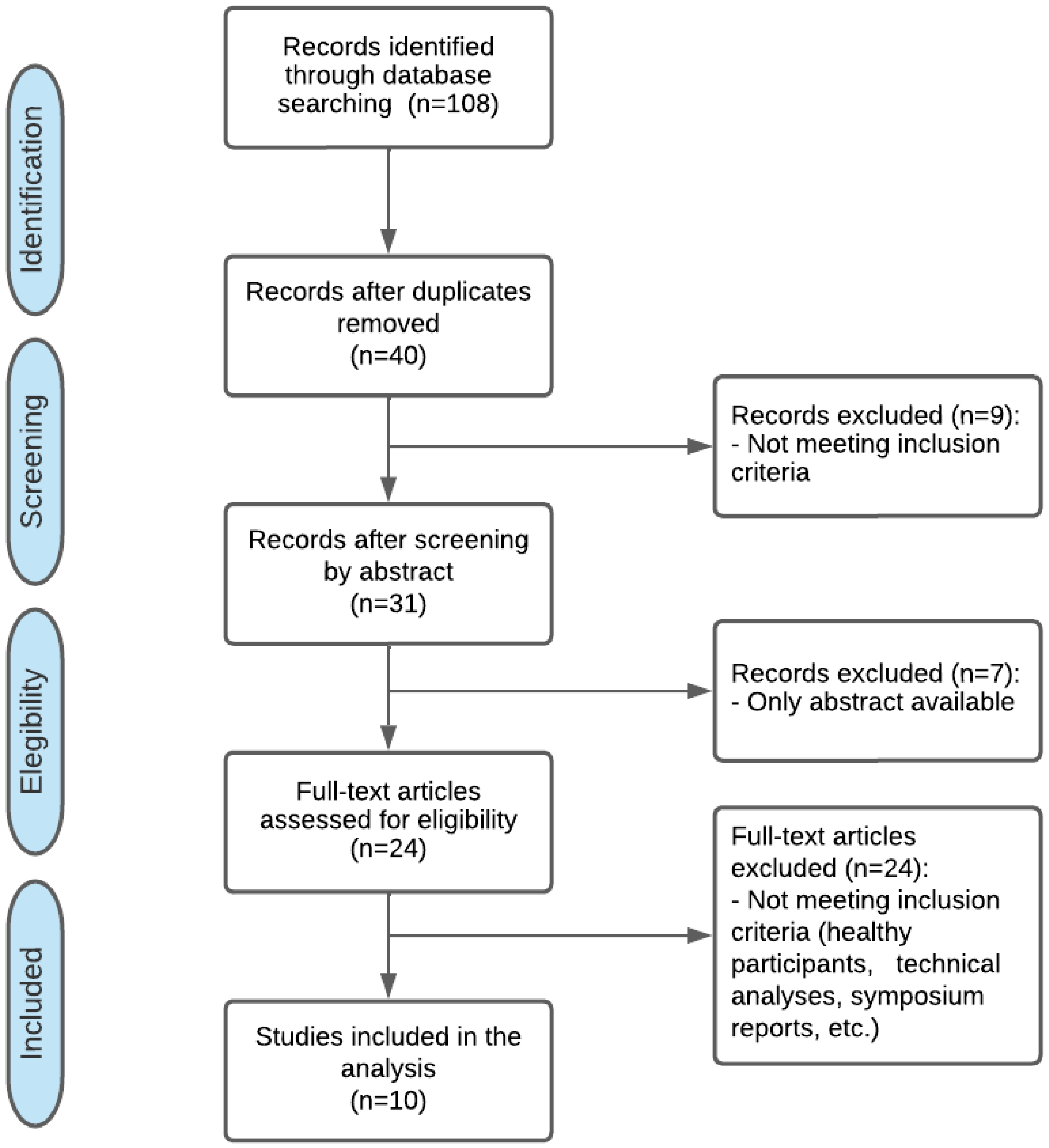
2. Materials and Methods
2.1. Design
2.2. Search Strategy
2.3. Study Selection
2.4. Participants
2.5. Interventions
2.6. Outcome Measures
2.7. Data Extraction and Analysis
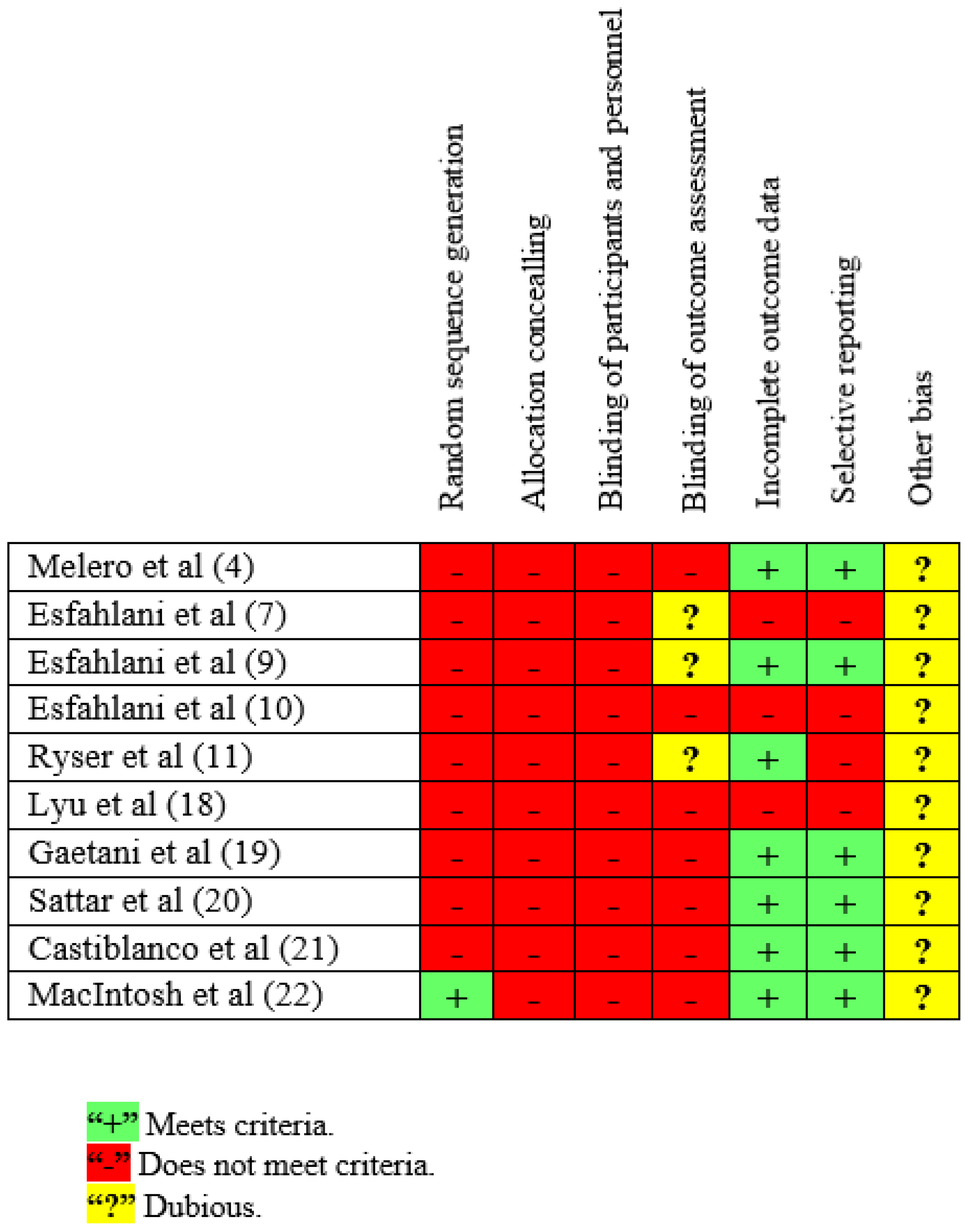
2.8. Assessment of Methodological Quality of the Studies and Risk of Bias
- (a)
- Selection bias: relates to recruiting process and participant allocation. To analyze it, randomization and allocation concealing must be considered.
- (b)
- Performance bias: refers to systematic differences between groups in the care that is provided, or in exposure to factors other than the interventions of interest. To analyze it, blinding procedures must be examined.
- (c)
- Detection bias: refers to systematic differences between groups in how outcomes are determined and may occur during intervention and follow-up. Blinding of outcome assessors must be considered when analyzing it, since it may reduce the risk.
- (d)
- Attrition bias: systematic differences between groups in withdrawals from a study. It occurs when there are withdrawals that lead to incomplete outcome data or when withdrawals in both groups differ significantly.
- (e)
- Reporting bias: refers to systematic differences between reported and unreported findings. This can occur once the study is finished and it is due to the selective report of results, reporting only statistically significant data.
- (f)
- Other biases: occur when reviewers include methodological aspects that are not assessed in the domains described before. They relate mainly to certain trial designs, such as crossover trials.
3. Results
3.1. Sample Characteristics
3.2. Intervention Characteristics
3.3. Outcome Measures
3.4. Accuracy of the System and Effects of the Interventions
3.5. Assessment of Methodological Quality of the Studies and Risk of Bias
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Totty, M.S.; Wade, E. Muscle Activation and Inertial Motion Data for Noninvasive Classification of Activities of Daily Living. IEEE Trans. Biomed. Eng. 2018, 65, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Yancosek, K.E.; Howell, D. A narrative review of dexterity assessments. J. Hand Ther. 2009, 22, 258–269. [Google Scholar] [CrossRef] [PubMed]
- Nasri, N.; Orts-Escolano, S.; Cazorla, M. An sEMG-Controlled 3D Game for Rehabilitation Therapies: Real-Time Time Hand Gesture Recognition Using Deep Learning Techniques. Sensors 2020, 20, 6451. [Google Scholar] [CrossRef] [PubMed]
- Melero, M.; Hou, A.; Cheng, E.; Tayade, A.; Lee, S.C.; Unbearth, M.; Navab, N. Upbeat: Augmented Reality-Guided Dancing for Prosthetic Rehabilitation of Upper Limb Amputees. J. Healthc. Eng. 2019, 2019, 2163705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waliño-Paniagua, C.N.; Gomez-Calero, C.; Jiménez-Trujillo, M.I.; Aguirre-Tejedor, L.; Bermejo-Franco, A.; Ortiz-Gutiérrez, R.M.; Cano-de-la-Cuerda, R. Effects of a Game-Based Virtual Reality Video Capture Training Program Plus Occupational Therapy on Manual Dexterity in Patients with Multiple Sclerosis: A Randomized Controlled Trial. J. Healthc. Eng. 2019, 2019, 2163705. [Google Scholar] [CrossRef] [Green Version]
- Perrochon, A.; Borel, B.; Istrate, D.; Compagnat, M.; Daviet, J.C. Exercise-based Games Interventions at Home in Individuals with a Neurological Disease: A Systematic Review and Meta-Analysis. Ann. Phys. Rehabil Med. 2019, 62, 366–378. [Google Scholar] [CrossRef]
- Esfahlani, S.S.; Thompson, T.; Parsa, A.D.; Brown, I.; Cirstea, S. ReHabgame: A non-immersive virtual reality rehabilitation system with applications in neuroscience. Heliyon 2018, 4, e00526. [Google Scholar] [CrossRef] [Green Version]
- Rutkowski, S.; Kiper, P.; Cacciante, L.; Cieślik, B.; Mazurek, J.; Turolla, A.; Szczepańska-Gieracha, J. Use of virtual reality-based training in different fields of rehabilitation: A systematic review and meta-analysis. J. Rehabil. Med. 2020, 52, jrm00121. [Google Scholar] [CrossRef]
- Esfahlani, S.S.; Muresan, B.; Sanaei, A.; Wilson, G. Validity of the Kinect and Myo armband in a serious game for assessing upper limb movement. Entertain. Comput. 2018, 27, 150–156. [Google Scholar] [CrossRef]
- Esfahlani, S.S.; Butt, J.; Shirvani, H. Fusion of Artificial Intelligence in Neuro-Rehabilitation Video Games. IEEE Access 2019, 7, 102617–102627. [Google Scholar] [CrossRef]
- Ryser, F.; Butzer, T.; Held, J.P.; Lambercy, O.; Gassert, R. Fully embedded myoelectric control for a wearable robotic hand orthosis. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 615–621. [Google Scholar] [CrossRef] [Green Version]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulz, K.F.; Altman, D.G.; Moher, D.; for the CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, 698–702. [Google Scholar] [CrossRef] [PubMed]
- Ciapponi, A. Guía de lectura crítica de estudios observacionales en epidemiología. Evid. Actual. Práct. Ambul. 2010, 13, 135–140. [Google Scholar] [CrossRef]
- Berra, S.; Elorza-Ricart, J.M.; Estrada, M.D.; Sánchez, E. Instrumento para la lectura crítica y la evaluación de estudios epidemiológicos transversales. Gac. Sanit. 2008, 22, 492–497. [Google Scholar] [CrossRef]
- Oxford CEBM. Oxford Centre for Evidence-Based Medicine: Levels of Evidence (March 2009). Available online: https://www.cebm.ox.ac.uk/resources/levels-of-evidence/oxford-centre-for-evidence-based-medicine-levels-of-evidence-march-2009 (accessed on 10 March 2022).
- Higgins, J.P.T.; Thomas, J. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Higgins, J.P.T., Savović, J., Page, M.J., Elbers, R.G., Sterne, J.A.C., Eds.; The Cochrane Collaboration; John Wiley & Sons, Ltd.: Oxford, UK, 2019; pp. 205–228. [Google Scholar] [CrossRef]
- Lyu, M.; Lambelet, C.; Woolley, D.; Zhang, X.; Chen, W.; Ding, X.; Gassert, R.; Wenderoth, N. Training wrist extensor function and detecting unwanted movement strategies in an EMG-controlled visuomotor task. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 1549–1555. [Google Scholar] [CrossRef]
- Gaetani, F.; de Fazio, R.; Zappatore, G.A.; Visconti, P. A prosthetic limb managed by sensors-based electronic system: Experimental results on amputees. Bull. Electr. Eng. Inform. 2020, 9, 514–524. [Google Scholar] [CrossRef]
- Sattar, N.Y.; Kausar, Z.; Usama, S.A.; Naseer, N.; Farooq, U.; Abdullah, A.; Mirtaheri, P. Enhancing Classification Accuracy of Transhumeral Prosthesis: A Hybrid sEMG and fNIRS Approach. IEEE Access. 2021, 9, 113246–113257. [Google Scholar] [CrossRef]
- Castiblanco, J.C.; Ortmann, S.; Mondragon, I.F.; Alvarado-Rojas, C. Myoelectric pattern recognition of hand motions for stroke rehabilitation. Biomed. Signal Process. Control 2020, 57, 101737. [Google Scholar] [CrossRef]
- MacIntosh, A.; Desailly, E.; Vignais, N.; Vigneron, V.; Biddiss, E. A biofeedback-enhanced therapeutic exercise video game intervention for young people with cerebral palsy: A randomized single-case experimental design feasibility study. PLoS ONE 2020, 15, e0234767. [Google Scholar] [CrossRef]
- Zasadzka, E.; Trzmiel, T.; Pieczyńska, A.; Hojan, K. Modern Technologies in the Rehabilitation of Patients with Multiple Sclerosis and Their Potential Application in Times of COVID-19. Medicina 2021, 57, 549. [Google Scholar] [CrossRef]
- Nizamis, K.; Athanasiou, A.; Almpani, S.; Dimitrousis, C.; Astaras, A. Converging Robotic Technologies in Targeted Neural Rehabilitation: A Review of Emerging Solutions and Challenges. Sensors 2021, 21, 2084. [Google Scholar] [CrossRef]
- Lopes, J.B.P.; Duarte, N.A.C.; Lazzari, R.D.; Oliveira, C.S. Virtual reality in the rehabilitation process for individuals with cerebral palsy and Down syndrome: A systematic review. J. Bodyw. Mov. Ther. 2020, 24, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Norouzi, E.; Gerber, M.; Pühse, U.; Vaezmosavi, M.; Brand, S. Combined virtual reality and physical training improved the bimanual coordination of women with multiple sclerosis. Neuropsychol. Rehabil. 2021, 31, 552–569. [Google Scholar] [CrossRef] [PubMed]
- Hwang, N.K.; Choi, J.B.; Choi, D.K.; Park, J.M.; Hong, C.W.; Park, J.S.; Yoon, T.H. Effects of Semi-Immersive Virtual Reality-Based Cognitive Training Combined with Locomotor Activity on Cognitive Function and Gait Ability in Community-Dwelling Older Adults. Healthcare 2021, 9, 814. [Google Scholar] [CrossRef] [PubMed]
- Luque-Moreno, C.; Ferragut-Garcías, A.; Rodríguez-Blanco, C.; Heredia-Rizo, A.M.; Oliva-Pascual-Vaca, J.; Kiper, P.; Oliva-Pascual-Vaca, A. A Decade of Progress Using Virtual Reality for Poststroke Lower Extremity Rehabilitation: Systematic Review of the Intervention Methods. BioMed Res. Int. 2015, 2015, 342529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortés-Pérez, I.; Zagalaz-Anula, N.; Montoro-Cárdenas, D.; Lomas-Vega, R.; Obrero-Gaitán, E.; Osuna-Pérez, M.C. Leap Motion Controller Video Game-Based Therapy for Upper Extremity Motor Recovery in Patients with Central Nervous System Diseases. A Systematic Review with Meta-Analysis. Sensors 2021, 21, 2065. [Google Scholar] [CrossRef] [PubMed]
- Avcil, E.; Tarakci, D.; Arman, N.; Tarakci, E. Upper extremity rehabilitation using video games in cerebral palsy: A randomized clinical trial. Acta Neurol. Belg. 2021, 121, 1053–1060. [Google Scholar] [CrossRef]
- Lamers, I.; Maris, A.; Severijns, D.; Dielkens, W.; Geurts, S.; van Wijmeersch, B.; Feys, P. Upper Limb Rehabilitation in People with Multiple Sclerosis: A Systematic Review. Neurorehabil. Neural Repair 2016, 30, 773–793. [Google Scholar] [CrossRef] [Green Version]
- Laver, K.E.; Lange, B.; George, S.; Deutsch, J.E.; Saposnik, G.; Crotty, M. Virtual reality for stroke rehabilitation. Cochrane Database Syst. Rev. 2017, 11, CD008349. [Google Scholar] [CrossRef] [Green Version]
- García-Bravo, S.; Cuesta-Gómez, A.; Campuzano-Ruiz, R.; López-Navas, M.J.; Domínguez-Paniagua, J.; Araújo-Narváez, A.; Barreñada-Copete, E.; García-Bravo, C.; Flórez-García, M.T.; Botas-Rodríguez, J.; et al. Virtual reality and video games in cardiac rehabilitation programs. A systematic review. Disabil. Rehabil. 2021, 43, 448–457. [Google Scholar] [CrossRef]
- Da Cruz, M.M.A.; Ricci-Vitor, A.L.; Borges, G.L.B.; da Silva, P.F.; Turri-Silva, N.; Takahashi, C.; Grace, S.L.; Vanderlei, L.C.M. A Randomized, Controlled, Crossover Trial of Virtual Reality in Maintenance Cardiovascular Rehabilitation in a Low-Resource Setting: Impact on Adherence, Motivation, and Engagement. Phys. Ther. 2021, 101, pzab071. [Google Scholar] [CrossRef]
- Cano-Mañas, M.J.; Collado-Vázquez, S.; Cano-de la Cuerda, R. Videojuegos comerciales en la rehabilitación de pacientes con ictus subagudo: Estudio piloto. Rev. Neurol. 2017, 65, 337–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cano-Mañas, M.J.; Collado-Vázquez, S.; Cano-de la Cuerda, R. Realidad Virtual Semi-Inmersiva en el Paciente con Accidente Cerebrovascular Subagudo. Ph.D. Thesis, Department of Physical Therapy, Occupational Therapy, Rehabilitation and Physical Medicine, Rey Juan Carlos University, Alcorcón, Spain, 2021. [Google Scholar]
- Fandim, J.V.; Saragiotto, B.T.; Porfírio, G.J.M.; Santana, R.F. Effectiveness of virtual reality in children and young adults with cerebral palsy: A systematic review of randomized controlled trial. Braz. J. Phys. Ther. 2021, 25, 369–386. [Google Scholar] [CrossRef] [PubMed]
- Luna-Oliva, L.; Ortiz-Gutiérrez, R.M.; Cano-de la Cuerda, R.; Piédrola, R.M.; Alguacil-Diego, I.M.; Sánchez-Camarero, C.; Martínez Culebras, M.C. Kinect Xbox 360 as a therapeutic modality for children with cerebral palsy in a school environment: A preliminary study. NeuroRehabilitation 2013, 33, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Diez-Alegre, M.I.; Cano-de la Cuerda, R. Empleo de un video juego como herramienta terapéutica en adultos con parálisis cerebral tipo tetraparesia espástica: Estudio piloto. Fisioterapia 2012, 34, 23–30. [Google Scholar] [CrossRef]
- Oña, E.D.; Balaguer, C.; Cano-de la Cuerda, R.; Collado-Vázquez, S.; Jardón, A. Effectiveness of Serious Games for Leap Motion on the Functionality of the Upper Limb in Parkinson’s Disease: A Feasibility Study. Comput. Intell. Neurosci. 2018, 2018, 7148427. [Google Scholar] [CrossRef]
- Moreno-Verdú, M.; Ferreira-Sánchez, M.R.; Cano-de la Cuerda, R.; Jiménez-Antona, C. Eficacia de la realidad virtual sobre el equilibrio y la marcha en esclerosis múltiple. Revisión sistemática de ensayos controlados aleatorizados. Rev. Neurol. 2019, 68, 357–368. [Google Scholar] [CrossRef]
- Garcia, L.M.; Birckhead, B.J.; Krishnamurthy, P.; Sackman, J.; Mackey, I.G.; Louis, R.G.; Salmasi, V.; Maddox, T.; Darnall, B.D. An 8-Week Self-Administered At-Home Behavioral Skills-Based Virtual Reality Program for Chronic Low Back Pain: Double-Blind, Randomized, Placebo-Controlled Trial Conducted During COVID-19. J. Med. Internet Res. 2021, 23, e26292. [Google Scholar] [CrossRef]
| Database | Search Filter |
|---|---|
| PubMed |
|
| Scopus |
|
| Web of Science |
|
| ScienceDirect |
|
| PEDro | No filter |
| Cochrane Library |
|
| Study | Participants (Disease and Sample Size) | Protocol | Data Collection Process | Outcome Measures | Results |
|---|---|---|---|---|---|
| Melero et al. [4] | Disease: amputation n: 3 | Dance game with visual feedback using Kinect® + MYO Armband® | 10 game trials for each patient | Detection time, reaction time and operating time. O = R + D | D = 0.24 s/R = 0.92 s/O = 1.15 s MD = 2.6 s Operating time (R + MD) = 3.56 s Initial expected operating time = 6 s |
| Ryser et al. [11] | Disease: stroke n: 3 | Assessing the accuracy of the MYO® to detect movement intention in order to control a dynamic hand orthosis device | Performing three gestures, each for 60 s | Classification algorithm | Accuracy for five gestures for all samples: 98%, Accuracy for three gestures related to ADL: 94.3% Accuracy in people with stroke: 78–99%. The system is suitable for stroke rehabilitation |
| Lyu et al. [18] | Disease: stroke n: 6 healthy + 2 stroke | Visuomotor training task using the MYO Armband® | Accuracy: four gestures, 25 repetitions per gesture, 4 s contraction, 2 s relaxation, 30 s rest Validation: 36 blocks of exercises, four trials per block | sEMG signals captured via MYO® | Accuracy: 99.3% for wrist extension, 82% for radial deviation, 100% for flexion Accuracy in healthy subjects: 92.5% Validation: task performance improves through training Stroke patients: no event was reported regarding calibration, donning, or executing tasks with the device. Lower accuracy than healthy subjects |
| Gaetani et al. [19] | Disease: transradial amputation n: 9 (8 healthy + 1 congenital amelia) | performing three different gestures with hand fingers to collect sEMG data with the MYO® and analyze accuracy and response time | 10 s of flexion, 10 s of extension, and 10 s of rest | Learning algorithm, analysis of sEMG signal | Average accuracy of gesture recognition: 90.4% Accuracy in subject with amputation: 93.3% Response time: <1 s The system works also on subjects with small not-trained muscles |
| Sattar et al. [20] | Disease: transhumeral amputation n: 18 (15 healthy + 3 amputees) | Creation of BCI to control upper limb prostheses: sEMG (MYO Armband®) + fNIRS The armband acquired the sEMG signals for four-arm motions: elbow extension, elbow flexion, wrist pronation, and wrist supination | Training session: resting period of 3 min to establish a data baseline. Data acquisition: initial 5-sec rest followed by a 20-sec task period | Data processing from sEMG and fNIRS using MATLAB® | The hybrid sEMG and fNIRS system is a feasible approach to improve the CA for transhumeral amputees, improving the control performances of multifunctional upper-limb prostheses. The average accuracy of 94.6% and 74% was achieved for elbow and wrist motions by sEMG for healthy and amputated subjects, respectively Simultaneously, the fNIRS modality showed an average accuracy of 96.9% and 94.5% for hand motions of healthy and amputated subjects |
| Castiblanco et al. [21] | Disease: stroke n: 10 (6 healthy + 4 stroke) | Healthy: collection of six sEMG signals (four from right arm and two from left). One trial. Stroke: 12 sEMG signals (eight from impaired side, two from non-impaired). Three trials. All with visual feedback | Maintaining each movement 3–5 s (open-close the hand, flexion-extension of the wrist, spread the fingers, and pinch-grip each finger) | Classification algorithms | Exercises with best performance: opening-closing hand Exercises with worst performance: pinch-grip finger it was possible to identify the hand movements from sEMG signals for subjects who had a motor disability due to stroke with a correct classification rate of 85% |
| Study | Participants (Disease and Sample Size) | Intervention or Protocol | Dosage | Outcome Measures | Results |
|---|---|---|---|---|---|
| Esfahlani et al. [7] | Disease: stroke n: 20 | 3D games controlled with Kinect® and MYO® | 8 weeks 1 h/day, (days per week not specified) | EQ (Rasch Analysis), MAS, angular velocity, acceleration, ROM | Flow, presence, and absorption EQ: participants enjoyed the sessions the activities covered a good ROM for the upper body Suggest audio feedback |
| Esfahlani et al. [9] | Disease: stroke, MA and TBI n: 23 (10 healthy CG; 2 stroke, 2 TBI and 9 MA IG) | Serious game controlled by Kinect® + MYO® + pedal | 45-minute sessions, no further information | ROM response time, electromyographic data, velocity, orientation, and inertial information | Improvement in performance reflected in response time and ROM High interest and engagement The combination of MYO® and Kinect® increase the accuracy to detect gestures |
| Esfahlani et al. [10] | Disease: MS n: 52 (40 MS IG; 12 healthy CG) | IG: video games using Kinect + MYO + Pedal GC: not specified | 10 weeks 5 days/week 1 h/day | MAS, ROM | Statistically significant differences in performance and ROM. High interest and engagement |
| MacIntosh et al. [22] | Disease: CP n: 19 | Video game controlled by completing therapeutic gestures detected via electromyography and inertial sensors on the forearm via the MYO® and custom software | 4 weeks 17 min/day | AHA, BBT, wrist extension, grip strength, COPM, SEAS | Moderate improvements in active writs extension, grip strength, COPM and BBT, small improvement in AHA Positive results in SEAS No adverse effects |
| Tool item | Melero et al. [4] | Esfahlani et al. [7] | Esfahlani et al. [9] | Esfahlani et al. [10] | Ryser et al. [11] | Lyu et al. [18] | Gaetani et al. [19] | Sattar et al. [20] | Castiblanco et al. [21] | MacIntosh et al. [22] | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Methodological analysis | 1 | VG | F | F | F | F | VG | F | VG | VG | VG |
| 2 | NS | B | B | NS | B | NS | B | F | B | G | |
| 3 | NS | B | B | F | B | NS | B | F | F | G | |
| 4 | NS | B | B | G | B | B | B | B | B | F | |
| 5 | NS | NS | B | NS | B | NS | B | B | B | G | |
| 6 | B | NS | NS | NS | B | G | F | NS | F | VG | |
| 7 | NA | NA | B | G | NA | F | NA | B | F | G | |
| 8 | NA | NA | F | B | NA | B | NA | G | F | G | |
| 9 | NA | NA | G | G | NA | B | NA | G | B | G | |
| 10 | NA | NA | NS | NS | NA | F | NA | NS | NS | G | |
| 11 | G | G | F | G | F | F | F | F | F | VG | |
| 12 | F | G | B | F | F | F | NS | F | F | VG | |
| 13 | NS | G | B | F | B | NS | G | F | NS | VG | |
| 14 | B | F | F | F | F | F | B | F | F | G | |
| 15 | F | B | VG | F | NS | F | B | B | G | F | |
| 16 | F | F | G | G | NS | G | NS | B | G | F | |
| 17 | G | NS | NS | NS | NS | NS | NS | B | NS | NS | |
| 18 | F | NS | NS | NS | NS | F | G | B | NS | NS | |
| 19 | VG | B | F | F | F | F | F | G | G | G | |
| 20 | F | F | G | G | G | G | F | G | G | VG | |
| 21 | F | B | F | G | F | F | B | G | G | VG | |
| 22 | NA | B | VG | G | B | F | G | NS | F | G | |
| 23 | G | F | G | G | VG | G | G | F | NS | G | |
| 24 | G | F | G | VG | VG | G | B | F | NS | G | |
| 25 | B | B | B | B | B | B | B | B | B | F | |
| 26 | NS | B | F | B | F | F | B | B | NS | B | |
| 27 | VG | VG | VG | NS | B | NS | F | NS | F | F | |
| 28 | NS | G | NS | G | NS | NS | NS | NS | NS | VG | |
| 29 | NS | NS | NS | NS | NS | NS | NS | NS | NS | VG | |
| 30 | NS | NS | NS | NS | NS | NS | NS | NS | NS | VG | |
| 31 | NS | NS | NS | NS | NS | NS | NS | NS | NS | NS | |
| Internal validity | LOW | LOW | LOW | MEDIUM | LOW | MEDIUM | LOW | LOW | MEDIUM | HIGH | |
| External validity | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Overall quality | LOW | LOW | LOW | MEDIUM | LOW | MEDIUM | LOW | LOW | MEDIUM | HIGH |
| Study | Level of Evidence | Grade of Recommendation |
|---|---|---|
| Melero et al. [4] | 4 | C |
| Esfahlani et al. [7] | 4 | C |
| Esfahlani et al. [9] | 4 | C |
| Esfahlani et al. [10] | 4 | C |
| Ryser et al. [11] | 4 | C |
| Lyu et al. [18] | 4 | C |
| Gaetani et al. [19] | 4 | C |
| Sattar et al. [20] | 4 | C |
| Castiblanco et al. [21] | 4 | C |
| MacIntosh et al. [22] | 4 | C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcos-Antón, S.; Gor-García-Fogeda, M.D.; Cano-de-la-Cuerda, R. An sEMG-Controlled Forearm Bracelet for Assessing and Training Manual Dexterity in Rehabilitation: A Systematic Review. J. Clin. Med. 2022, 11, 3119. https://doi.org/10.3390/jcm11113119
Marcos-Antón S, Gor-García-Fogeda MD, Cano-de-la-Cuerda R. An sEMG-Controlled Forearm Bracelet for Assessing and Training Manual Dexterity in Rehabilitation: A Systematic Review. Journal of Clinical Medicine. 2022; 11(11):3119. https://doi.org/10.3390/jcm11113119
Chicago/Turabian StyleMarcos-Antón, Selena, María Dolores Gor-García-Fogeda, and Roberto Cano-de-la-Cuerda. 2022. "An sEMG-Controlled Forearm Bracelet for Assessing and Training Manual Dexterity in Rehabilitation: A Systematic Review" Journal of Clinical Medicine 11, no. 11: 3119. https://doi.org/10.3390/jcm11113119
APA StyleMarcos-Antón, S., Gor-García-Fogeda, M. D., & Cano-de-la-Cuerda, R. (2022). An sEMG-Controlled Forearm Bracelet for Assessing and Training Manual Dexterity in Rehabilitation: A Systematic Review. Journal of Clinical Medicine, 11(11), 3119. https://doi.org/10.3390/jcm11113119







