Sex-Related Differences in Left Atrial Low-Voltage Areas According to CHA2DS2-VA Scores among Patients with Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort Creation
2.2. Electro-Anatomical Mapping and Ablation Procedure
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Baseline Demographics
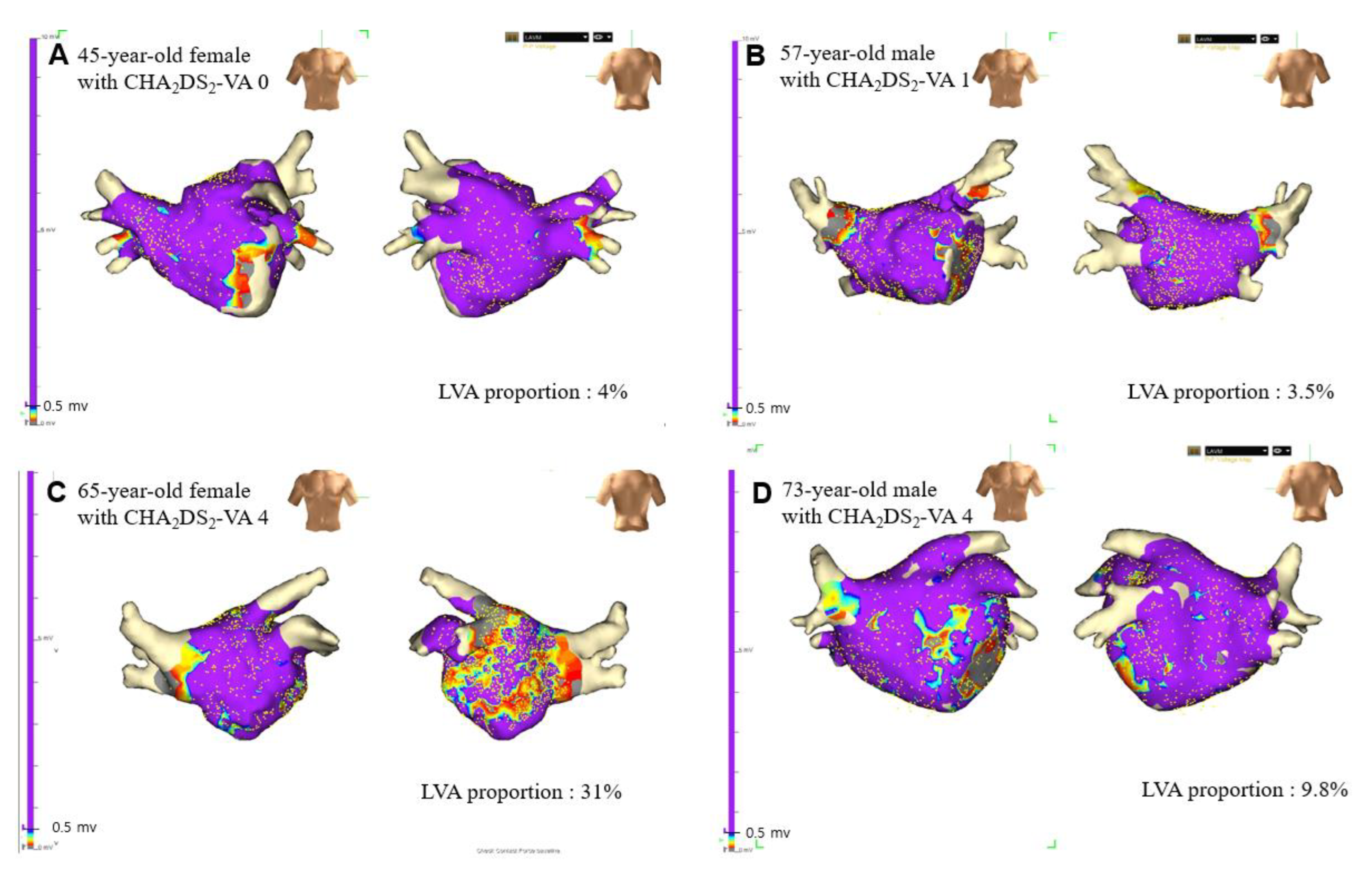
3.2. LA LVAs According to Number of Non-Sex Category Risk Factors (CHA2DS2-VA Scores)
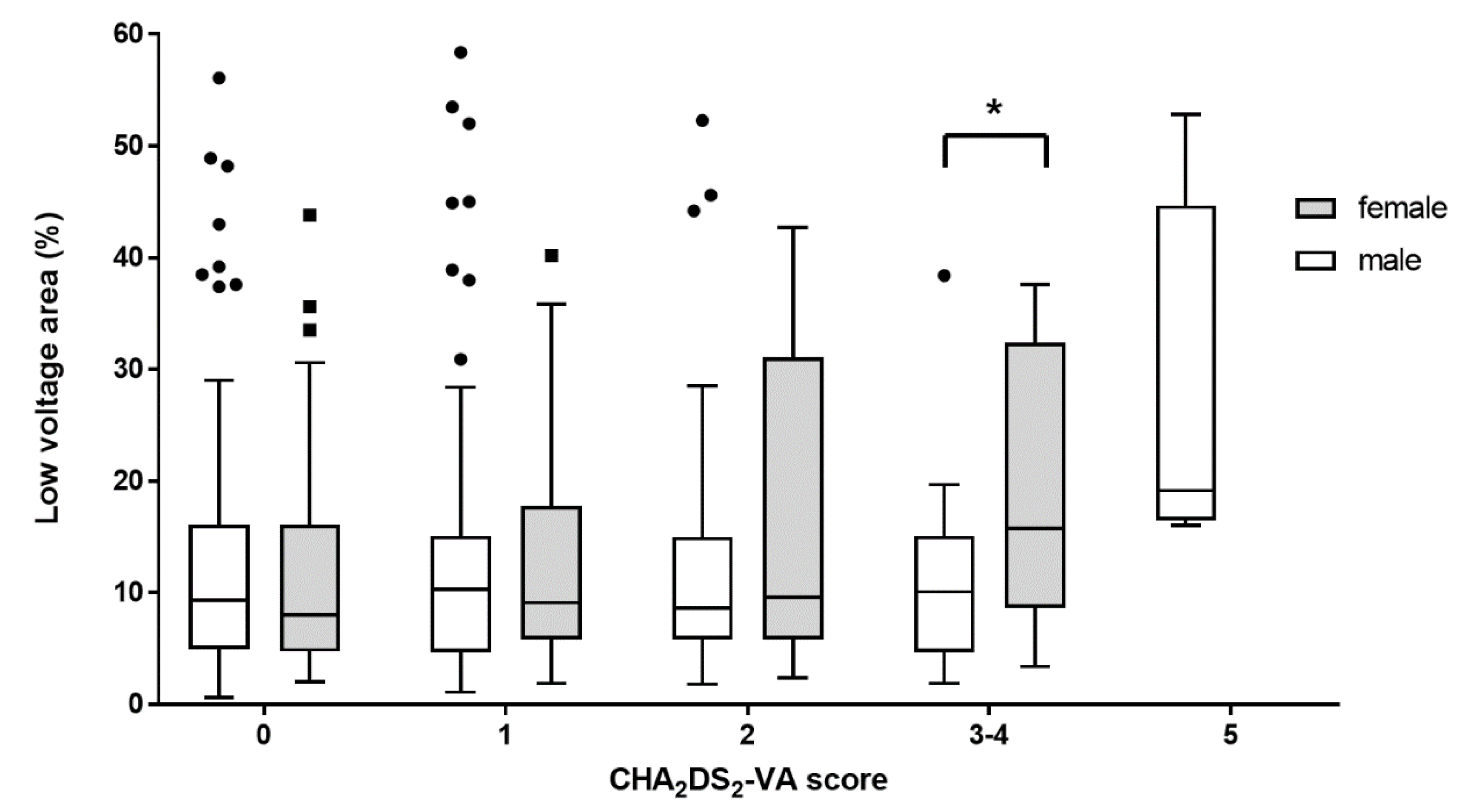
3.3. Sex-Related Difference of Proportion of LVAs According to CHA2DS2-VA Scores
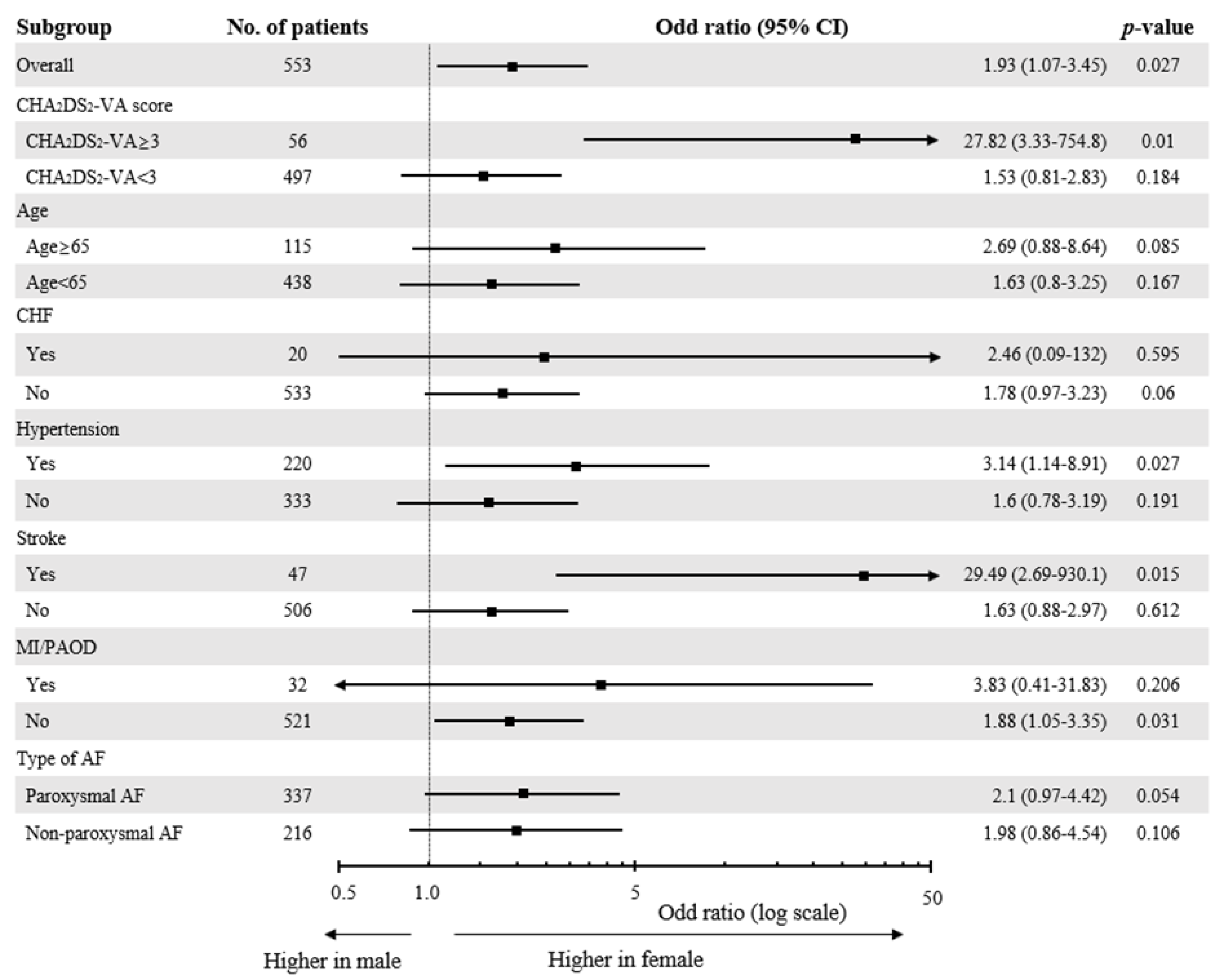
3.4. Differential Effect of Female Sex on the Extent of LVAs
4. Discussion
4.1. Left Atrial Fibrosis in Patients with AF
4.2. Sex-Related Difference in the Extent of LA Fibrosis among Patients with AF
4.3. Female Sex as a Risk Modifier on Stroke Risk and LA Fibrosis
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lip, G.; Freedman, B.; De Caterina, R.; Potpara, T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017, 117, 1230–1239. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, W.M.; Blackshear, J.L.; Laupacis, A.; Kronmal, R.; Hart, R.G. Prevalence, age distribution, and gender of patients with atrial fibrillation. Analysis and implications. Arch. Intern. Med. 1995, 155, 469–743. [Google Scholar] [CrossRef] [PubMed]
- Emdin, C.A.; Wong, C.X.; Hsiao, A.J.; Altman, D.G.; Peters, S.A.; Woodward, M.; Odutayo, A.A. Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: Systematic review and meta-analysis of cohort studies. BMJ 2016, 532, h7013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, M.C.; Singer, D.E.; Chang, Y.; Hylek, E.M.; Henault, L.E.; Jensvold, N.G.; Go, A.S. Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: The AnTicoagulation and Risk factors In Atrial fibrillation (ATRIA) study. Circulation 2005, 112, 1687–1691. [Google Scholar] [CrossRef]
- Friberg, J.; Scharling, H.; Gadsbøll, N.; Truelsen, T.; Jensen, G.B. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (The Copenhagen City Heart Study). Am. J. Cardiol. 2004, 94, 889–894. [Google Scholar] [CrossRef]
- Martin, R.C.; Burgin, W.S.; Schabath, M.B.; Kirby, B.; Chae, S.H.; Fradley, M.G.; Rose, D.Z.; Labovitz, A.J. Gender-Specific differences for risk of disability and death in atrial fibrillation-related stroke. Am. J. Cardiol. 2017, 119, 256–261. [Google Scholar] [CrossRef]
- Mikkelsen, A.P.; Lindhardsen, J.; Lip, G.Y.; Gislason, G.H.; Torp-Pedersen, C.; Olesen, J.B. Female sex as a risk factor for stroke in atrial fibrillation: A nationwide cohort study. J. Thromb. Haemost. 2012, 10, 1745–1751. [Google Scholar] [CrossRef]
- Wagstaff, A.J.; Overvad, T.F.; Lip, G.Y.; Lane, D.A. Is female sex a risk factor for stroke and thromboembolism in patients with atrial fibrillation? A systematic review and meta-analysis. QJM 2014, 107, 955–967. [Google Scholar] [CrossRef]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The Euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Schnabel, R.B.; Sullivan, L.M.; Levy, D.; Pencina, M.J.; Massaro, J.M.; D’Agostino, R.B.S.; Newton-Cheh, C.; Yamamoto, J.F.; Magnani, J.W.; Tadros, T.M.; et al. Development of a risk score for atrial fibrillation (Framingham Heart Study): A community-based cohort study. Lancet 2009, 373, 739–745. [Google Scholar] [CrossRef] [Green Version]
- Friberg, L.; Benson, L.; Rosenqvist, M.; Lip, G.Y. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: Nationwide retrospective cohort study. BMJ 2012, 344, e3522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renoux, C.; Coulombe, J.; Suissa, S. Revisiting sex differences in outcomes in non-valvular atrial fibrillation: A population-based cohort study. Eur. Heart J. 2017, 38, 1473–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, P.B.; Skjøth, F.; Overvad, T.F.; Larsen, T.B.; Lip, G.Y. Female sex is a risk modifier rather than a risk factor for stroke in atrial fibrillation: Should we use a CHA2DS2-VA score rather than CHA2DS2-VASc? Circulation 2018, 137, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Chao, T.F.; Liu, C.J.; Wang, K.L.; Lin, Y.J.; Chang, S.L.; Lo, L.W.; Hu, Y.F.; Tuan, T.C.; Chen, T.J.; Lip, G.Y.; et al. Should atrial fibrillation patients with 1 additional risk factor of the CHA2DS2-VASc score (beyond sex) receive oral anticoagulation? J. Am. Coll. Cardiol. 2015, 65, 635–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camm, A.J.; Savelieva, I. Female gender as a risk factor for stroke associated with atrial fibrillation. Eur. Heart J. 2017, 38, 1480–1484. [Google Scholar] [CrossRef]
- Nattel, S.; Harada, M. Atrial remodeling and atrial fibrillation: Recent advances and translational perspectives. J. Am. Coll. Cardiol. 2014, 63, 2335–2345. [Google Scholar] [CrossRef] [Green Version]
- Müller, P.; Makimoto, H.; Dietrich, J.W.; Fochler, F.; Nentwich, K.; Krug, J.; Duncker, D.; Blockhaus, C.; Kelm, M.; Fürnkranz, A.; et al. Association of left atrial low-voltage area and thromboembolic risk in patients with atrial fibrillation. Europace 2018, 20, f359–f365. [Google Scholar] [CrossRef]
- Kim, Y.G.; Shim, J.; Oh, S.K.; Park, H.S.; Lee, K.N.; Hwang, S.H.; Choi, J.I.; Kim, Y.H. Different responses of left atrium and left atrial appendage to radiofrequency catheter ablation of atrial fibrillation: A follow up MRI study. Sci. Rep. 2018, 8, 7871. [Google Scholar] [CrossRef]
- Verma, A.; Wazni, O.M.; Marrouche, N.F.; Martin, D.O.; Kilicaslan, F.; Minor, S.; Schweikert, R.A.; Saliba, W.; Cummings, J.; Burkhardt, J.D.; et al. Pre-Existent left atrial scarring in patients undergoing pulmonary vein antrum isolation: An independent predictor of procedural failure. J. Am. Coll. Cardiol. 2005, 45, 285–292. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.N.; Choi, J.I.; Kim, Y.G.; Oh, S.K.; Kim, D.H.; Lee, D.I.; Roh, S.Y.; Ahn, J.H.; Shim, J.; Park, S.W.; et al. Comparison between linear and focal ablation of complex fractionated atrial electrograms in patients with non-paroxysmal atrial fibrillation: A prospective randomized trial. Europace 2019, 21, 598–606. [Google Scholar] [CrossRef]
- Kornej, J.; Büttner, P.; Sommer, P.; Dagres, N.; Dinov, B.; Schumacher, K.; Bollmann, A.; Hindricks, G. Prediction of electro-anatomical substrate using APPLE score and biomarkers. Europace 2019, 21, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, T.; Tsuchiya, T.; Fukui, A.; Kawano, Y.; Otsubo, T.; Takahashi, Y.; Hirota, K.; Murotani, K.; Eshima, K.; Takahashi, N. Impact of the extent of low-voltage zone on outcomes after voltage-based catheter ablation for persistent atrial fibrillation. J. Cardiol. 2018, 72, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daccarett, M.; Badger, T.J.; Akoum, N.; Burgon, N.S.; Mahnkopf, C.; Vergara, G.; Kholmovski, E.; McGann, C.J.; Parker, D.; Brachmann, J.; et al. Association of left atrial fibrosis detected by delayed-enhancement magnetic resonance imaging and the risk of stroke in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2011, 57, 831–838. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.Y.; Kim, Y.G.; Choi, J.I.; Choi, H.Y.; Choi, Y.Y.; Boo, K.Y.; Lee, K.N.; Rho, S.Y.; Shim, J.; Kim, J.S.; et al. A novel predictive model for late recurrence after catheter ablation for atrial fibrillation using left appendage volume measured by cardiac computed tomography. Int. J. Cardiovasc. Imaging 2021, 37, 2063–2070. [Google Scholar] [CrossRef] [PubMed]
- Burstein, B.; Comtois, P.; Michael, G.; Nishida, K.; Villeneuve, L.; Yeh, Y.H.; Nattel, S. Changes in connexin expression and the atrial fibrillation substrate in congestive heart failure. Circ. Res. 2009, 105, 1213–1222. [Google Scholar] [CrossRef] [Green Version]
- Cochet, H.; Mouries, A.; Nivet, H.; Sacher, F.; Derval, N.; Denis, A.; Merle, M.; Relan, J.; Hocini, M.; Haïssaguerre, M.; et al. Age, atrial fibrillation, and structural heart disease are the main determinants of left atrial fibrosis detected by delayed-enhanced magnetic resonance imaging in a general cardiology population. J. Cardiovasc. Electrophysiol. 2015, 26, 484–492. [Google Scholar] [CrossRef]
- Akoum, N.; Mahnkopf, C.; Kholmovski, E.G.; Brachmann, J.; Marrouche, N.F. Age and sex differences in atrial fibrosis among patients with atrial fibrillation. Europace 2018, 20, 1086–1092. [Google Scholar] [CrossRef]
- Fonseca, A.C.; Marto, J.P.; Alves, P.N.; Inácio, N.; Viana-Baptista, M.; Pinho e Melo, T.; Ferro, J.M.; Almeida, A.G. Women who have ischemic strokes have a higher burden of left atrial fibrosis than men. Stroke 2018, 49, 2584–2589. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Z.; Yin, Z.; Zhang, Y.; Xue, X.; Han, J.; Zhu, Y.; Zhang, J.; Emmert, M.Y.; Wang, H. Gender differences in fibrosis remodeling in patients with long-standing persistent atrial fibrillation. Oncotarget 2017, 8, 53714–53729. [Google Scholar] [CrossRef]
- Pellman, J.; Lyon, R.C.; Sheikh, F. Extracellular matrix remodeling in atrial fibrosis: Mechanisms and implications in atrial fibrillation. J. Mol. Cell. Cardiol. 2010, 48, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Dworatzek, E.; Baczko, I.; Kararigas, G. Effects of aging on cardiac extracellular matrix in men and women. Proteom. Clin. Appl. 2016, 10, 84–91. [Google Scholar] [CrossRef] [PubMed]
- King, J.B.; Azadani, P.N.; Suksaranjit, P.; Bress, A.P.; Witt, D.M.; Han, F.T.; Chelu, M.G.; Silver, M.A.; Biskupiak, J.; Wilson, B.D.; et al. Left atrial fibrosis and risk of cerebrovascular and cardiovascular events in patients with atrial fibrillation. J. Am. Coll. Cardiol. 2017, 70, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.A. Atrial stunning: Basics and clinical considerations. Int. J. Cardiol. 2003, 92, 113–128. [Google Scholar] [CrossRef]
| Male | Female | Total | p-Value | |
|---|---|---|---|---|
| (n = 444) | (n = 109) | (n = 553) | ||
| Age, years | 54.9 ± 10.6 | 59.9 ± 9.6 | 55.9 ± 10.6 | <0.001 |
| BMI, kg/m2 | 25.2 ± 3.0 | 24.5 ± 3.2 | 25.1 ± 3.1 | 0.022 |
| CHF, n (%) | 17 (3.8) | 3 (2.8) | 20 (3.6) | 0.8 |
| HTN, n (%) | 174 (39.1) | 46 (42.2) | 220 (39.8) | 0.641 |
| DM, n (%) | 29 (6.5) | 8 (7.3) | 37 (6.7) | 0.929 |
| MI/PAOD, n (%) | 26 (5.9) | 6 (5.5) | 32 (5.8) | 1 |
| CHA2DS2-VA, n (%) | 0.176 | |||
| 0 | 200 (45.1) | 44 (40.4) | 244 (44.1) | |
| 1 | 126 (28.3) | 30 (27.5) | 156 (28.2) | |
| 2 | 78 (17.6) | 19 (17.4) | 97 (17.5) | |
| 3 | 32 (7.2) | 12 (11.0) | 44 (8.0) | |
| 4 | 4 (0.9) | 4 (3.7) | 8 (1.4) | |
| 5 | 4 (0.9) | 0 (0.0) | 4 (0.7) | |
| eGFR, ml/min per 1.73 m2 | 78.1 ± 13.9 | 73.7 ± 18.2 | 77.3 ± 15.0 | 0.019 |
| Non-paroxysmal AF, n (%) | 187 (42.1) | 29 (26.6) | 216 (39.1) | 0.004 |
| Duration of AF, years | 3.0 (1.0–6.0) | 3.0 (1.0–7.5) | 3.0 (1.0–6.0) | 0.96 |
| LA diameter, mm | 41.0 (37.5–44.5) | 40.2 (36.0–42.8) | 40.7 (37.4–44.3) | 0.056 |
| LVEF, % | 57.5 (54.5–57.5) | 57.5 (54.5–57.5) | 57.5 (54.5–57.5) | 0.249 |
| LA low-voltage area (%) | 9.6 (5.2–15.2) | 9.8 (5.8–20.9) | 9.7 (5.4–16.4) | 0.152 |
| Univariate Analysis | Multiple Analysis Model | |||||
|---|---|---|---|---|---|---|
| Beta | 95% CI | p-Value | Beta | 95% CI | p-Value | |
| Intercept | 7.95 | 5.96–9.95 | <0.001 | |||
| Age per 10 years | 1.37 | −0.03–2.76 | 0.054 | |||
| Female with CHA2DS2-VA ≥ 3 | 6.46 | −2.33–15.25 | 0.149 | 8.52 | 0.44–16.6 | 0.039 |
| Non-PAF | 12.93 | 10.09–15.77 | <0.001 | 10.88 | 7.90–13.86 | <0.001 |
| AF duration, years | 0.27 | 0.07–0.47 | 0.009 | 0.24 | 0.05–0.43 | 0.012 |
| LA diameter ≥ 45 mm | 11.37 | 7.89–14.85 | <0.001 | 6.96 | 3.43–10.49 | <0.001 |
| LVEF, % | −0.33 | −0.59–−0.07 | 0.014 | |||
| eGFR < 60 mL/min per 1.73 m2 | 6.13 | 1.14–11.12 | 0.016 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.Y.; Kim, Y.G.; Choi, H.Y.; Choi, Y.Y.; Boo, K.Y.; Lee, K.-N.; Roh, S.-Y.; Shim, J.; Choi, J.-I.; Kim, Y.-H. Sex-Related Differences in Left Atrial Low-Voltage Areas According to CHA2DS2-VA Scores among Patients with Atrial Fibrillation. J. Clin. Med. 2022, 11, 3111. https://doi.org/10.3390/jcm11113111
Kim DY, Kim YG, Choi HY, Choi YY, Boo KY, Lee K-N, Roh S-Y, Shim J, Choi J-I, Kim Y-H. Sex-Related Differences in Left Atrial Low-Voltage Areas According to CHA2DS2-VA Scores among Patients with Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(11):3111. https://doi.org/10.3390/jcm11113111
Chicago/Turabian StyleKim, Do Young, Yun Gi Kim, Ha Young Choi, Yun Young Choi, Ki Yung Boo, Kwang-No Lee, Seung-Young Roh, Jaemin Shim, Jong-Il Choi, and Young-Hoon Kim. 2022. "Sex-Related Differences in Left Atrial Low-Voltage Areas According to CHA2DS2-VA Scores among Patients with Atrial Fibrillation" Journal of Clinical Medicine 11, no. 11: 3111. https://doi.org/10.3390/jcm11113111
APA StyleKim, D. Y., Kim, Y. G., Choi, H. Y., Choi, Y. Y., Boo, K. Y., Lee, K.-N., Roh, S.-Y., Shim, J., Choi, J.-I., & Kim, Y.-H. (2022). Sex-Related Differences in Left Atrial Low-Voltage Areas According to CHA2DS2-VA Scores among Patients with Atrial Fibrillation. Journal of Clinical Medicine, 11(11), 3111. https://doi.org/10.3390/jcm11113111






