Safety and Efficacy of Left Atrial Catheter Ablation in Patients with Left Atrial Appendage Occlusion Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
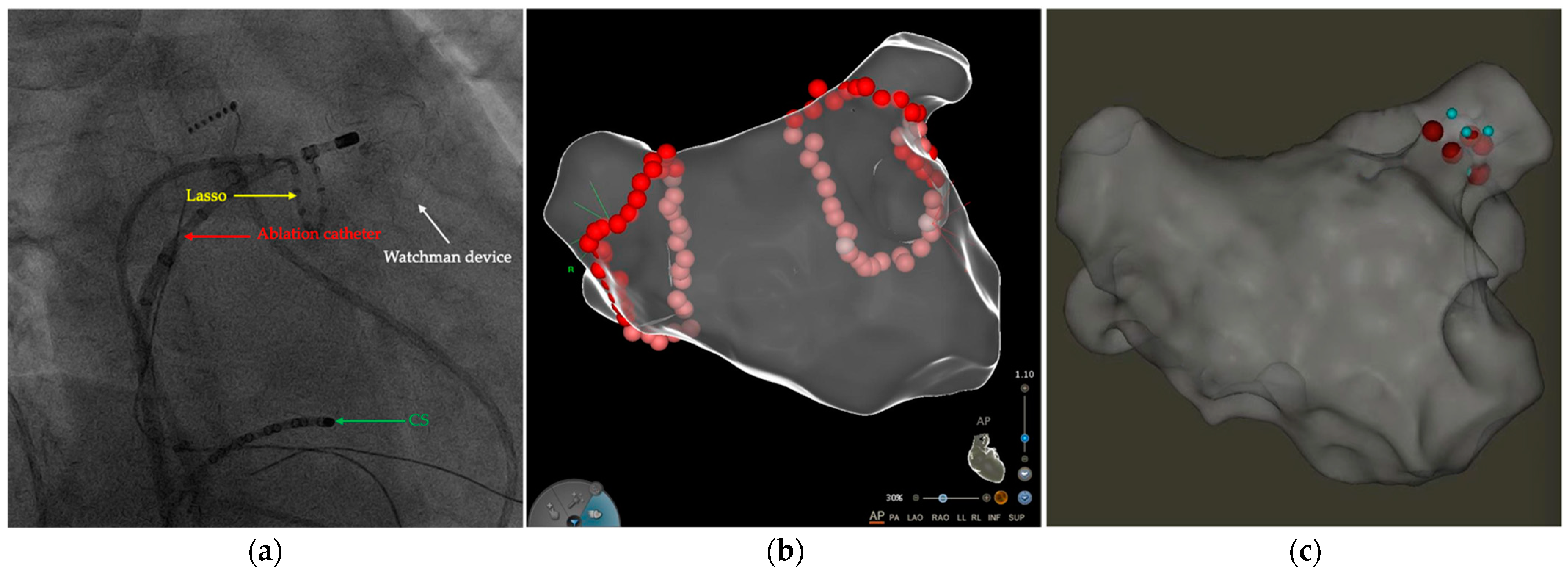
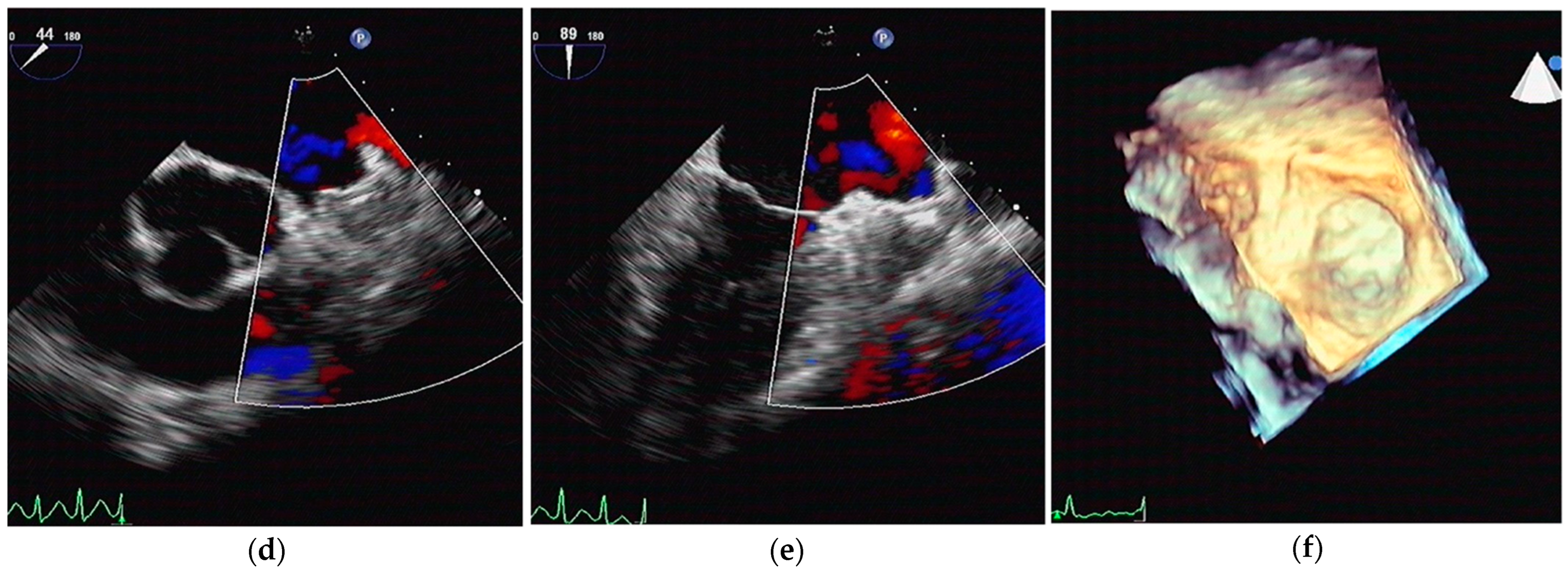
2.2. LA CA Procedure
2.3. Follow-Up
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Periprocedural Data
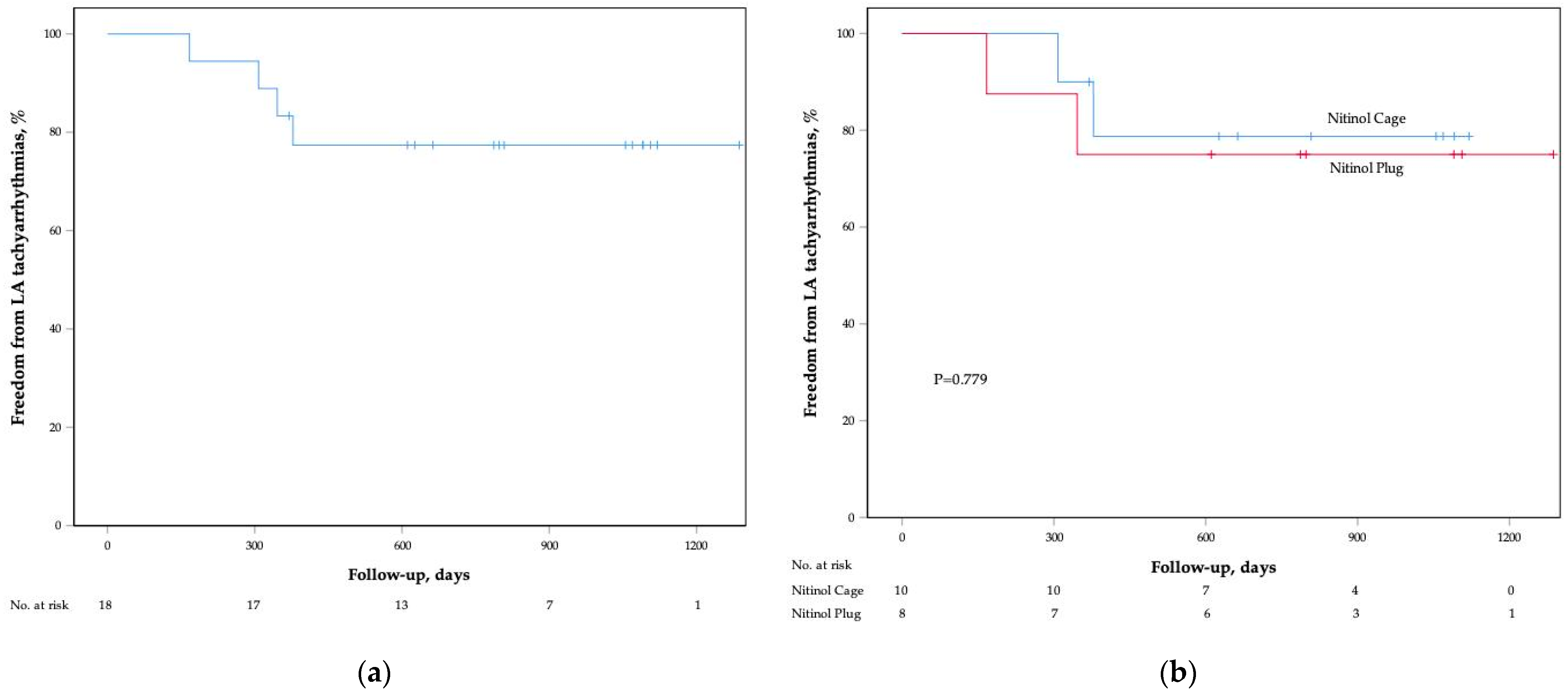
3.3. Follow-Up Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hindricks, G.; Potpara, T.; Dagres, N.; Arbelo, E.; Bax, J.J.; Blomstrom-Lundqvist, C.; Boriani, G.; Castella, M.; Dan, G.A.; Dilaveris, P.E.; et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 42, 373–498. [Google Scholar] [CrossRef] [PubMed]
- Blackshear, J.L.; Odell, J.A. Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann. Thorac. Surg. 1996, 61, 755–759. [Google Scholar] [CrossRef]
- Reddy, V.Y.; Doshi, S.K.; Kar, S.; Gibson, D.N.; Price, M.J.; Huber, K.; Horton, R.P.; Buchbinder, M.; Neuzil, P.; Gordon, N.T.; et al. 5-Year Outcomes After Left Atrial Appendage Closure: From the PREVAIL and PROTECT AF Trials. J. Am. Coll. Cardiol. 2017, 70, 2964–2975. [Google Scholar] [CrossRef] [PubMed]
- Osmancik, P.; Herman, D.; Neuzil, P.; Hala, P.; Taborsky, M.; Kala, P.; Poloczek, M.; Stasek, J.; Haman, L.; Branny, M.; et al. 4-Year Outcomes After Left Atrial Appendage Closure Versus Nonwarfarin Oral Anticoagulation for Atrial Fibrillation. J. Am. Coll. Cardiol. 2022, 79, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wilber, D.J.; Pappone, C.; Neuzil, P.; De Paola, A.; Marchlinski, F.; Natale, A.; Macle, L.; Daoud, E.G.; Calkins, H.; Hall, B.; et al. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: A randomized controlled trial. JAMA 2010, 303, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Chu, H.; Ye, P.; He, B.; Xu, H.; Jiang, S.; Lin, M.; Lin, R.; Liu, J.; Wang, B.; et al. Combination of left atrial appendage closure and catheter ablation in a single procedure for patients with atrial fibrillation: Multicenter experience. J. Formos. Med. Assoc. 2019, 118, 891–897. [Google Scholar] [CrossRef]
- Fassini, G.; Conti, S.; Moltrasio, M.; Maltagliati, A.; Tundo, F.; Riva, S.; Dello Russo, A.; Casella, M.; Majocchi, B.; Zucchetti, M.; et al. Concomitant cryoballoon ablation and percutaneous closure of left atrial appendage in patients with atrial fibrillation. Europace 2016, 18, 1705–1710. [Google Scholar] [CrossRef]
- Steckman, D.A.; Nguyen, D.T.; Sauer, W.H. Catheter ablation of atrial fibrillation and left atrial flutter in a patient with a left atrial appendage occlusion device. Europace 2014, 16, 651. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.D.; Patel, V.M.; Sharma, P.S.; Jameria, Z.; Lazar, S.; Trohman, R.; Wissner, E. Cryoballoon pulmonary vein isolation and voltage mapping for symptomatic atrial fibrillation 9 months after Watchman device implantation. HeartRhythm Case Rep. 2018, 4, 6–9. [Google Scholar] [CrossRef]
- Pietrasik, G.M.; Huang, H.D.; Rodriguez, J.M.; Sharma, P.S.; Trohman, R.G.; Krishnan, K. Safety and feasibility of radiofrequency redo pulmonary vein isolation ablation for atrial fibrillation after Amulet implantation and device electrical characteristics. HeartRhythm Case Rep. 2020, 6, 415–418. [Google Scholar] [CrossRef]
- Heeger, C.H.; Rillig, A.; Lin, T.; Mathew, S.; Deiss, S.; Lemes, C.; Botros, M.; Metzner, A.; Wissner, E.; Kuck, K.H.; et al. Feasibility and clinical efficacy of left atrial ablation for the treatment of atrial tachyarrhythmias in patients with left atrial appendage closure devices. Heart Rhythm 2015, 12, 1524–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walker, D.T.; Phillips, K.P. Left atrial catheter ablation subsequent to Watchman(R) left atrial appendage device implantation: A single centre experience. Europace 2015, 17, 1402–1406. [Google Scholar] [CrossRef]
- Wintgens, L.I.S.; Klaver, M.N.; Swaans, M.J.; Alipour, A.; Balt, J.C.; van Dijk, V.F.; Rensing, B.; Wijffels, M.; Boersma, L.V.A. Left atrial catheter ablation in patients with previously implanted left atrial appendage closure devices. Europace 2019, 21, 428–433. [Google Scholar] [CrossRef] [PubMed]
- Turagam, M.K.; Lavu, M.; Afzal, M.R.; Vuddanda, V.; Jazayeri, M.A.; Parikh, V.; Atkins, D.; Bommana, S.; Di Biase, L.; Horton, R.; et al. Catheter Ablation for Atrial Fibrillation in Patients with Watchman Left Atrial Appendage Occlusion Device: Results from a Multicenter Registry. J. Cardiovasc. Electrophysiol. 2017, 28, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.D.; Krishnan, K.; Sharma, P.S.; Kavinsky, C.J.; Rodriguez, J.; Ravi, V.; Larsen, T.R.; Trohman, R.G. Cryoballoon Ablation and Bipolar Voltage Mapping in Patients with Left Atrial Appendage Occlusion Devices. Am. J. Cardiol. 2020, 135, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Lip, G.Y.; Nieuwlaat, R.; Pisters, R.; Lane, D.A.; Crijns, H.J. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: The euro heart survey on atrial fibrillation. Chest 2010, 137, 263–272. [Google Scholar] [CrossRef]
- Pisters, R.; Lane, D.A.; Nieuwlaat, R.; de Vos, C.B.; Crijns, H.J.; Lip, G.Y. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: The Euro Heart Survey. Chest 2010, 138, 1093–1100. [Google Scholar] [CrossRef] [Green Version]
- Mehran, R.; Rao, S.V.; Bhatt, D.L.; Gibson, C.M.; Caixeta, A.; Eikelboom, J.; Kaul, S.; Wiviott, S.D.; Menon, V.; Nikolsky, E.; et al. Standardized bleeding definitions for cardiovascular clinical trials: A consensus report from the Bleeding Academic Research Consortium. Circulation 2011, 123, 2736–2747. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, R.S.; Holmes, D.R.; Van Tassel, R.A.; Hauser, R.; Henry, T.D.; Mooney, M.; Matthews, R.; Doshi, S.; Jones, R.M.; Virmani, R. Left atrial appendage obliteration: Mechanisms of healing and intracardiac integration. JACC Cardiovasc. Interv. 2010, 3, 870–877. [Google Scholar] [CrossRef] [Green Version]
- Bass, J.L. Transcatheter occlusion of the left atrial appendage--experimental testing of a new Amplatzer device. Catheter. Cardiovasc. Interv. 2010, 76, 181–185. [Google Scholar] [CrossRef]
- Aminian, A.; Chouchane, I.; Compagnie, M.; Decubber, M.; Lalmand, J. Delayed and fatal embolization of a left atrial appendage closure device. Circ. Cardiovasc. Interv. 2014, 7, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroeter, M.R.; Danner, B.C.; Hunlich, M.; Schillinger, W. Uncommon delayed and late complications after percutaneous left atrial appendage closure with Amplatzer((R)) Cardiac Plug. Clin. Res. Cardiol. 2014, 103, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, F.; Tilz, R.; Chun, J.; Schmidt, B.; Wissner, E.; Zerm, T.; Neven, K.; Kokturk, B.; Konstantinidou, M.; Metzner, A.; et al. Long-term results of catheter ablation in paroxysmal atrial fibrillation: Lessons from a 5-year follow-up. Circulation 2010, 122, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Tilz, R.R.; Rillig, A.; Thum, A.M.; Arya, A.; Wohlmuth, P.; Metzner, A.; Mathew, S.; Yoshiga, Y.; Wissner, E.; Kuck, K.H.; et al. Catheter ablation of long-standing persistent atrial fibrillation: 5-year outcomes of the Hamburg Sequential Ablation Strategy. J. Am. Coll. Cardiol. 2012, 60, 1921–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Sanchez, J.; Mohanty, S.; Horton, R.; Gallinghouse, G.J.; Bailey, S.M.; Zagrodzky, J.D.; Santangeli, P.; et al. Left atrial appendage: An underrecognized trigger site of atrial fibrillation. Circulation 2010, 122, 109–118. [Google Scholar] [CrossRef] [Green Version]
- Di Biase, L.; Burkhardt, J.D.; Mohanty, P.; Mohanty, S.; Sanchez, J.E.; Trivedi, C.; Gunes, M.; Gokoglan, Y.; Gianni, C.; Horton, R.P.; et al. Left Atrial Appendage Isolation in Patients with Longstanding Persistent AF Undergoing Catheter Ablation: BELIEF Trial. J. Am. Coll. Cardiol. 2016, 68, 1929–1940. [Google Scholar] [CrossRef]
| Variable | Total | Nitinol Cage | Nitinol Plug | p Value |
|---|---|---|---|---|
| n | 18 | 10 | 8 | - |
| Age, years | 70 (60, 74) | 64 (57, 78) | 71 (68, 74) | 0.450 |
| Male, n (%) | 14 (78) | 9 (90) | 5 (63) | 0.275 |
| Type of arrhythmias, n (%) | ||||
| Paroxysmal AF | 5 (28) | 3 (30) | 2 (25) | 1.000 |
| Persistent AF | 10 (56) | 6 (60) | 4 (50) | 1.000 |
| AFL | 1 (6) | 0 | 1 (13) | 0.444 |
| AT | 2 (11) | 1 (10) | 1 (13) | 1.000 |
| CHA2DS2-VASc score, points | 4.5 (3, 6) | 4 (3, 6) | 4.5 (4, 6) | 0.752 |
| HAS-BLED score, points | 3 (2.5, 4) | 3 (2, 4) | 3 (3, 5) | 0.483 |
| Prior stroke/TIA, n (%) | 16 (89) | 10 (100) | 6 (75) | 0.183 |
| Prior bleeding, n (%) | 7 (39) | 5 (50) | 2 (25) | 0.367 |
| Prior LA CA, n (%) | 5 (28) | 3 (30) | 2 (25) | 1.000 |
| LA diameter, mm | 42 (39, 45) | 41 (36, 47) | 43 (40, 45) | 0.532 |
| LVEF, % | 64 (63, 67) | 65 (63, 68) | 64 (63, 67) | 0.788 |
| Variable | Total | Nitinol Cage | Nitinol Plug | p Value |
|---|---|---|---|---|
| No. of LA CA | 20 | 11 | 9 | - |
| Strategy of CA, n (%) | ||||
| CPVI | 16 (80) | 9 (82) | 7 (78) | 1.000 |
| Linear lesions | 14 (70) | 6 (55) | 8 (89) | 0.157 |
| CFAE | 6 (30) | 3 (27) | 3 (33) | 1.000 |
| Procedural time, min | 119 (89, 130) | 112 (99, 126) | 122 (79, 131) | 0.894 |
| Ablation time, min | 34 (23, 46) | 31 (20, 39) | 40 (25, 52) | 0.328 |
| X-ray exposure time, min | 5.0 (4.0, 6.1) | 5.0 (3.8, 6.1) | 4.9 (4.1, 6.0) | 1.000 |
| X-ray exposure dose, mGy | 25 (20, 36) | 23 (19, 40) | 28 (24, 34) | 0.534 |
| Complications, n (%) | ||||
| Stroke/TIA | 0 | 0 | 0 | 1.000 |
| Systemic embolism | 0 | 0 | 0 | 1.000 |
| Pericardial effusion | 0 | 0 | 0 | 1.000 |
| Major bleeding events | 0 | 0 | 0 | 1.000 |
| Minor bleeding events | 2 (10) | 1 (9) | 1 (11) | 1.000 |
| Interference with device | 0 | 0 | 0 | 1.000 |
| Device dislodgement | 0 | 0 | 0 | 1.000 |
| Variable | Total | Nitinol Cage | Nitinol Plug | p Value |
|---|---|---|---|---|
| n | 18 | 10 | 8 | - |
| Recurrent LA tachyarrhythmias, n (%) | 4 (22) | 2 (20) | 2 (25) | 1.000 |
| Redo ablation, n (%) | 2 (11) | 1 (10) | 1 (13) | 1.000 |
| Stroke/TIA/systemic embolism, n (%) | 0 | 0 | 0 | 1.000 |
| Major bleeding events, n (%) | 0 | 0 | 0 | 1.000 |
| Minor bleeding events, n (%) | 1 (6) | 1 (10) | 0 | 1.000 |
| TEE follow-up, n (%) | 14 (78) | 8 (80) | 6 (75) | 1.000 |
| DRT | 0 | 0 | 0 | 1.000 |
| Newly developed PDL | 0 | 0 | 0 | 1.000 |
| LAAO device dislodgement | 0 | 0 | 0 | 1.000 |
| OAC therapy, n (%) | ||||
| Warfarin | 2 (11) | 2 (20) | 0 | 0.477 |
| Dabigatran | 8 (44) | 4 (40) | 4 (50) | 1.000 |
| Rivaroxaban | 8 (44) | 4 (40) | 4 (50) | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; He, B.; Fu, G.; Feng, M.; Du, X.; Liu, J.; Yu, Y.; Chu, H. Safety and Efficacy of Left Atrial Catheter Ablation in Patients with Left Atrial Appendage Occlusion Devices. J. Clin. Med. 2022, 11, 3110. https://doi.org/10.3390/jcm11113110
Wang B, He B, Fu G, Feng M, Du X, Liu J, Yu Y, Chu H. Safety and Efficacy of Left Atrial Catheter Ablation in Patients with Left Atrial Appendage Occlusion Devices. Journal of Clinical Medicine. 2022; 11(11):3110. https://doi.org/10.3390/jcm11113110
Chicago/Turabian StyleWang, Binhao, Bin He, Guohua Fu, Mingjun Feng, Xianfeng Du, Jing Liu, Yibo Yu, and Huimin Chu. 2022. "Safety and Efficacy of Left Atrial Catheter Ablation in Patients with Left Atrial Appendage Occlusion Devices" Journal of Clinical Medicine 11, no. 11: 3110. https://doi.org/10.3390/jcm11113110
APA StyleWang, B., He, B., Fu, G., Feng, M., Du, X., Liu, J., Yu, Y., & Chu, H. (2022). Safety and Efficacy of Left Atrial Catheter Ablation in Patients with Left Atrial Appendage Occlusion Devices. Journal of Clinical Medicine, 11(11), 3110. https://doi.org/10.3390/jcm11113110








