ABO Blood Type Is Associated with Thrombotic Risk in Patients with Nonvalvular Atrial Fibrillation
Abstract
:1. Introduction
2. Materials and Methods
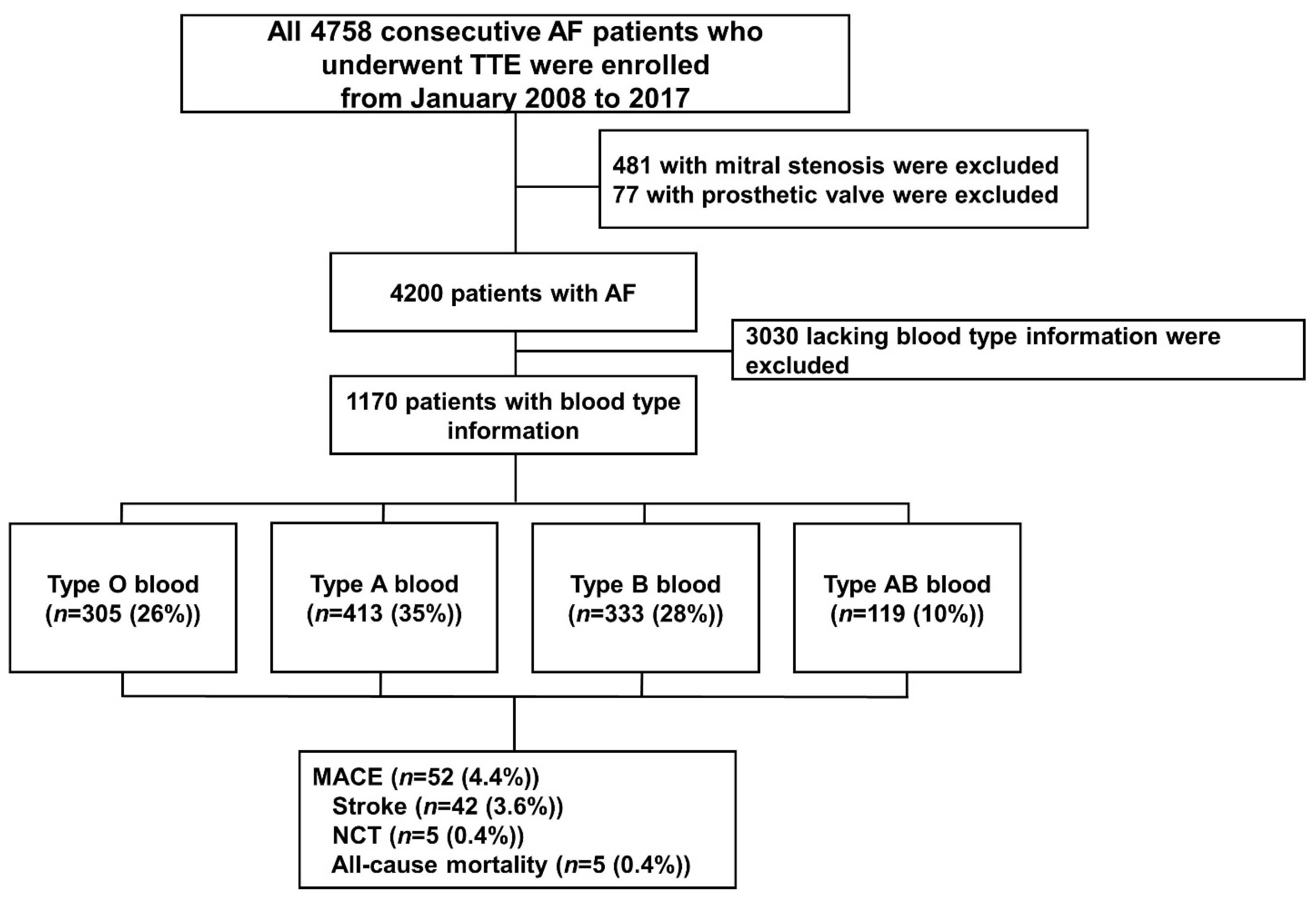
2.1. Study Sample
2.2. Clinical Outcome Assessment
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
3.2. Clinical Outcomes
3.3. Multivariable Cox Regression for Analyzing Predictors for Long-Term Adverse Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heeringa, J.; van der Kuip, D.A.; Hofman, A.; Kors, J.A.; van Herpen, G.; Stricker, B.H.; Stijnen, T.; Lip, G.Y.; Witteman, J.C. Prevalence, incidence and lifetime risk of atrial fibrillation: The Rotterdam study. Eur. Heart J. 2006, 27, 949–953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marini, C.; De Santis, F.; Sacco, S.; Russo, T.; Olivieri, L.; Totaro, R.; Carolei, A. Contribution of atrial fibrillation to incidence and outcome of ischemic stroke: Results from a population-based study. Stroke 2005, 36, 1115–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, D.; Yang, P.S.; Lip, G.Y.H.; Joung, B. Atrial fibrillation increases the risk of early-onset dementia in the general population: Data from a population-based cohort. J. Clin. Med. 2020, 9, 3665. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Lip, G.Y.; De Caterina, R.; Savelieva, I.; Atar, D.; Hohnloser, S.H.; Hindricks, G.; Kirchhof, P. ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: An update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur. Heart J. 2012, 33, 2719–2747. [Google Scholar] [CrossRef] [Green Version]
- Jang, A.Y.; Yu, J.; Park, Y.M.; Shin, M.S.; Chung, W.J.; Moon, J. Cardiac structural or functional changes associated with CHA2DS2-VASc Scores in Nonvalvular Atrial Fibrillation: A cross-sectional study using echocardiography. J. Cardiovasc. Imaging 2018, 26, 135–143. [Google Scholar] [CrossRef]
- Corban, M.T.; Godo, S.; Burczak, D.R.; Noseworthy, P.A.; Toya, T.; Lewis, B.R.; Lerman, L.O.; Gulati, R.; Lerman, A. coronary endothelial dysfunction is associated with increased risk of incident atrial fibrillation. J. Am. Heart Assoc. 2020, 9, e014850. [Google Scholar] [CrossRef]
- Wankowicz, P.; Staszewski, J.; Debiec, A.; Nowakowska-Kotas, M.; Szylinska, A.; Rotter, I. Ischemic stroke risk factors in patients with atrial fibrillation treated with new oral anticoagulants. J. Clin. Med. 2021, 10, 1223. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, S.H.; Xu, H.; Li, J.J. ABO blood group system and the coronary artery disease: An updated systematic review and meta-analysis. Sci. Rep. 2016, 6, 23250. [Google Scholar] [CrossRef]
- Vasan, S.K.; Rostgaard, K.; Majeed, A.; Ullum, H.; Titlestad, K.E.; Pedersen, O.B.; Erikstrup, C.; Nielsen, K.R.; Melbye, M.; Nyren, O.; et al. ABO blood group and risk of thromboembolic and arterial disease: A study of 1.5 million blood donors. Circulation 2016, 133, 1449–1457. [Google Scholar] [CrossRef]
- Jang, A.Y.; Kang, W.C.; Park, Y.M.; Ha, K.; Seo, J.; Oh, P.C.; Lee, K.; Moon, J. The thromboembolic predictability of CHA2DS2-VASc scores using different echocardiographic criteria for congestive heart failure in korean patients with nonvalvular atrial fibrillation. J. Clin. Med. 2022, 11, 300. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, C.; Ke, X.; Xiong, L.; Shi, Y.; Li, J.; Tan, X.; Ye, S. Analysis of circulating cholesterol levels as a mediator of an association between ABO blood group and coronary heart disease. Circ. Cardiovasc. Genet. 2014, 7, 43–48. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wolpin, B.; Rexrode, K.; Manson, J.E.; Rimm, E.; Hu, F.B.; Qi, L. ABO blood group and risk of coronary heart disease in two prospective cohort studies. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2314–2320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakai, N.A.; Judd, S.E.; Alexander, K.; McClure, L.A.; Kissela, B.M.; Howard, G.; Cushman, M. ABO blood type and stroke risk: The reasons for geographic and racial differences in stroke study. J. Thromb. Haemost. 2014, 12, 564–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Crestani, S.; Franchini, M. ABO blood group and vascular disease: An update. Semin. Thromb. Hemost. 2014, 40, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Camm, A.J.; Accetta, G.; Ambrosio, G.; Atar, D.; Bassand, J.P.; Berge, E.; Cools, F.; Fitzmaurice, D.A.; Goldhaber, S.Z.; Goto, S.; et al. Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation. Heart 2017, 103, 307–314. [Google Scholar] [CrossRef]
- Huisman, M.V.; Rothman, K.J.; Paquette, M.; Teutsch, C.; Diener, H.C.; Dubner, S.J.; Halperin, J.L.; Ma, C.S.; Zint, K.; Elsaesser, A.; et al. The Changing Landscape for Stroke Prevention in AF: Findings from the GLORIA-AF registry Phase 2. J. Am. Coll. Cardiol. 2017, 69, 777–785. [Google Scholar] [CrossRef]
- Steffel, J.; Heidbuchel, H. 2021 European heart rhythm association practical guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation: Comment-Authors’ reply. Europace 2021, 23, 1685–1686. [Google Scholar] [CrossRef]
- Ogilvie, I.M.; Newton, N.; Welner, S.A.; Cowell, W.; Lip, G.Y. Underuse of oral anticoagulants in atrial fibrillation: A systematic review. Am. J. Med. 2010, 123, 638–645.e4. [Google Scholar] [CrossRef]
- Lee, S.R.; Choi, E.K. Prevalence of Atrial Fibrillation in Korean Population. Int. J. Arrhythm. 2017, 18, 195–204. [Google Scholar] [CrossRef] [Green Version]
- Storry, J.R.; Olsson, M.L. The ABO blood group system revisited: A review and update. Immunohematology 2009, 25, 48–59. [Google Scholar] [CrossRef]
- Franchini, M.; Lippi, G. The intriguing relationship between the ABO blood group, cardiovascular disease, and cancer. BMC Med. 2015, 13, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medalie, J.H.; Levene, C.; Papier, C.; Goldbourt, U.; Dreyfuss, F.; Oron, D.; Neufeld, H.; Riss, E. Blood groups, myocardial infarction and angina pectoris among 10,000 adult males. N. Engl. J. Med. 1971, 285, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Reilly, M.P.; Li, M.; He, J.; Ferguson, J.F.; Stylianou, I.M.; Mehta, N.N.; Burnett, M.S.; Devaney, J.M.; Knouff, C.W.; Thompson, J.R.; et al. Identification of ADAMTS7 as a novel locus for coronary atherosclerosis and association of ABO with myocardial infarction in the presence of coronary atherosclerosis: Two genome-wide association studies. Lancet 2011, 377, 383–392. [Google Scholar] [CrossRef] [Green Version]
- Franchini, M.; Crestani, S.; Frattini, F.; Sissa, C.; Bonfanti, C. ABO blood group and von Willebrand factor: Biological implications. Clin. Chem. Lab. Med. 2014, 52, 1273–1276. [Google Scholar] [CrossRef]
- Jenkins, P.V.; O’onnell, J.S. ABO blood group determines plasma von Willebrand factor levels: A biologic function after all? Transfusion 2006, 46, 1836–1844. [Google Scholar] [CrossRef]
- Franchini, M.; Mannucci, P.M. ABO blood group and thrombotic vascular disease. Thromb. Haemost. 2014, 112, 1103–1109. [Google Scholar] [CrossRef] [Green Version]
- Barbalic, M.; Dupuis, J.; Dehghan, A.; Bis, J.C.; Hoogeveen, R.C.; Schnabel, R.B.; Nambi, V.; Bretler, M.; Smith, N.L.; Peters, A.; et al. Large-scale genomic studies reveal central role of ABO in sP-selectin and sICAM-1 levels. Hum. Mol. Genet. 2010, 19, 1863–1872. [Google Scholar] [CrossRef] [Green Version]
- Pare, G.; Chasman, D.I.; Kellogg, M.; Zee, R.Y.; Rifai, N.; Badola, S.; Miletich, J.P.; Ridker, P.M. Novel association of ABO histo-blood group antigen with soluble ICAM-1: Results of a genome-wide association study of 6578 women. PLoS Genet. 2008, 4, e1000118. [Google Scholar] [CrossRef] [Green Version]
- Qi, L.; Cornelis, M.C.; Kraft, P.; Jensen, M.; van Dam, R.M.; Sun, Q.; Girman, C.J.; Laurie, C.C.; Mirel, D.B.; Hunter, D.J.; et al. Genetic variants in ABO blood group region, plasma soluble E-selectin levels and risk of type 2 diabetes. Hum. Mol. Genet. 2010, 19, 1856–1862. [Google Scholar] [CrossRef] [Green Version]
- Alpoim, P.N.; de Barros Pinheiro, M.; Junqueira, D.R.; Freitas, L.G.; das Gracas Carvalho, M.; Fernandes, A.P.; Komatsuzaki, F.; Gomes, K.B.; Sant’na Dusse, L.M. Preeclampsia and ABO blood groups: A systematic review and meta-analysis. Mol. Biol. Rep. 2013, 40, 2253–2261. [Google Scholar] [CrossRef]
- Li, J.; Zhou, J.; Wan, Y.; Liu, L.; Ou, C. Association between ABO blood type and postoperative cognitive dysfunction in elderly patients undergoing unilateral total hip arthroplasty surgery in China. Med. Sci. Monit. 2017, 23, 2584–2589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagherazzi, G.; Gusto, G.; Clavel-Chapelon, F.; Balkau, B.; Bonnet, F. ABO and Rhesus blood groups and risk of type 2 diabetes: Evidence from the large E3N cohort study. Diabetologia 2015, 58, 519–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchini, M.; Bonfanti, C. Evolutionary aspects of ABO blood group in humans. Clin. Chim. Acta 2015, 444, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Teslovich, T.M.; Musunuru, K.; Smith, A.V.; Edmondson, A.C.; Stylianou, I.M.; Koseki, M.; Pirruccello, J.P.; Ripatti, S.; Chasman, D.I.; Willer, C.J.; et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature 2010, 466, 707–713. [Google Scholar] [CrossRef]
- Dentali, F.; Sironi, A.P.; Ageno, W.; Turato, S.; Bonfanti, C.; Frattini, F.; Crestani, S.; Franchini, M. Non-O blood type is the commonest genetic risk factor for VTE: Results from a meta-analysis of the literature. Semin. Thromb. Hemost. 2012, 38, 535–548. [Google Scholar] [CrossRef]
- Chung, C.M.; Wang, R.Y.; Chen, J.W.; Fann, C.S.; Leu, H.B.; Ho, H.Y.; Ting, C.T.; Lin, T.H.; Sheu, S.H.; Tsai, W.C.; et al. A genome-wide association study identifies new loci for ACE activity: Potential implications for response to ACE inhibitor. Pharmacogenom. J. 2010, 10, 537–544. [Google Scholar] [CrossRef] [Green Version]
- Gasso, P.; Ritter, M.A.; Mas, S.; Lafuente, A. Influence of ABO genotype and phenotype on angiotensin-converting enzyme plasma activity. J. Renin Angiotensin Aldosterone Syst. 2014, 15, 580–584. [Google Scholar] [CrossRef] [Green Version]
- Gotsman, I.; Keren, A.; Zwas, D.R.; Lotan, C.; Admon, D. Clinical impact of ABO and rhesus D blood type groups in patients with chronic heart failure. Am. J. Cardiol. 2018, 122, 413–419. [Google Scholar] [CrossRef]

| All (n = 1170) | Type O Blood (n = 305 (26%)) | Type A Blood (n = 413 (35%)) | Type B Blood (n = 333 (28%)) | Type AB Blood (n = 119 (10%)) | p | |
|---|---|---|---|---|---|---|
| Demographic data | ||||||
| Age (years) | 70 ± 11 | 70 ± 11 | 70 ± 11 | 70 ± 11 | 72 ± 9 | 0.026 |
| Men, n (%) | 673 (58) | 175 (57) | 233 (56) | 201 (60) | 64 (54) | 0.575 |
| Previous medical history | ||||||
| Congestive heart failure, n (%) | 222 (19) | 71 (23) | 63 (15) | 61 (18) | 27 (23) | 0.036 |
| Hypertension, n (%) | 197 (17) | 51 (17) | 74 (18) | 49 (15) | 23 (19) | 0.584 |
| Diabetes mellitus, n (%) | 264 (23) | 71 (23) | 91 (22) | 72 (22) | 30 (25) | 0.849 |
| Secondary prevention for TE, n (%) | 257 (22) | 61 (20) | 99 (24) | 73 (22) | 24 (20) | 0.725 |
| Ischemic stroke, n (%) | 195 (17) | 54 (18) | 71 (17) | 54 (16) | 16 (13) | 0.740 |
| TIA, n (%) | 25 (2) | 9 (3) | 5 (1) | 9 (3) | 2 (2) | 0.349 |
| Systemic/pulmonary TE, n (%) | 40 (3) | 7 (2) | 14 (13) | 15 (5) | 4 (3) | 0.502 |
| Peripheral arterial disease, n (%) | 26 (2) | 8 (3) | 8 (2) | 8 (2) | 2 (2) | 0.899 |
| Myocardial infarction, n (%) | 54 (5) | 12 (4) | 18 (4) | 19 (6) | 5 (4) | 0.722 |
| CHA2DS2-VASc score | 2.79 ± 1.86 | 2.82 ± 1.82 | 2.79 ± 1.83 | 2.72 ± 1.92 | 2.95 ± 1.87 | 0.712 |
| Concurrent medication | ||||||
| OAC, n (%) | 541 (46) | 139 (46) | 192 (46) | 153 (46) | 57 (48) | 0.860 |
| VKA, n (%) | 301 (26) | 75 (25) | 111 (27) | 82 (25) | 33 (28) | |
| NOAC, n (%) | 240 (21) | 64 (21) | 81 (20) | 71 (21) | 24 (20) | |
| OAC in CHA2DS2-VASc ≥ 2, n (%) | 403 (48) | 99 (44) | 136 (46) | 109 (47) | 42 (47) | 0.737 |
| VKA, n (%) | 204 (24) | 49 (22) | 72 (26) | 55 (24) | 24 (27) | |
| NOAC, n (%) | 199 (24) | 52 (23) | 64 (22) | 54 (24) | 18 (20) | |
| Duration of anticoagulation (months) | 7.6 ± 13.7 | 7.2 ± 13.2 | 7.9 ± 14.4 | 8.0 ± 13.9 | 6.9 ± 12.4 | 0.788 |
| Beta-blockers, n (%) | 332 (28) | 84 (28) | 127 (31) | 88 (26) | 33 (28) | 0.594 |
| ACEi/ARBs, n (%) | 458 (39) | 120 (39) | 167 (40) | 131 (39) | 40 (34) | 0.609 |
| Diuretics, n (%) | 435 (37) | 116 (38) | 147 (36) | 131 (39) | 41 (35) | 0.663 |
| All (n = 1170) | Type O Blood (n = 305 (26%)) | Type A Blood (n = 413 (35%)) | Type B Blood (n = 333 (28%)) | Type AB Blood (n = 119 (10%)) | p | |
|---|---|---|---|---|---|---|
| Mean follow-up (months) | 17 ± 13 | 16 ± 13 | 17 ± 13 | 18 ± 13 | 17 ± 13 | 0.132 |
| MACE, n (%) | 52 (4.4) | 10 (3.3) | 16 (3.9) | 16 (4.8) | 10 (8.4) | 0.096 |
| Ischemic stroke, n (%) | 42 (3.6) | 9 (3.0) | 14 (3.4) | 12 (3.6) | 7 (5.9) | 0.529 |
| NCT, n (%) | 5 (0.4) | 0 (0.0) | 1 (0.2) | 3 (0.9) | 1 (0.8) | 0.266 |
| All-cause mortality, n (%) | 5 (0.4) | 1 (0.3) | 1 (0.2) | 1 (0.3) | 2 (1.7) | 0.178 |
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| Adjusted HR (95% CI) | p | Adjusted HR (95% CI) | p | |
| Type AB vs. non-AB blood | 2.01 (1.01–4.00) | 0.048 | - | - |
| Type O vs. non-O blood | - | - | 0.75 (0.38–1.50) | 0.422 |
| CHA2DS2-VASc score | 1.02 (0.92–1.22) | 0.450 | 1.07 (0.92–1.23) | 0.386 |
| Anticoagulation duration (months) | 0.97 (0.94–0.99) | 0.008 | 0.97 (0.94–0.99) | 0.008 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jang, A.Y.; Seo, J.; Park, Y.M.; Shin, Y.H.; Lee, J.; Oh, P.C.; Kang, W.C.; Chung, W.-J.; Moon, J. ABO Blood Type Is Associated with Thrombotic Risk in Patients with Nonvalvular Atrial Fibrillation. J. Clin. Med. 2022, 11, 3064. https://doi.org/10.3390/jcm11113064
Jang AY, Seo J, Park YM, Shin YH, Lee J, Oh PC, Kang WC, Chung W-J, Moon J. ABO Blood Type Is Associated with Thrombotic Risk in Patients with Nonvalvular Atrial Fibrillation. Journal of Clinical Medicine. 2022; 11(11):3064. https://doi.org/10.3390/jcm11113064
Chicago/Turabian StyleJang, Albert Youngwoo, Jeongduk Seo, Yae Min Park, Yong Hoon Shin, Joonpyo Lee, Pyung Chun Oh, Woong Chol Kang, Wook-Jin Chung, and Jeonggeun Moon. 2022. "ABO Blood Type Is Associated with Thrombotic Risk in Patients with Nonvalvular Atrial Fibrillation" Journal of Clinical Medicine 11, no. 11: 3064. https://doi.org/10.3390/jcm11113064
APA StyleJang, A. Y., Seo, J., Park, Y. M., Shin, Y. H., Lee, J., Oh, P. C., Kang, W. C., Chung, W.-J., & Moon, J. (2022). ABO Blood Type Is Associated with Thrombotic Risk in Patients with Nonvalvular Atrial Fibrillation. Journal of Clinical Medicine, 11(11), 3064. https://doi.org/10.3390/jcm11113064







