Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Standard Protocol Approvals, Registrations, and Patient Consents
2.2. Study Design and Subjects
2.3. Data Analysis
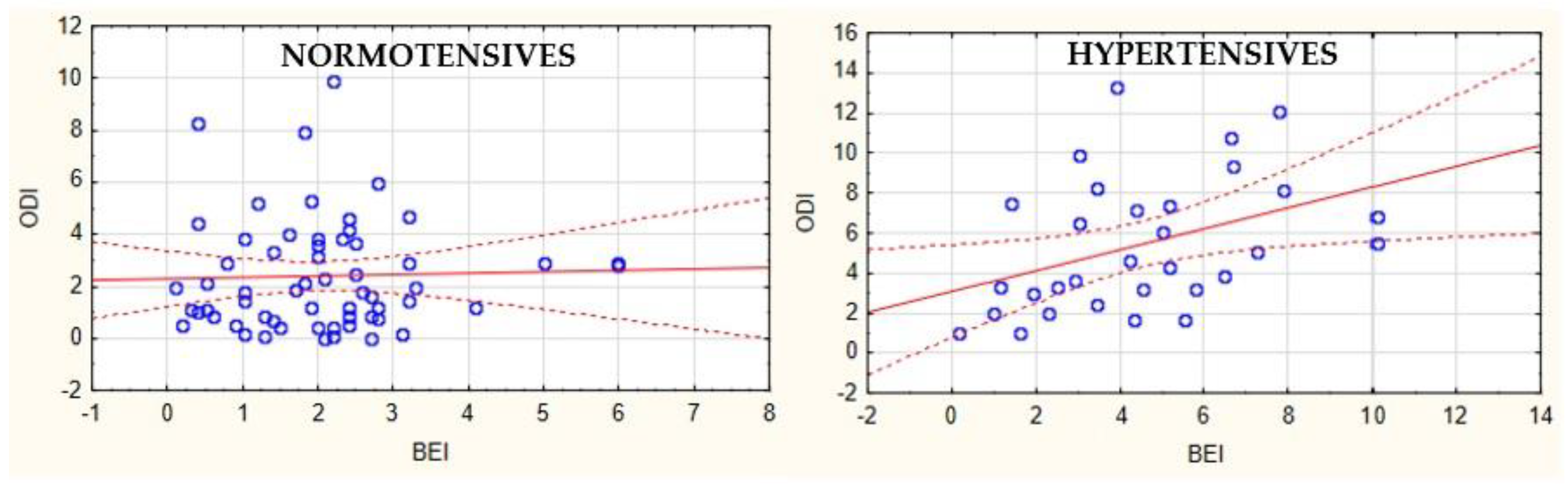
3. Results
4. Discussion
5. Conclusions
- Nonapneic hypertensives had higher SB intensity, altered sleep architecture, increased snoring, and decreased mean oxygen saturation compared to normotensives.
- The association between oxygen desaturations and bruxism episodes exists only in hypertensives, but not in normotensives.
- Dental screening is necessary for patients with arterial hypertension, especially those presenting with the symptoms of SB.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lobbezoo, F.; Ahlberg, J.; Glaros, A.G.; Kato, T.; Koyano, K.; Lavigne, G.J.; de Leeuw, R.; Manfredini, D.; Svensson, P.; Winocur, E. Bruxism defined and graded: An international consensus. J. Oral Rehabil. 2013, 40, 2–4. [Google Scholar] [CrossRef] [PubMed]
- De la Hoz-Aizpurua, J.L.; Diaz-Alonso, E.; LaTouche-Arbizu, R.; Mesa-Jimenez, J. Sleep bruxism. Conceptual review and update. Med. Oral Patol. Oral Cir. Bucal 2011, 16, e231–e238. [Google Scholar] [CrossRef] [PubMed]
- American Sleep Disorders Association. The International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual; American Sleep Disorders Association: Darien, IL, USA, 1997; ISBN 0965722015. [Google Scholar]
- Sateia, M.J. International classification of sleep disorders-third edition: Highlights and modifications. Chest 2014, 146, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Lobbezoo, F.; Ahlberg, J.; Raphael, K.G.; Wetselaar, P.; Glaros, A.G.; Kato, T.; Santiago, V.; Winocur, E.; de Laat, A.; de Leeuw, R.; et al. International consensus on the assessment of bruxism: Report of a work in progress. J. Oral Rehabil. 2018, 45, 837–844. [Google Scholar] [CrossRef]
- Yap, A.U.J.; Chua, A. Sleep bruxism: Current knowledge and contemporary management. J. Conserv. Dent. 2016, 19, 383. [Google Scholar] [CrossRef]
- Michalek-Zrabkowska, M.; Martynowicz, H.; Wieckiewicz, M.; Smardz, J.; Poreba, R.; Mazur, G. Cardiovascular implications of sleep bruxism—A systematic review with narrative summary and future perspectives. J. Clin. Med. 2021, 10, 2245. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Kato, T.; Kolta, A.; Sessle, B.J. Neurobiological mechanisms involved in sleep bruxism. Crit. Rev. Oral Biol. Med. 2003, 14, 30–46. [Google Scholar] [CrossRef]
- Sjöholm, T.T.; Piha, S.J.; Lehtinen, I. Cardiovascular autonomic control is disturbed in nocturnal teethgrinders. Clin. Physiol. 1995, 15, 349–354. [Google Scholar] [CrossRef]
- Nukazawa, S.; Yoshimi, H.; Sato, S. Autonomic nervous activities associated with bruxism events during sleep. CRANIO® 2018, 36, 106–112. [Google Scholar] [CrossRef]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Gac, P.; Smardz, J.; Poreba, R.; Wojakowska, A.; Goslawska, K.; Mazur, G.; Martynowicz, H. Effect of sleep bruxism intensity on blood pressure in normotensives. J. Clin. Med. 2021, 10, 1304. [Google Scholar] [CrossRef]
- Kato, T.; Rompré, P.; Montplaisir, J.Y.; Sessle, B.J.; Lavigne, G.J. Sleep bruxism: An oromotor activity secondary to micro-arousal. J. Dent. Res. 2001, 80, 1940–1944. [Google Scholar] [CrossRef] [PubMed]
- Macaluso, G.M.; Guerra, P.; di Giovanni, G.; Boselli, M.; Parrino, L.; Terzano, M.G. Sleep bruxism is a disorder related to periodic arousals during sleep. J. Dent. Res. 1998, 77, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef] [PubMed]
- Van den Eeden, S.K.; Albers, K.B.; Davidson, J.E.; Kushida, C.A.; Leimpeter, A.D.; Nelson, L.M.; Popat, R.; Tanner, C.M.; Bibeau, K.; Quesenberry, C.P. Risk of cardiovascular disease associated with a restless legs syndrome diagnosis in a retrospective cohort study from Kaiser permanente northern California. Sleep 2015, 38, 1009–1015. [Google Scholar] [CrossRef]
- Jarrin, D.C.; Alvaro, P.K.; Bouchard, M.-A.; Jarrin, S.D.; Drake, C.L.; Morin, C.M. Insomnia and hypertension: A systematic review. Sleep Med. Rev. 2018, 41, 3–38. [Google Scholar] [CrossRef]
- Van Ryswyk, E.M.; Mukherjee, S.; Chai-Coetzer, C.L.; Vakulin, A.; McEvoy, R.D. Sleep disorders, including sleep apnea, and hypertension. Am. J. Hypertens. 2018, 31, 857–864. [Google Scholar] [CrossRef]
- Martynowicz, H.; Dymczyk, P.; Dominiak, M.; Kazubowska, K.; Skomro, R.; Poreba, R.; Gac, P.; Wojakowska, A.; Mazur, G.; Wieckiewicz, M. Evaluation of intensity of sleep bruxism in arterial hypertension. J. Clin. Med. 2018, 7, 327. [Google Scholar] [CrossRef]
- Nashed, A.; Lanfranchi, P.; Rompré, P.; Carra, M.C.; Mayer, P.; Colombo, R.; Huynh, N.; Lavigne, G. Sleep bruxism is associated with a rise in arterial blood pressure. Sleep 2012, 35, 529–536. [Google Scholar] [CrossRef]
- Abe, Y.; Suganuma, T.; Ishii, M.; Yamamoto, G.; Gunji, T.; Clark, G.T.; Tachikawa, T.; Kiuchi, Y.; Igarashi, Y.; Baba, K. Association of genetic, psychological and behavioral factors with sleep bruxism in a Japanese population. J. Sleep Res. 2012, 21, 289–296. [Google Scholar] [CrossRef]
- Lobbezoo, F.; Naeije, M. Bruxism is mainly regulated centrally, not peripherally. J. Oral Rehabil. 2001, 28, 1085–1091. [Google Scholar] [CrossRef]
- Wieckiewicz, M.; Bogunia-Kubik, K.; Mazur, G.; Danel, D.; Smardz, J.; Wojakowska, A.; Poreba, R.; Dratwa, M.; Chaszczewska-Markowska, M.; Winocur, E.; et al. Genetic basis of sleep bruxism and sleep apnea—Response to a medical puzzle. Sci. Rep. 2020, 10, 7497. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH guidelines for the management of arterial hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef] [PubMed]
- Michalek-Zrabkowska, M.; Wieckiewicz, M.; Smardz, J.; Gac, P.; Poreba, R.; Wojakowska, A.; Mazur, G.; Martynowicz, H. Determination of inflammatory markers, hormonal disturbances, and sleepiness associated with sleep bruxism among adults. Nat. Sci. Sleep 2020, 12, 969–979. [Google Scholar] [CrossRef] [PubMed]
- Keskinruzgar, A.; Kalenderoglu, A.; Yapici Yavuz, G.; Koparal, M.; Simsek, A.; Karadag, A.S.; Utkun, M. Investigation of neurodegenerative and inflammatory processes in sleep bruxism. CRANIO® 2020, 38, 358–364. [Google Scholar] [CrossRef]
- Wieczorek, T.; Wieckiewicz, M.; Smardz, J.; Wojakowska, A.; Michalek-Zrabkowska, M.; Mazur, G.; Martynowicz, H. Sleep structure in sleep bruxism: A polysomnographic study including bruxism activity phenotypes across sleep stages. J. Sleep Res. 2020, 29, e13028. [Google Scholar] [CrossRef] [PubMed]
- Castroflorio, T.; Bargellini, A.; Rossini, G.; Cugliari, G.; Deregibus, A. Sleep bruxism and related risk factors in adults: A systematic literature review. Arch. Oral Biol. 2017, 83, 25–32. [Google Scholar] [CrossRef]
- Manfredini, D.; Winocur, E.; Guarda-Nardini, L.; Paesani, D.; Lobbezoo, F. Epidemiology of bruxism in adults: A systematic review of the literature. J. Orofac. Pain 2013, 27, 99–110. [Google Scholar] [CrossRef]
- Oksenberg, A.; Arons, E. Sleep bruxism related to obstructive sleep apnea: The effect of continuous positive airway pressure. Sleep Med. 2002, 3, 513–515. [Google Scholar] [CrossRef]
- Ohayon, M.M.; Li, K.K.; Guilleminault, C. Risk factors for sleep bruxism in the general population. Chest 2001, 119, 53–61. [Google Scholar] [CrossRef]
- Konecny, T.; Kara, T.; Somers, V.K. Obstructive sleep apnea and hypertension. Hypertension 2014, 63, 203–209. [Google Scholar] [CrossRef]
- Gonzaga, C.; Bertolami, A.; Bertolami, M.; Amodeo, C.; Calhoun, D. Obstructive sleep apnea, hypertension and cardiovascular diseases. J. Hum. Hypertens. 2015, 29, 705–712. [Google Scholar] [CrossRef] [PubMed]
- Phillips, C.L.; O’Driscoll, D.M. Hypertension and obstructive sleep apnea. Nat. Sci. Sleep 2013, 5, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Loke, Y.K.; Brown, J.W.L.; Kwok, C.S.; Niruban, A.; Myint, P.K. Association of obstructive sleep apnea with risk of serious cardiovascular events. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 720–728. [Google Scholar] [CrossRef] [PubMed]
- Dopp, J.M.; Reichmuth, K.J.; Morgan, B.J. Obstructive sleep apnea and hypertension: Mechanisms, evaluation, and management. Curr. Hypertens. Rep. 2007, 9, 529–534. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, W.; Wei, S.; Yang, L.; Wu, Y.; Yan, B. Fragmented sleep and the prevalence of hypertension in middle-aged and older individuals. Nat. Sci. Sleep 2021, 13, 2273–2280. [Google Scholar] [CrossRef]
- Huynh, N.; Kato, T.; Rompre, P.H.; Okura, K.; Saber, M.; Lanfranchi, P.A.; Montiplaisir, J.Y.; Lavigne, G.J. Sleep bruxism is associated to micro-arousals and an increase in cardiac sympathetic activity. J. Sleep Res. 2006, 15, 339–346. [Google Scholar] [CrossRef]
- Lavigne, G.J.; Huynh, N.; Kato, T.; Okura, K.; Adachi, K.; Yao, D.; Sessle, B. Genesis of sleep bruxism: Motor and autonomic-cardiac interactions. Arch. Oral Biol. 2007, 52, 381–384. [Google Scholar] [CrossRef]
- Marthol, H.; Reich, S.; Jacke, J.; Lechner, K.-H.; Wichmann, M.; Hilz, M.J. Enhanced sympathetic cardiac modulation in bruxism patients. Clin. Auton. Res. 2006, 16, 276–280. [Google Scholar] [CrossRef]
- Mizumori, T.; Sumiya, M.; Kobayashi, Y.; Inano, S.; Yatani, H. Prediction of sleep bruxism events by increased heart rate. Int. J. Prosthodont. 2013, 26, 239–243. [Google Scholar] [CrossRef][Green Version]
- Salman, L.A.; Shulman, R.; Cohen, J.B. Obstructive sleep apnea, hypertension, and cardiovascular risk: Epidemiology, pathophysiology, and management. Curr. Cardiol. Rep. 2020, 22, 6. [Google Scholar] [CrossRef]
- Stepanski, E.J. The effect of sleep fragmentation on daytime function. Sleep 2002, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- De Trindade, M.O.; Rodriguez, A.G. Polysomnographic analysis of bruxism. Gen. Dent. 2014, 62, 56–60. [Google Scholar]
- Nieto, F.J. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA 2000, 283, 1829. [Google Scholar] [CrossRef] [PubMed]
- Milde-Busch, A.; Blaschek, A.; Heinen, F.; Borggräfe, I.; Koerte, I.; Straube, A.; Schankin, C.; von Kries, R. Associations between stress and migraine and tension-type headache: Results from a school-based study in adolescents from grammar schools in Germany. Cephalalgia 2011, 31, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; de Siqueira, J.T.T.; de Gonçalves, D.A.G.; Camparis, C.M. Association between painful temporomandibular disorders, sleep bruxism and tinnitus. Braz. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dumais, I.E.; Lavigne, G.J.; Carra, M.C.; Rompré, P.H.; Huynh, N.T. Could transient hypoxia be associated with rhythmic masticatory muscle activity in sleep bruxism in the absence of sleep-disordered breathing? A preliminary report. J. Oral Rehabil. 2015, 42, 810–818. [Google Scholar] [CrossRef]
- Suzuki, Y.; Rompré, P.; Mayer, P.; Kato, T.; Okura, K.; Lavigne, G.J. Changes in oxygen and carbon dioxide in the genesis of sleep bruxism: A mechanism study. J. Prosthodont. Res. 2020, 64, 43–47. [Google Scholar] [CrossRef]

| Hypertensives (n = 31) | Normotensives (n = 60) | Total | |
|---|---|---|---|
| Female | 17 | 46 | 63 |
| Male | 14 | 14 | 28 |
| Age ± SD | 48.06 ± 14.76 | 34.88 ± 11.24 | 39.37 ± 13.96 |
| Parameter | Mean | Parameter | Mean |
|---|---|---|---|
| BEI (/h) | 2.86 ± 2.13 | SL (min) | 21.93 ± 22.93 |
| Phasic BEI (/h) | 1.62 ± 3.77 | AHI (/h) | 2.95 ± 2.48 |
| Tonic BEI (/h) | 1.08 ± 1.20 | ODI (/h) | 3.42 ± 2.96 |
| Mixed BEI (/h) | 0.66 ± 0.99 | Mean SpO2 (%) | 94.22 ± 1.66 |
| WASO (min) | 38.71 ± 38.25 | Mean heart rate (/min) | 62.87 ± 11.55 |
| N1 (%) | 3.65 ± 4.57 | Snore supine (% TST) | 12.90 ± 19.97 |
| N2 (%) | 49.18 ± 9.43 | Snore nonsupine (% TST) | 7.46 ± 14.88 |
| N3 (%) | 23.96 ± 7.94 | REM Snore (% TST) | 5.22 ± 9.90 |
| REM (%) | 23.18 ± 6.41 | NREM Snore (% TST) | 11.49 ± 16.67 |
| Bruxism Episode Index (/h) | Hypertensives n = 31 | Normotensives n = 60 | p-Value |
|---|---|---|---|
| Mean | 4.47 ± 2.55 | 2.04 ± 1.24 | <0.001 |
| Phasic | 3.23 ± 6.09 | 0.79 ± 085 | <0.01 |
| Tonic | 1.69 ± 1.75 | 0.77 ± 0.59 | <0.001 |
| Mixed | 1.08 ± 1.52 | 0.45 ± 0.43 | <0.01 |
| Hypertensives n = 31 | Normotensives n = 60 | p-Value | |
|---|---|---|---|
| N1 (%TST) | 3.98 ± 3.50 | 3.48 ± 5.06 | >0.05 |
| N2 (%TST) | 46.48 ± 10.69 | 50.57 ± 8.47 | <0.001 |
| N3 (%TST) | 24.96 ± 9.11 | 23.44 ± 7.28 | >0.05 |
| R (%TST) | 24.54 ± 6.91 | 22.48 ± 6.08 | >0.05 |
| Arousals (/h) | 4.35 ± 3.29 | 2.79 ± 1.86 | <0.01 |
| AHI (/h) | 4.77 ± 2.85 | 2.01 ± 1.62 | <0.001 |
| ODI (/h) | 5.42 ± 3.32 | 2.39 ± 2.12 | <0.001 |
| Mean SpO2 (%) | 93.25 ± 1.87 | 94.71 ± 1.31 | <0.001 |
| Minimal SpO2 (%) | 84.26 ± 8.14 | 90.15 ± 3.96 | <0.001 |
| SpO2 < 90% | 6.20 ± 12.38 | 0.78 ± 4.08 | <0.01 |
| Average desaturation drop (%) | 3.51 ± 0.53 | 3.12 ± 1.08 | >0.05 |
| HR (/min) | 61.82 ± 14.00 | 63.42 ± 10.14 | >0.05 |
| HR maximum (/min) | 100.44 ± 34.65 | 81.75 ± 55.51 | >0.05 |
| HR minimum (/min) | 50.60 ± 9.60 | 50.47 ± 7.94 | >0.05 |
| Hypertensives n = 31 | Normotensives n = 60 | p-Value | |
|---|---|---|---|
| Supine snore | 24.04 ± 23.28 | 7.14 ± 15.29 | <0.001 |
| Nonsupine snore | 15.48 ± 19.78 | 3.32 ± 9.35 | <0.001 |
| REM snore | 10.61 ± 13.94 | 2.43 ± 5.24 | <0.001 |
| NREM snore | 22.15 ± 19.62 | 5.99 ± 11.72 | <0.001 |
| Total snore | 18.80 ± 17.67 | 5.25 ± 10.03 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanclerska, J.; Wieckiewicz, M.; Poreba, R.; Szymanska-Chabowska, A.; Gac, P.; Wojakowska, A.; Frosztega, W.; Michalek-Zrabkowska, M.; Mazur, G.; Martynowicz, H. Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study. J. Clin. Med. 2022, 11, 3113. https://doi.org/10.3390/jcm11113113
Kanclerska J, Wieckiewicz M, Poreba R, Szymanska-Chabowska A, Gac P, Wojakowska A, Frosztega W, Michalek-Zrabkowska M, Mazur G, Martynowicz H. Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study. Journal of Clinical Medicine. 2022; 11(11):3113. https://doi.org/10.3390/jcm11113113
Chicago/Turabian StyleKanclerska, Justyna, Mieszko Wieckiewicz, Rafal Poreba, Anna Szymanska-Chabowska, Pawel Gac, Anna Wojakowska, Weronika Frosztega, Monika Michalek-Zrabkowska, Grzegorz Mazur, and Helena Martynowicz. 2022. "Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study" Journal of Clinical Medicine 11, no. 11: 3113. https://doi.org/10.3390/jcm11113113
APA StyleKanclerska, J., Wieckiewicz, M., Poreba, R., Szymanska-Chabowska, A., Gac, P., Wojakowska, A., Frosztega, W., Michalek-Zrabkowska, M., Mazur, G., & Martynowicz, H. (2022). Polysomnographic Evaluation of Sleep Bruxism Intensity and Sleep Architecture in Nonapneic Hypertensives: A Prospective, Observational Study. Journal of Clinical Medicine, 11(11), 3113. https://doi.org/10.3390/jcm11113113









