Effects of Coffee on Sirtuin-1, Homocysteine, and Cholesterol of Healthy Adults: Does the Coffee Powder Matter?
Abstract
:1. Introduction
2. Materials and Methods
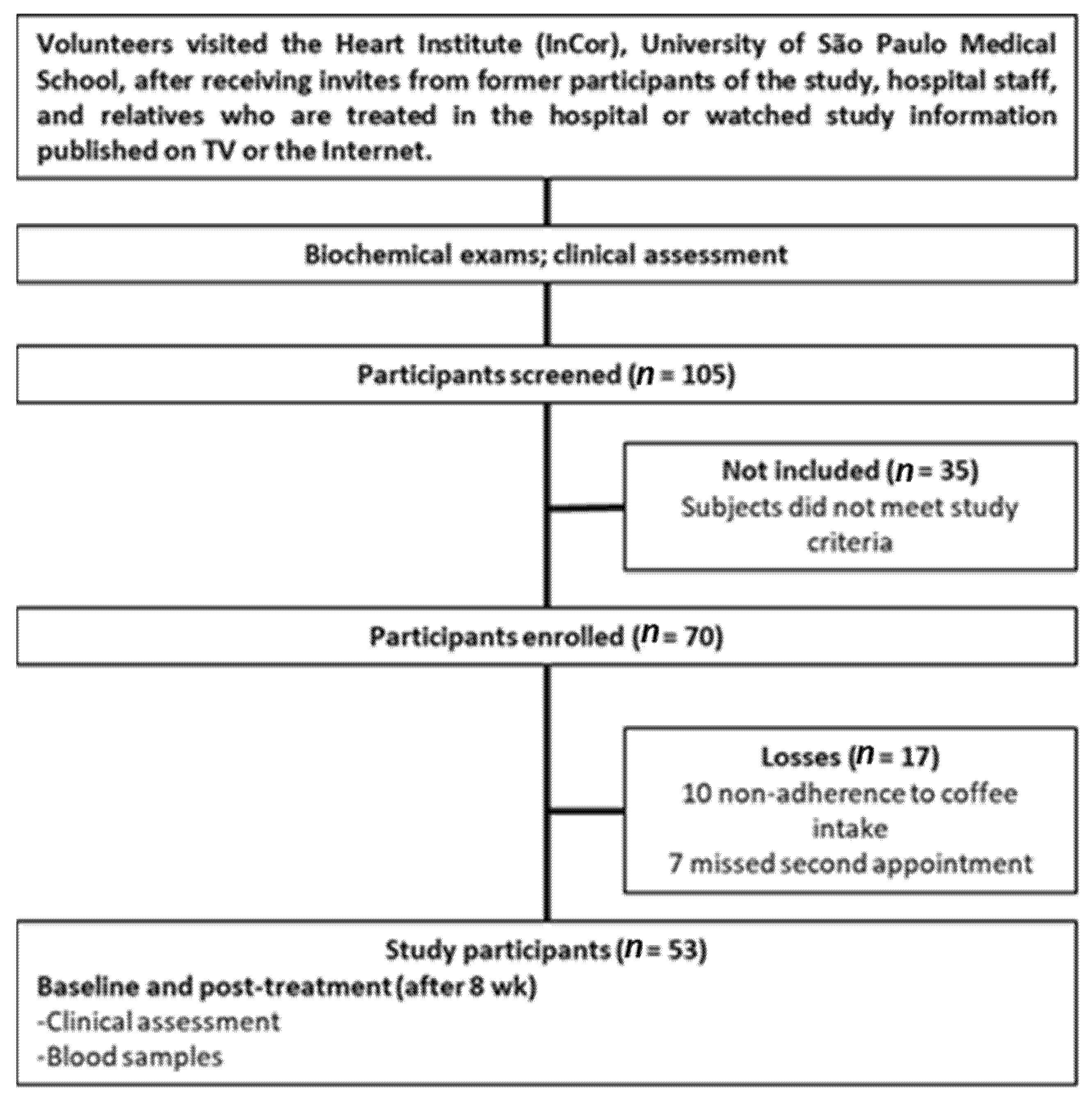
2.1. Study Design, Participants, and Intervention
2.2. Biochemical Analyses
2.3. Statistical Analyses
3. Results
3.1. Baseline Data
3.2. Post-Treatment Data
3.3. Adjusted Effects of Different Coffee Powders
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Coffee Species | ||||
|---|---|---|---|---|
| Chemical Composition | Arabica | Robusta | ||
| Mean | SD | Mean | SD | |
| Nicotinic acid | 0.03 | 0.01 | 0.02 | 0.00 |
| Trigonelline | 0.49 | 0.20 | 0.22 | 0.14 |
| 5-CQA | 0.29 | 0.13 | 0.21 | 0.15 |
| Caffeine | 1.22 | 0.09 | 2.01 | 0.03 |
| Kahweol | 0.82 | 0.10 | n.d. | n.d. |
| Cafestol | 0.37 | 0.08 | 0.21 | 0.03 |
References
- Kane, A.E.; Sinclair, D.A. Sirtuins and NAD+ in the development and treatment of metabolic and cardiovascular diseases. Circ. Res. 2018, 123, 868–885. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Ott, M. The ups and downs of SIRT1. Trends Biochem. Sci. 2008, 33, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Beher, D.; Wu, J.; Cumine, S.; Kim, K.W.; Lu, S.C.; Atangan, L.; Wang, M. Resveratrol is not a direct activator of sirt1 enzyme activity. Chem. Biol. Drug Des. 2009, 74, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Palacios, J.A.; Herranz, D.; De Bonis, M.L.; Velasco, S.; Serrano, M.; Blasco, M.A. SIRT1 contributes to telomere maintenance and augments global homologous recombination. J. Cell Biol. 2010, 191, 1299–1313. [Google Scholar] [CrossRef] [Green Version]
- Weindruch, R.; Chia, D.; Barnett, E.V.; Walford, R.L. Dietary restriction in mice beginning at 1 year of age: Effects on serum immune complex levels. Age 1982, 5, 111–112. [Google Scholar] [CrossRef]
- Mansur, A.P.; Roggerio, A.; Goes, M.F.S.; Avakian, S.D.; Leal, D.P.; Maranhão, R.C.; Strunz, C.M.C. Serum concentrations and gene expression of sirtuin 1 in healthy and slightly overweight subjects after caloric restriction or resveratrol supplementation: A randomized trial. Int. J. Cardiol. 2017, 227, 788–794. [Google Scholar] [CrossRef]
- Roggerio, A.; Cassaro Strunz, C.M.; Pacanaro, A.P.; Leal, D.P.; Takada, J.Y.; Avakian, S.D.; Mansur, A.D. Gene expression of sirtuin-1 and endogenous secretory receptor for advanced glycation end products in healthy and slightly overweight subjects after caloric restriction and resveratrol administration. Nutrients 2018, 10, 937. [Google Scholar] [CrossRef] [Green Version]
- Gonçalinho, G.H.F.; Roggerio, A.; Goes, M.F.d.S.; Avakian, S.D.; Leal, D.P.; Strunz, C.M.C.; Mansur, A.D. Comparison of Resveratrol Supplementation and Energy Restriction Effects on Sympathetic Nervous System Activity and Vascular Reactivity: A Randomized Clinical Trial. Molecules 2021, 26, 3168. [Google Scholar] [CrossRef]
- Timmers, S.; Konings, E.; Bilet, L.; Houtkooper, R.H.; Van De Weijer, T.; Goossens, G.H.; Hoeks, J.; Van Der Krieken, S.; Ryu, D.; Kersten, S.; et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011, 14, 612–622. [Google Scholar] [CrossRef] [Green Version]
- Gomes, A.P.; Price, N.L.; Ling, A.J.Y.; Moslehi, J.J.; Montgomery, M.K.; Rajman, L.; White, J.P.; Teodoro, J.S.; Wrann, C.D.; Hubbard, B.P.; et al. Declining NAD+ induces a pseudohypoxic state disrupting nuclear-mitochondrial communication during aging. Cell 2013, 155, 1624–1638. [Google Scholar] [CrossRef] [Green Version]
- Vachharajani, V.T.; Liu, T.; Wang, X.; Hoth, J.J.; Yoza, B.K.; McCall, C.E. Sirtuins Link Inflammation and Metabolism. J. Immunol. Res. 2016, 2016, 8167273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef] [PubMed]
- de Boer, V.C.J.; de Goffau, M.C.; Arts, I.C.W.; Hollman, P.C.H.; Keijer, J. SIRT1 stimulation by polyphenols is affected by their stability and metabolism. Mech. Ageing Dev. 2006, 127, 618–627. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; Van Dam, R.M.; Hu, F.B. Long-term coffee consumption and risk of cardiovascular disease: A systematic review and a dose-response meta-analysis of prospective cohort studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef] [Green Version]
- Poole, R.; Kennedy, O.J.; Roderick, P.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. Coffee consumption and health: Umbrella review of meta-analyses of multiple health outcomes. BMJ 2017, 359, j5024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, G.L.; Wang, X.; Zhang, L.; Qiu, M.H. The sources and mechanisms of bioactive ingredients in coffee. Food Funct. 2019, 10, 3113–3126. [Google Scholar] [CrossRef]
- Du, Y.; Lv, Y.; Zha, W.; Hong, X.; Luo, Q. Effect of coffee consumption on dyslipidemia: A meta-analysis of randomized controlled trials. Nutr. Metab. Cardiovasc. Dis. 2020, 30, 2159–2170. [Google Scholar] [CrossRef]
- Ludwig, I.A.; Mena, P.; Calani, L.; Cid, C.; Del Rio, D.; Lean, M.E.J.; Crozier, A. Variations in caffeine and chlorogenic acid contents of coffees: What are we drinking? Food Funct. 2014, 5, 1718–1726. [Google Scholar] [CrossRef] [Green Version]
- Wongsa, P.; Khampa, N.; Horadee, S.; Chaiwarith, J.; Rattanapanone, N. Quality and bioactive compounds of blends of Arabica and Robusta spray-dried coffee. Food Chem. 2019, 283, 579–587. [Google Scholar] [CrossRef]
- Mensink, R.P.; Lebbink, W.J.; Lobbezoo, I.E.; der Wouw, M.P.W.V.; Zock, P.L.; Katan, M.B. Diterpene composition of oils from Arabica and Robusta coffee beans and their effects on serum lipids in man. J. Intern. Med. 1995, 237, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Ciaramelli, C.; Palmioli, A.; Airoldi, C. Coffee variety, origin and extraction procedure: Implications for coffee beneficial effects on human health. Food Chem. 2019, 278, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.; Arbillaga, L.; De Peña, M.P.; Cid, C. Antioxidant and genoprotective effects of spent coffee extracts in human cells. Food Chem. Toxicol. 2013, 60, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Bakuradze, T.; Lang, R.; Hofmann, T.; Stiebitz, H.; Bytof, G.; Lantz, I.; Baum, M.; Eisenbrand, G.; Janzowski, C. Antioxidant effectiveness of coffee extracts and selected constituents in cell-free systems and human colon cell lines. Mol. Nutr. Food Res. 2010, 54, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekar, V.; Viswanathan, R. Physical and thermal properties of soybean. J. Agric. Engng Res 1999, 73, 227–234. [Google Scholar] [CrossRef]
- Eira, M.T.S.; Amaral Da Silva, E.A.; De Castro, R.D.; Dussert, S.; Walters, C.; Bewley, J.D.; Hilhorst, H.W.M. Coffee seed physiology. Braz. J. Plant Physiol. 2006, 18, 149–163. [Google Scholar] [CrossRef] [Green Version]
- Cagliani, L.R.; Pellegrino, G.; Giugno, G.; Consonni, R. Quantification of Coffea arabica and Coffea canephora var. robusta in roasted and ground coffee blends. Talanta 2013, 106, 169–173. [Google Scholar] [CrossRef]
- De Souza, R.M.N.; Benassi, M.T. Discrimination of commercial roasted and ground coffees according to chemical composition. J. Braz. Chem. Soc. 2012, 23, 1347–1354. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Zheng, J.; Liu, C. Low serum level of sirtuin 1 predicts coronary atherosclerosis plaques during computed tomography angiography among an asymptomatic cohort. Coron. Artery Dis. 2019, 30, 621–625. [Google Scholar] [CrossRef]
- Rebollo-Hernanz, M.; Zhang, Q.; Aguilera, Y.; Martín-Cabrejas, M.A.; Gonzalez de Mejia, E. Phenolic compounds from coffee by-products modulate adipogenesis-related inflammation, mitochondrial dysfunction, and insulin resistance in adipocytes, via insulin/PI3K/AKT signaling pathways. Food Chem. Toxicol. 2019, 132, 110672. [Google Scholar] [CrossRef]
- Ho, L.; Varghese, M.; Wang, J.; Zhao, W.; Chen, F.; Knable, L.A.; Ferruzzi, M.; Pasinetti, G.M. Dietary supplementation with decaffeinated green coffee improves diet-induced insulin resistance and brain energy metabolism in mice. Nutr. Neurosci. 2012, 15, 37–45. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Mousavi, K.; Khalili, M.; Jamshidzadeh, A.; Heidari, R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia 2020, 75, 1221–1230. [Google Scholar] [CrossRef]
- de Oliveira, M.R.; de Souza, I.C.C.; Fürstenau, C.R. Mitochondrial Protection Promoted by the Coffee Diterpene Kahweol in Methylglyoxal-Treated Human Neuroblastoma SH-SY5Y Cells. Neurotox. Res. 2020, 37, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Fürstenau, C.R.; de Souza, I.C.C.; de Oliveira, M.R. The effects of kahweol, a diterpene present in coffee, on the mitochondria of the human neuroblastoma SH-SY5Y cells exposed to hydrogen peroxide. Toxicol. In Vitro 2019, 61, 104601. [Google Scholar] [CrossRef] [PubMed]
- Lagouge, M.; Argmann, C.; Gerhart-Hines, Z.; Meziane, H.; Lerin, C.; Daussin, F.; Messadeq, N.; Milne, J.; Lambert, P.; Elliott, P.; et al. Resveratrol Improves Mitochondrial Function and Protects against Metabolic Disease by Activating SIRT1 and PGC-1α. Cell 2006, 127, 1109–1122. [Google Scholar] [CrossRef]
- van Dam, R.M.; Hu, F.B.; Willett, W.C. Coffee, Caffeine, and Health. N. Engl. J. Med. 2020, 383, 369–378. [Google Scholar] [CrossRef]
- Torres-collado, L.; Compañ-gabucio, L.M.; González-palacios, S.; Notario-barandiaran, L.; Oncina-cánovas, A.; Vioque, J.; García-de la Hera, M. Coffee consumption and all-cause, cardiovascular, and cancer mortality in an adult mediterranean population. Nutrients 2021, 13, 1241. [Google Scholar] [CrossRef]
- Gunter, M.J.; Murphy, N.; Cross, A.J.; Dossus, L.; Dartois, L.; Fagherazzi, G.; Kaaks, R.; Kühn, T.; Boeing, H.; Aleksandrova, K.; et al. Coffee drinking and mortality in 10 European countries: A multinational cohort study. Ann. Intern. Med. 2017, 167, 236–247. [Google Scholar] [CrossRef] [Green Version]
- Tsai, K.L.; Hung, C.H.; Chan, S.H.; Hsieh, P.L.; Ou, H.C.; Cheng, Y.H.; Chu, P.M. Chlorogenic Acid Protects Against oxLDL-Induced Oxidative Damage and Mitochondrial Dysfunction by Modulating SIRT1 in Endothelial Cells. Mol. Nutr. Food Res. 2018, 62, 1700928. [Google Scholar] [CrossRef]
- Hada, Y.; Uchida, H.A.; Otaka, N.; Onishi, Y.; Okamoto, S.; Nishiwaki, M.; Takemoto, R.; Takeuchi, H.; Wada, J. The protective effect of chlorogenic acid on vascular senescence via the Nrf2/HO-1 pathway. Int. J. Mol. Sci. 2020, 21, 4527. [Google Scholar] [CrossRef]
- Yang, L.; Wei, J.; Sheng, F.; Li, P. Attenuation of Palmitic Acid–Induced Lipotoxicity by Chlorogenic Acid through Activation of SIRT1 in Hepatocytes. Mol. Nutr. Food Res. 2019, 63, 1801432. [Google Scholar] [CrossRef]
- Wanke, V.; Cameroni, E.; Uotila, A.; Piccolis, M.; Urban, J.; Loewith, R.; De Virgilio, C. Caffeine extends yeast lifespan by targeting TORC1. Mol. Microbiol. 2008, 69, 277–285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, L.; Zhang, W.; Zhang, Y.; Zhang, M.; Zhang, Y.; Niu, X.; Zhao, Q.; Liu, Z.; Li, Y.; Diao, A. Caffeine promotes the expression of telomerase reverse transcriptase to regulate cellular senescence and aging. Food Funct. 2021, 12, 2914–2924. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Qian, L.; Zhang, J.; Zhang, W.; Morrison, A.; Hayes, P.; Wilson, S.; Chen, T.; Zhao, J. Sirt1 overexpression in neurons promotes neurite outgrowth and cell survival through inhibition of the mTOR signaling. J. Neurosci. Res. 2011, 89, 1723–1736. [Google Scholar] [CrossRef] [PubMed]
- Amano, H.; Chaudhury, A.; Rodriguez-Aguayo, C.; Lu, L.; Akhanov, V.; Catic, A.; Popov, Y.V.; Verdin, E.; Johnson, H.; Stossi, F.; et al. Telomere Dysfunction Induces Sirtuin Repression that Drives Telomere-Dependent Disease. Cell Metab. 2019, 29, 1274–1290.e9. [Google Scholar] [CrossRef]
- Xu, H.; Gan, C.; Gao, Z.; Huang, Y.; Wu, S.; Zhang, D.; Wang, X.; Sheng, J. Caffeine Targets SIRT3 to Enhance SOD2 Activity in Mitochondria. Front. Cell Dev. Biol. 2020, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Li, Y.F.; Wang, G.E.; Tan, R.R.; Tsoi, B.; Mao, G.W.; Zhai, Y.J.; Cao, L.F.; Chen, M.; Kurihara, H.; et al. Caffeine ameliorates high energy diet-induced hepatic steatosis: Sirtuin 3 acts as a bridge in the lipid metabolism pathway. Food Funct. 2015, 6, 2578–2587. [Google Scholar] [CrossRef]
- Urgert, R.; Katan, M.B. The cholesterol-raising factor from coffee beans. J. R. Soc. Med. 1996, 89, 618–623. [Google Scholar] [CrossRef] [Green Version]
- Godos, J.; Pluchinotta, F.R.; Marventano, S.; Buscemi, S.; Volti, G.L.; Galvano, F.; Grosso, G. Coffee components and cardiovascular risk: Beneficial and detrimental effects. Int. J. Food Sci. Nutr. 2014, 65, 925–936. [Google Scholar] [CrossRef]
- Superko, H.R.; Bortz, W.; Williams, P.T.; Albers, J.J.; Wood, P.D. Caffeinated and decaffeinated coffee effects on plasma lipoprotein cholesterol, apolipoproteins, and lipase activity: A controlled, randomized trial. Am. J. Clin. Nutr. 1991, 54, 599–605. [Google Scholar] [CrossRef]
- Uto-Kondo, H.; Ayaori, M.; Ogura, M.; Nakaya, K.; Ito, M.; Suzuki, A.; Takiguchi, S.I.; Yakushiji, E.; Terao, Y.; Ozasa, H.; et al. Coffee consumption enhances high-density lipoprotein-mediated cholesterol efflux in macrophages. Circ. Res. 2010, 106, 779–787. [Google Scholar] [CrossRef] [Green Version]
- Christensen, B.; Mosdol, A.; Retterstol, L.; Landaas, S.; Thelle, D.S. Abstention from filtered coffee reduces the concentrations of plasma homocysteine and serum cholesterol—A randomized controlled trial. Am. J. Clin. Nutr. 2001, 74, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Mioto, B.M.; Tarasoutchi, D.; Tuda, V.L.; César, L.A.M.; Torres, E.A.F.S. Paper-filtered coffee increases cholesterol and inflammation biomarkers independent of roasting degree: A clinical trial. Nutrition 2013, 29, 977–981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muzykiewicz-Szymańska, A.; Nowak, A.; Wira, D.; Klimowicz, A. The Effect of Brewing Process Parameters on Antioxidant Activity and Caffeine Content in Infusions of Roasted and Unroasted Arabica Coffee Beans Originated from Different Countries. Molecules 2021, 26, 3681. [Google Scholar] [CrossRef] [PubMed]
- Fears, R. The hypercholesterolaemic effect of caffeine in rats fed on diets with and without supplementary cholesterol. Br. J. Nutr. 1978, 39, 363–374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Liu, K.; Luo, H.; Xu, D.; Chen, L.; Zhang, L.; Wang, H. Caffeine programs hepatic SIRT1-related cholesterol synthesis and hypercholesterolemia via A2AR/cAMP/PKA pathway in adult male offspring rats. Toxicology 2019, 418, 11–21. [Google Scholar] [CrossRef]
- Hung, C.H.; Chan, S.H.; Chu, P.M.; Tsai, K.L. Homocysteine facilitates LOX-1 activation and endothelial death through the PKCβ and SIRT1/HSF1 mechanism: Relevance to human hyperhomocysteinaemia. Clin. Sci. 2015, 129, 477–487. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Wang, X.; Zhang, H.; Liu, E.; Su, X. Homocysteine up-regulates endothelin type A receptor in vascular smooth muscle cells through Sirt1/ERK1/2 signaling pathway. Microvasc. Res. 2017, 114, 34–40. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zhang, H.; Liu, E.; Xu, C.B.; Su, X. The sirt1/NF-kB signaling pathway is involved in regulation of endothelin type B receptors mediated by homocysteine in vascular smooth muscle cells. Biomed. Pharmacother. 2016, 84, 1979–1985. [Google Scholar] [CrossRef]
- Ganguly, P.; Alam, S.F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 2015, 14, 6. [Google Scholar] [CrossRef] [Green Version]
- Urgert, R.; Van Vliet, T.; Zock, P.L.; Katan, M.B. Heavy coffee consumption and plasma homocysteine: A randomized controlled trial in healthy volunteers. Am. J. Clin. Nutr. 2000, 72, 1107–1110. [Google Scholar] [CrossRef]
- Verhoef, P.; Pasman, W.J.; Van Vliet, T.; Urgert, R.; Katan, M.B. Contribution of caffeine to the homocysteine-raising effect of coffee: A randomized controlled trial in humans. Am. J. Clin. Nutr. 2002, 76, 1244–1248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mursu, J.; Voutilainen, S.; Nurmi, T.; Alfthan, G.; Virtanen, J.K.; Rissanen, T.H.; Happonen, P.; Nyyssönen, K.; Kaikkonen, J.; Salonen, R.; et al. The effects of coffee consumption on lipid peroxidation and plasma total homocysteine concentrations: A clinical trial. Free Radic. Biol. Med. 2005, 38, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Gonçalinho, G.H.F.; Sampaio, G.R.; Soares-Freitas, R.A.M.; Damasceno, N.R.T. Omega-3 Fatty Acids in Erythrocyte Membranes as Predictors of Lower Cardiovascular Risk in Adults without Previous Cardiovascular Events. Nutrients 2021, 13, 1919. [Google Scholar] [CrossRef] [PubMed]
- Kida, Y.; Goligorsky, M.S. Sirtuins, Cell Senescence, and Vascular Aging. Can. J. Cardiol. 2016, 32, 634–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, Y.; Chen, A.F.; Zhao, J.; Gu, Y.J.; Fu, G.X. Serum levels of cathepsin D, sirtuin1, and endothelial nitric oxide synthase are correlatively reduced in elderly healthy people. Aging Clin. Exp. Res. 2016, 28, 641–645. [Google Scholar] [CrossRef]
- Gok, O.; Karaali, Z.; Ergen, A.; Ekmekci, S.S.; Abaci, N. Serum sirtuin 1 protein as a potential biomarker for type 2 diabetes: Increased expression o sirtuin 1 and the correlation with microRNAs. J. Res. Med. Sci. 2019, 24, 1–5. [Google Scholar] [CrossRef]
- Kumar, R.; Chaterjee, P.; Sharma, P.K.; Singh, A.K.; Gupta, A.; Gill, K.; Tripathi, M.; Dey, A.B.; Dey, S. Sirtuin1: A Promising Serum Protein Marker for Early Detection of Alzheimer’s Disease. PLoS ONE 2013, 8, 4–9. [Google Scholar] [CrossRef] [Green Version]
| Blended Coffee Group | p | Arabica Coffee Group | p | Baseline p | Post-Treatment p | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Final | Baseline | Final | |||||||||
| n = 33 | n = 33 | n = 20 | n = 20 | |||||||||
| Variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Age, years | 45.5 | 12.2 | 45.5 | 12.2 | N/A | 48.7 | 14.1 | 48.7 | 14.1 | N/A | 0.392 | N/A |
| Sex (male/female), n (%) | 8/25 (24.2/75.8) | N/A | 5/15 (25.0/75.0) | N/A | N/A | N/A | ||||||
| Body weight, kg | 68.0 | 10.3 | 67.9 | 10.2 | 0.523 | 69.0 | 11.5 | 68.7 | 11.2 | 0.230 | 0.755 | 0.784 |
| Body mass index, kg/m2 | 26.9 | 4.1 | 27.0 | 4.1 | 0.230 | 26.2 | 3.9 | 26.1 | 3.8 | 0.132 | 0.517 | 0.399 |
| Total cholesterol, mmol/L | 4.70 | 0.79 | 5.17 | 0.89 | <0.001 | 5.01 | 1.19 | 4.82 | 1.36 | 0.279 | 0.269 | 0.268 |
| HDL-c, mmol/L | 1.26 | 0.23 | 1.36 | 0.28 | <0.001 | 1.38 | 0.33 | 1.36 | 0.27 | 0.515 | 0.118 | 0.972 |
| LDL-c, mmol/L | 2.98 | 0.65 | 3.32 | 0.73 | <0.001 | 3.21 | 1.02 | 3.18 | 1.08 | 0.693 | 0.316 | 0.570 |
| LDL-c/HDL-c | 2.46 | 0.78 | 2.55 | 0.80 | 0.120 | 2.41 | 0.83 | 2.41 | 0.89 | 0.959 | 0.815 | 0.554 |
| Triglycerides, mmol/L | 1.06 | 0.45 | 1.08 | 0.52 | 0.776 | 0.91 | 0.46 | 0.93 | 0.46 | 0.652 | 0.251 | 0.298 |
| Glucose, mmol/L | 4.8 | 0.51 | 4.87 | 0.56 | 0.336 | 4.96 | 0.45 | 5.03 | 0.39 | 0.355 | 0.251 | 0.253 |
| Insulin, µIU/mL * | 2.3 | 1.0; 4.2 | 3.1 | 1.0; 5.5 | 0.119 | 3.0 | 1.0; 3.9 | 3.6 | 1.0; 5.1 | 0.762 | 0.925 | 0.857 |
| HOMA-IR | 0.8 | 0.8 | 1.0 | 1.0 | 0.105 | 0.8 | 0.9 | 1.1 | 1.3 | 0.403 | 0.908 | 0.837 |
| hs-CRP, mg/L * | 1.3 | 1.0; 1.5 | 1.5 | 1.2; 2.3 | 0.477 | 0.8 | 0.6; 1.4 | 1.0 | 0.6; 1.7 | 0.447 | 0.598 | 0.163 |
| Lp(a), mg/dL * | 12.2 | 7.6; 20.0 | 14.0 | 7.2; 20.2 | 0.931 | 15.5 | 9.1; 27.4 | 15.9 | 10.1; 26.6 | 0.548 | 0.710 | 0.491 |
| Homocysteine, µmol/L | 8.5 | 3.1 | 8.4 | 3.4 | 0.893 | 7.9 | 2.1 | 7.8 | 2.2 | 0.856 | 0.438 | 0.456 |
| Folic acid, µ/L | 13.3 | 4.5 | 13.6 | 5.1 | 0.650 | 17.3 | 5.5 | 16.7 | 5.2 | 0.448 | 0.006 | 0.039 |
| Sirtuin-1, ng/mL | 0.40 | 0.13 | 0.49 | 0.16 | 0.003 | 0.51 | 0.10 | 0.58 | 0.14 | 0.004 | 0.003 | 0.043 |
| Change % | |||||
|---|---|---|---|---|---|
| Blended Coffee Group | Arabica Coffee Group | p | |||
| n = 33 | n = 20 | ||||
| Variables | % Mean Change | SD | % Mean Change | SD | |
| Body weight, kg | −0.2 | 1.6 | −0.3 | 1.3 | 0.738 |
| Body mass index, kg/m2 | 0.4 | 1.8 | −0.4 | 1.3 | 0.092 |
| Total cholesterol, mmol/L | 10.7 | 14.8 | −3.5 | 15.3 | 0.002 |
| HDL-c, mmol/L | 8.0 | 11.6 | −0.6 | 10.6 | 0.009 |
| LDL-c, mmol/L | 12.7 | 18.3 | −1.1 | 10.3 | 0.003 |
| LDL-c/HDL-c | 4.6 | 13.6 | −0.1 | 2.08 | 0.183 |
| Triglycerides, mmol/L | 9.8 | 47.9 | 9.4 | 37.8 | 0.975 |
| Glucose, mmol/L | 1.7 | 8.3 | 1.8 | 6.9 | 0.970 |
| Insulin, µIU/mL * | 0.0 | 0.0; 54.9 | 0.0 | −8.75; 21.4 | 0.448 |
| HOMA-IR | 7.9 | −0.7; 58.9 | 4.1 | −8.75; 23.0 | 0.509 |
| hs-CRP, mg/L * | 13.3 | −12.3; 56.9 | 10.0 | −7.6; 39.3 | 0.985 |
| Lp(a), mg/dL | 3.6 | 29.9 | −5.2 | 17.2 | 0.233 |
| Homocysteine, µmol/L | 1.9 | 29.5 | 1.5 | 22.2 | 0.956 |
| Folic acid, µ/L | 3.4 | 21.6 | −0.8 | 20.3 | 0.487 |
| Sirtuin-1, ng/mL * | 15.1 | 6.5; 29.5 | 15.8 | 5.5; 24.5 | 0.846 |
| Tests of Fixed Effects * | ||
|---|---|---|
| Outcome | F | p-Value |
| Sirt-1 | ||
| Intercept | 938.542 | <0.001 |
| Time | 17.641 | <0.001 |
| Coffee type | 9.071 | 0.004 |
| Time*Coffee type | 0.133 | 0.717 |
| Total Cholesterol | ||
| Intercept | 69.891 | <0.001 |
| Time | 2.270 | 0.138 |
| Coffee type | 0.192 | 0.663 |
| Time*Coffee type | 12.399 | 0.001 |
| LDL-c | ||
| Intercept | 28.211 | <0.001 |
| Time | 5.950 | 0.018 |
| Coffee type | 0.039 | 0.844 |
| Time*Coffee type | 8.992 | 0.004 |
| HDL-c | ||
| Intercept | 1382.005 | <0.001 |
| Time | 3.224 | 0.078 |
| Coffee type | 0.832 | 0.366 |
| Time*Coffee type | 8.495 | 0.005 |
| Estimate Fixed Effects | Pairwise Comparisons | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 95% CI | Mean Effect | 95% CI | |||||||
| Outcome | Estimate | SE | t | Lower | Upper | SE | Lower | Upper | |
| Sirt-1, ng/mL | |||||||||
| Intercept | 0.584 | 0.033 | 17.426 | 0.516 | 0.651 | ||||
| Time (pre vs. post) | −0.080 | 0.033 | −2.425 | −0.147 | −0.014 | 0.088 | 0.021 | 0.046 | 0.130 |
| Coffee type (Arabica vs. blend) | −0.090 | 0.042 | −2.112 | −0.175 | −0.005 | 0.097 | 0.032 | 0.032 | 0.162 |
| Time*Coffee type | −0.015 | 0.042 | −0.364 | -0.099 | 0.069 | ||||
| Total Cholesterol, mmol/L | |||||||||
| Intercept | 3.469 | 0.482 | 7.199 | 2.499 | 4.439 | ||||
| Time (pre vs. post) | 0.186 | 0.146 | 1.277 | −0.106 | 0.479 | 0.139 | 0.092 | −0.046 | 0.325 |
| Coffee type (Arabica vs. blend) | 0.434 | 0.281 | 1.548 | −0.129 | 0.998 | −0.109 | 0.249 | −0.608 | 0.390 |
| Time*Coffee type | −0.651 | 0.185 | −3.521 | −1.022 | −0.280 | ||||
| LDL-c, mmol/L | |||||||||
| Intercept | 1.871 | 0.397 | 4.719 | 1.075 | 2.668 | ||||
| Time (pre vs. post) | 0.035 | 0.098 | 0.355 | −0.163 | 0.232 | 0.152 | 0.062 | 0.027 | 0.277 |
| Coffee type (Arabica vs. blend) | 0.227 | 0.221 | 1.027 | −0.217 | 0.671 | −0.040 | 0.203 | −0.447 | 0.367 |
| Time*Coffee type | −0.374 | 0.125 | −2.999 | −0.625 | −0.124 | ||||
| HDL-c, mmol/L | |||||||||
| Intercept | 1.412 | 0.057 | 24.627 | 1.297 | 1.527 | ||||
| Time (pre vs. post) | 0.023 | 0.033 | 0.709 | −0.043 | 0.089 | 0.037 | 0.021 | −0.004 | 0.079 |
| Coffee type (Arabica vs. blend) | −0.001 | 0.070 | −0.008 | −0.140 | 0.139 | 0.061 | 0.067 | −0.073 | 0.196 |
| Time*Coffee type | −0.121 | 0.042 | −2.915 | −0.205 | −0.038 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalinho, G.H.F.; Nascimento, J.R.d.O.; Mioto, B.M.; Amato, R.V.; Moretti, M.A.; Strunz, C.M.C.; César, L.A.M.; Mansur, A.d.P. Effects of Coffee on Sirtuin-1, Homocysteine, and Cholesterol of Healthy Adults: Does the Coffee Powder Matter? J. Clin. Med. 2022, 11, 2985. https://doi.org/10.3390/jcm11112985
Gonçalinho GHF, Nascimento JRdO, Mioto BM, Amato RV, Moretti MA, Strunz CMC, César LAM, Mansur AdP. Effects of Coffee on Sirtuin-1, Homocysteine, and Cholesterol of Healthy Adults: Does the Coffee Powder Matter? Journal of Clinical Medicine. 2022; 11(11):2985. https://doi.org/10.3390/jcm11112985
Chicago/Turabian StyleGonçalinho, Gustavo Henrique Ferreira, José Rafael de Oliveira Nascimento, Bruno Mahler Mioto, Reynaldo Vicente Amato, Miguel Antonio Moretti, Célia Maria Cassaro Strunz, Luiz Antonio Machado César, and Antonio de Padua Mansur. 2022. "Effects of Coffee on Sirtuin-1, Homocysteine, and Cholesterol of Healthy Adults: Does the Coffee Powder Matter?" Journal of Clinical Medicine 11, no. 11: 2985. https://doi.org/10.3390/jcm11112985
APA StyleGonçalinho, G. H. F., Nascimento, J. R. d. O., Mioto, B. M., Amato, R. V., Moretti, M. A., Strunz, C. M. C., César, L. A. M., & Mansur, A. d. P. (2022). Effects of Coffee on Sirtuin-1, Homocysteine, and Cholesterol of Healthy Adults: Does the Coffee Powder Matter? Journal of Clinical Medicine, 11(11), 2985. https://doi.org/10.3390/jcm11112985






