Implementation of EHMRG Risk Model in an Italian Population of Elderly Patients with Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
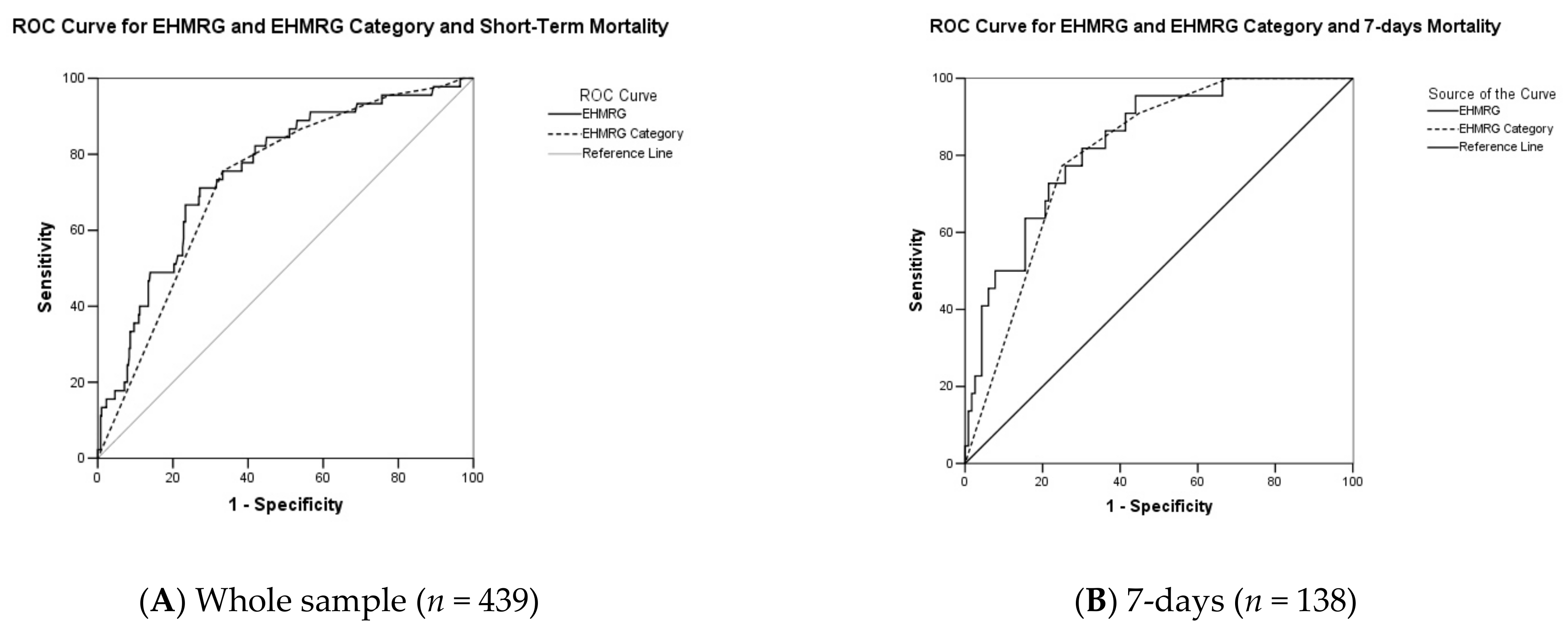
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Jencks, S.F.; Williams, M.V.; Coleman, E.A. Rehospitalizations among Patients in the Medicare Fee-for-Service Program. N. Engl. J. Med. 2009, 360, 1418–1428. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, J.E.; Tse, T. Heart failure: A global disease requiring a global response. Heart 2003, 89, 585–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, S.P.; Schauer, D.P.; Gupta, A.; Brunner, H.; Storrow, A.B.; Eckman, M.H. Cost-effectiveness analysis of ED decision making in patients with non-high-risk heart failure. Am. J. Emerg. Med. 2009, 27, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Ezekowitz, J.A.; Bakal, J.A.; Kaul, P.; Westerhout, C.M.; Armstrong, P.W. Acute heart failure in the emergency department: Short and long-term outcomes of elderly patients with heart failure. Eur. J. Heart Fail. 2008, 10, 308–314. [Google Scholar] [CrossRef] [Green Version]
- Butler, J.; Hanumanthu, S.; Chomsky, D.; Wilson, J.R. Frequency of low-risk hospital admissions for heart failure. Am. J. Cardiol. 1998, 81, 41–44. [Google Scholar] [CrossRef]
- Lee, D.S.; Schull, M.J.; Alter, D.A.; Austin, P.C.; Laupacis, A.; Chong, A.; Tu, J.V.; Stukel, T.A. Early deaths in patients with heart failure discharged from the emergency department: A population-based analysis. Circ. Heart Fail. 2010, 3, 228–235. [Google Scholar] [CrossRef] [Green Version]
- Peacock, W.F.; Braunwald, E.; Abraham, W.; Albert, N.; Burnett, J.; Christenson, R.; Collins, S.; Diercks, D.; Fonarow, G.; Hollander, J.; et al. National Heart, Lung, and Blood Institute working group on emergency department management of acute heart failure: Research challenges and opportunities. J. Am. Coll. Cardiol. 2010, 56, 343–351. [Google Scholar] [CrossRef] [Green Version]
- Gheorghiade, M.; Braunwald, E. A proposed model for initial assessment and management of acute heart failure syndromes. JAMA 2011, 305, 1702–1703. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Adams, K.F.; Abraham, W.T.; Yancy, C.W.; Boscardin, W.J.; ADHERE Scientific Advisory Committee, Study Group and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: Classification and regression tree analysis. JAMA 2005, 293, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Chin, M.H.; Goldman, L. Correlates of major complications or death in patients admitted to the hospital with congestive heart failure. Arch. Intern. Med. 1996, 156, 1814–1820. [Google Scholar] [CrossRef] [PubMed]
- Auble, T.E.; Hsieh, M.; Gardner, W.; Cooper, G.F.; Stone, R.A.; McCausland, J.B.; Yealy, D.M. A prediction rule to identify low-risk patients with heart failure. Acad. Emerg. Med. 2005, 12, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.S.; Austin, P.C.; Rouleau, J.L.; Liu, P.P.; Naimark, D.; Tu, J.V. Predicting mortality among patients hospitalized for heart failure: Derivation and validation of a clinical model. JAMA 2003, 290, 2581–2587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Justice, A.C.; Covinsky, K.E.; Berlin, J.A. Assessing the generalizability of prognostic information. Ann. Intern. Med. 1999, 130, 515–524. [Google Scholar] [CrossRef]
- Furlan, L.; Gianni, F.; Costantino, G. Prediction tools in clinical practice: Carefully read instructions before use. Eur. J. Intern. Med. 2022, 98, 37–38. [Google Scholar] [CrossRef]
- Adams, S.T.; Leveson, S.H. Clinical prediction rules. Bmj 2012, 344, d8312. [Google Scholar] [CrossRef] [Green Version]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Gattis, W.A.; Gheorghiade, M.; Greenberg, B.; O’Connor, C.M.; Yancy, C.W.; Young, J. Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure (OPTIMIZE-HF): Rationale and design. Am. Heart J. 2004, 148, 43–51. [Google Scholar] [CrossRef]
- Fonarow, G.C.; ADHERE Scientific Advisory Committee. The Acute Decompensated Heart Failure National Registry (ADHERE): Opportunities to improve care of patients hospitalized with acute decompensated heart failure. Rev. Cardiovasc. Med. 2003, 4 (Suppl. S7), S21–S30. [Google Scholar] [PubMed]
- Win, S.; Hussain, I.; Hebl, V.B.; Dunlay, S.M.; Redfield, M.M. Inpatient Mortality Risk Scores and Postdischarge Events in Hospitalized Heart Failure Patients. Circ. Heart Fail. 2017, 10, e003926. [Google Scholar] [CrossRef]
- Stiell, I.G.; Clement, C.M.; Brison, R.J.; Rowe, B.H.; Borgundvaag, B.; Aaron, S.D.; Lang, E.; Calder, L.A.; Perry, J.J.; Forster, A.J.; et al. A risk scoring system to identify emergency department patients with heart failure at high risk for serious adverse events. Acad. Emerg. Med. 2013, 20, 17–26. [Google Scholar] [CrossRef]
- Miró, Ò.; Rosselló, X.; Gil, V.; Martín-Sánchez, F.J.; Llorens, P.; Herrero, P.; Jacob, J.; López-Grima, M.L.; Gil, C.; Lucas Imbernón, F.J.; et al. The Usefulness of the MEESSI Score for Risk Stratification of Patients With Acute Heart Failure at the Emergency Department. Rev. Española Cardiol. (Engl. Ed.) 2019, 72, 198–207. [Google Scholar] [CrossRef]
- Lee, D.S.; Stitt, A.; Austin, P.C.; Stukel, T.A.; Schull, M.J.; Chong, A.; Newton, G.E.; Lee, J.S.; Tu, J.V. Prediction of heart failure mortality in emergent care: A cohort study. Ann. Intern. Med. 2012, 156, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Gil, V.; Miró, Ò.; Schull, M.J.; Llorens, P.; Herrero-Puente, P.; Jacob, J.; Ríos, J.; Lee, D.S.; Martín-Sánchez, F.J. Emergency Heart Failure Mortality Risk Grade score performance for 7-day mortality prediction in patients with heart failure attended at the emergency department: Validation in a Spanish cohort. Eur. J. Emerg. Med. 2018, 25, 169–177. [Google Scholar] [CrossRef]
- Falsetti, L.; Zaccone, V.; Viticchi, G.; Fioranelli, A.; Diblasi, I.; Guerrieri, E.; Ferrini, C.; Scarponi, M.; Giuliani, L.; Scalpelli, C.; et al. Improving the EHMRG Prognostic Evaluation of Acute Heart Failure with TAPSE/PASp: A Sequential Approach. Diagnostics 2022, 12, 478. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.-P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, L.; Proietti, M.; Zaccone, V.; Guerra, F.; Nitti, C.; Salvi, A.; Viticchi, G.; Riccomi, F.; Sampaolesi, M.; Silvestrini, M.; et al. Impact of atrial fibrillation in critically-ill patients admitted to a stepdown unit. Eur. J. Clin. Investig. 2020, 50, e13317. [Google Scholar] [CrossRef]
- Falsetti, L.; Rucco, M.; Proietti, M.; Viticchi, G.; Zaccone, V.; Scarponi, M.; Giovenali, L.; Moroncini, G.; Nitti, C.; Salvi, A. Risk prediction of clinical adverse outcomes with machine learning in a cohort of critically ill patients with atrial fibrillation. Sci. Rep. 2021, 11, 18925. [Google Scholar] [CrossRef]
| Variable | Units | Factor |
|---|---|---|
| Age | Years | 2 × age |
| ED arrival by ambulance | If “yes” | +60 |
| SBP | mmHg | −1 × SBP |
| Heart rate | beats/min | 1 × HR |
| Oxygen saturation | % | −2 × Oxygen Saturation |
| Creatinine | mg/dL | 20 × Creatinine |
| Serum potassium |
|
|
| Serum troponin | >ULN | +60 |
| Active cancer | If “yes” | +45 |
| Metolazone at home | If “yes” | +60 |
| Adjustment factor | +12 | |
| Total |
| Clinical Variables | Full Cohort (n = 439) | 7 Days (n = 138) |
|---|---|---|
| Age, years, (±SD) | 84.6 (±7.7) | 84.1 (± 8.3) |
| Males (n, %) | 180 (41.0%) | 64 (46.4%) |
| In-hospital death (n, %) | 45 (10.3%) | 22 (15.9%) |
| NYHA class, [IQR] | 4 [1] | 3 [1] |
| Length of hospitalization, days, [IQR] | 10 [7] | -- |
| BNP on admission, pg/mL, [IQR] | 600.5 [805] | 560.5 [846] |
| SBP, mmHg, (±SD) | 127.5 (±28.1) | 128.0 (±28.2) |
| HR, bpm, (±SD) | 89.4 (±24.6) | 90.4 (±23.9) |
| SpO2, %, (±SD) | 91.8 (±7.3) | 92.0 (±7.07) |
| Creatinine, mg/dl, (±SD) | 1.6 (±1.0) | 1.45 (±0.99) |
| Potassium, mmol/l, (±SD) | 4.00 (±0.69) | 4.04 (±0.65) |
| Out of range Potassium, (n, %) | 180 (41.1%) | 74 (53.6%) |
| Troponin, ng/mL, [IQR] | 0.05 [0.10] | 0.05 [0.11] |
| Increased troponin, (n, %) | 204 (46.5%) | 63 (45.7%) |
| ED arrival by ambulance, (n, %) | 284 (64.7%) | 83 (60.1%) |
| Active cancer, (n, %) | 77 (17.9%) | 16 (11.6%) |
| Metolazone use, (n, %) | 11 (2.6%) | 1 (0.72%) |
| EHMRG, [IQR] | 69 [98.4] | 60,8 [99.3] |
| EHMRG Class, [IQR] | 5 [2] | 5 [3] |
| AHF characteristics | ||
| ADHF (n, %) | 370 (84.2%) | 109 (78.9%) |
AHF de novo (n, %)
|
|
|
| EHMRG Category In-Hospital Death | Full Sample (n = 439) | 7-Days Observation (n = 138) |
|---|---|---|
| EHMRG Category 1 (n, %) | 0 (0.0%) | 0 (0.0%) |
| EHMRG Category 2 (n, %) | 1 (0.2%) | 0 (0.0%) |
| EHMRG Category 3 (n, %) | 1 (0.2%) | 0 (0.0%) |
| EHMRG Category 4 (n, %) | 4 (4.1%) | 2 (1.4%) |
| EHMRG Category 5a (n, %) | 5 (1.1%) | 3 (2.2%) |
| EHMRG Category 5b (n, %) | 34 (7.7%) | 17 (12.3%) |
| Total | 45 (10.3%) | 22 (15.9%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Falsetti, L.; Zaccone, V.; Guerrieri, E.; Perrotta, G.; Diblasi, I.; Giuliani, L.; Palma, L.E.G.; Viticchi, G.; Fioranelli, A.; Moroncini, G.; et al. Implementation of EHMRG Risk Model in an Italian Population of Elderly Patients with Acute Heart Failure. J. Clin. Med. 2022, 11, 2982. https://doi.org/10.3390/jcm11112982
Falsetti L, Zaccone V, Guerrieri E, Perrotta G, Diblasi I, Giuliani L, Palma LEG, Viticchi G, Fioranelli A, Moroncini G, et al. Implementation of EHMRG Risk Model in an Italian Population of Elderly Patients with Acute Heart Failure. Journal of Clinical Medicine. 2022; 11(11):2982. https://doi.org/10.3390/jcm11112982
Chicago/Turabian StyleFalsetti, Lorenzo, Vincenzo Zaccone, Emanuele Guerrieri, Giulio Perrotta, Ilaria Diblasi, Luca Giuliani, Linda Elena Gialluca Palma, Giovanna Viticchi, Agnese Fioranelli, Gianluca Moroncini, and et al. 2022. "Implementation of EHMRG Risk Model in an Italian Population of Elderly Patients with Acute Heart Failure" Journal of Clinical Medicine 11, no. 11: 2982. https://doi.org/10.3390/jcm11112982
APA StyleFalsetti, L., Zaccone, V., Guerrieri, E., Perrotta, G., Diblasi, I., Giuliani, L., Palma, L. E. G., Viticchi, G., Fioranelli, A., Moroncini, G., Pansoni, A., Luccarini, M., Martino, M., Scalpelli, C., Burattini, M., & Tarquinio, N. (2022). Implementation of EHMRG Risk Model in an Italian Population of Elderly Patients with Acute Heart Failure. Journal of Clinical Medicine, 11(11), 2982. https://doi.org/10.3390/jcm11112982








