Prognostic Implications of a Modified Seattle Heart Failure Model Score Following Transcatheter Aortic Valve Replacement
Abstract
:1. Background
2. Methods
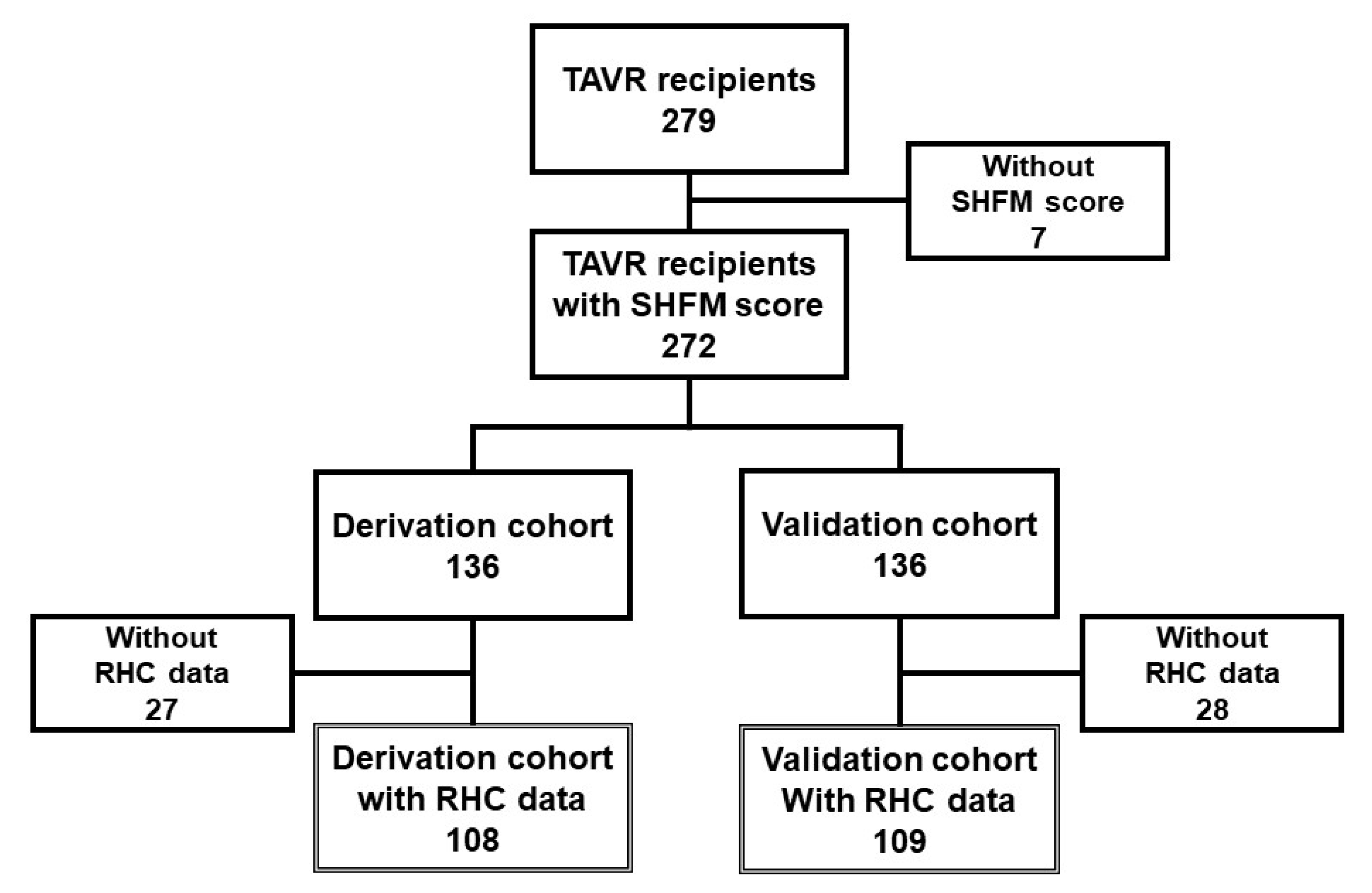
2.1. Patient Selection
2.2. TAVR Procedure
2.3. Clinical Variables
2.4. Clinical Outcomes
2.5. Study Protocol
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
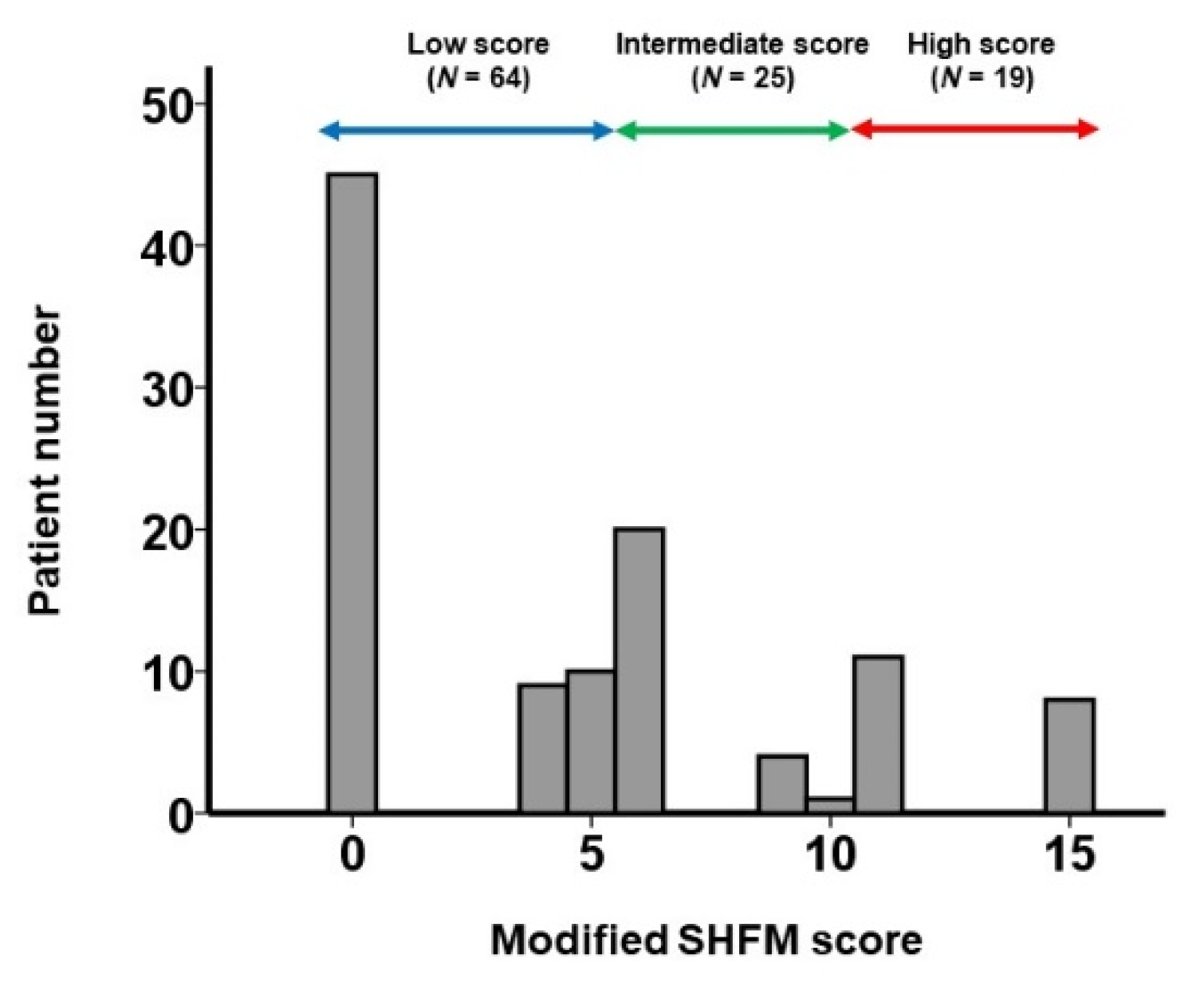
3.2. Derivation of Modified SHFM Score
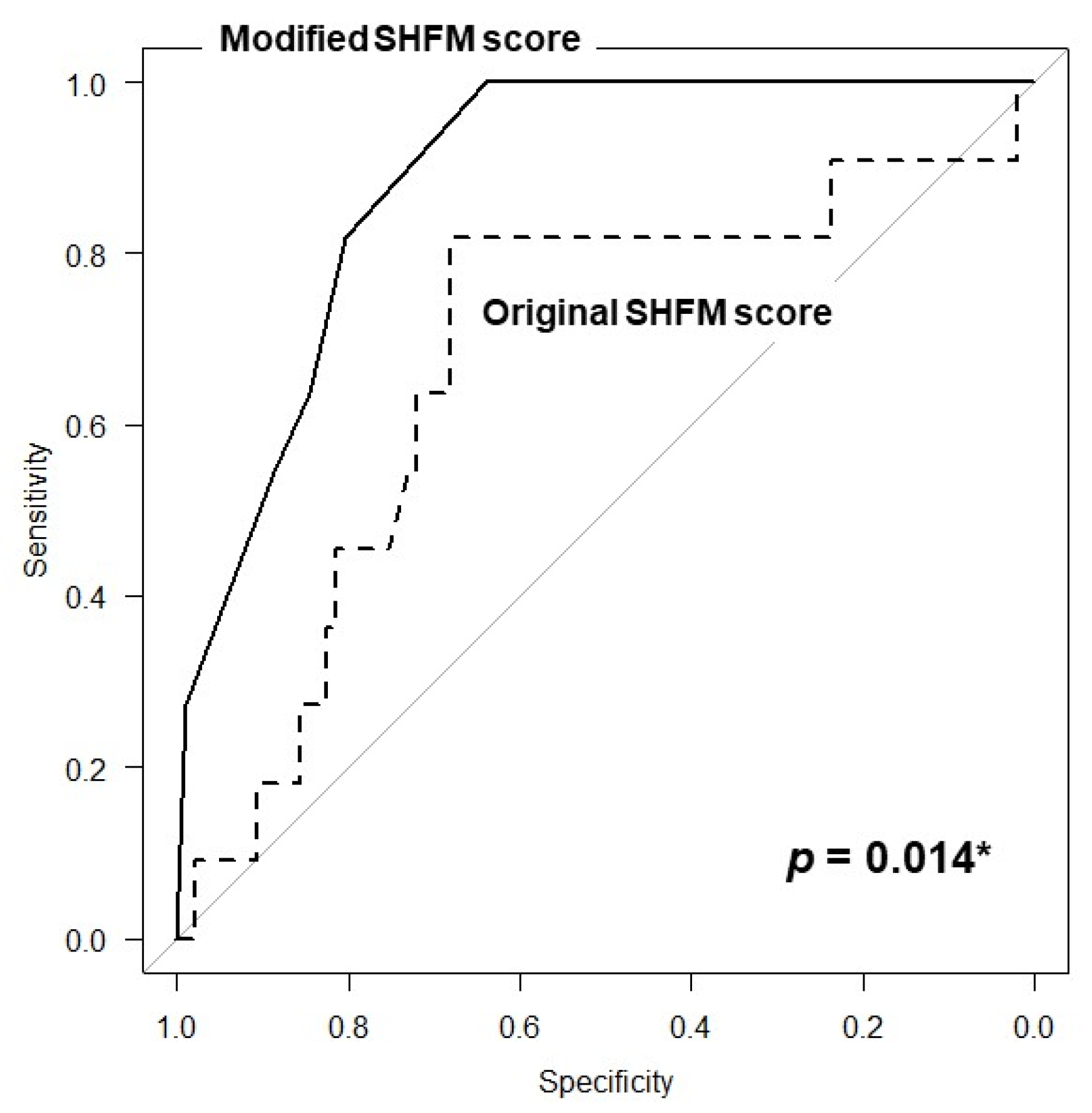
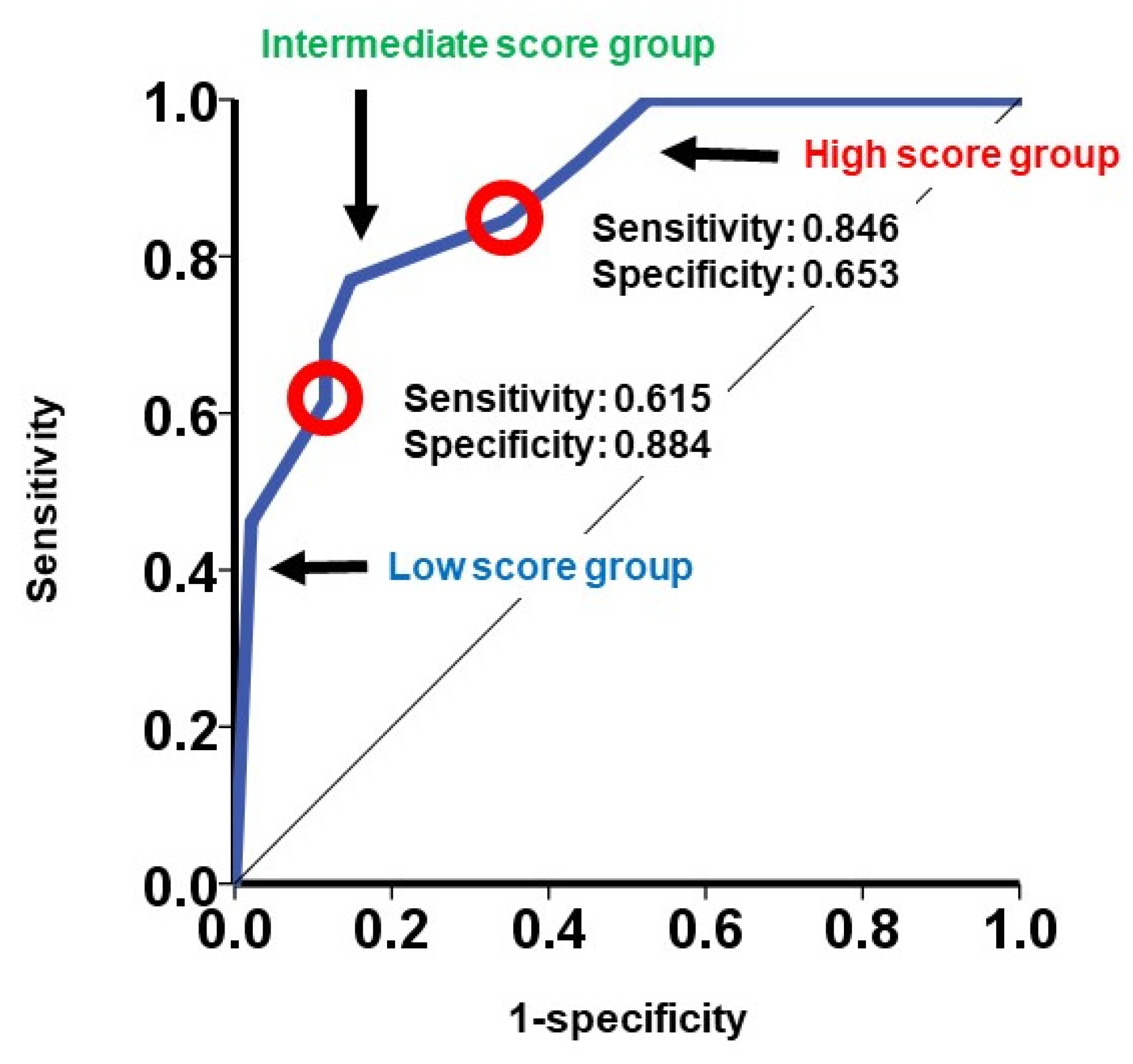
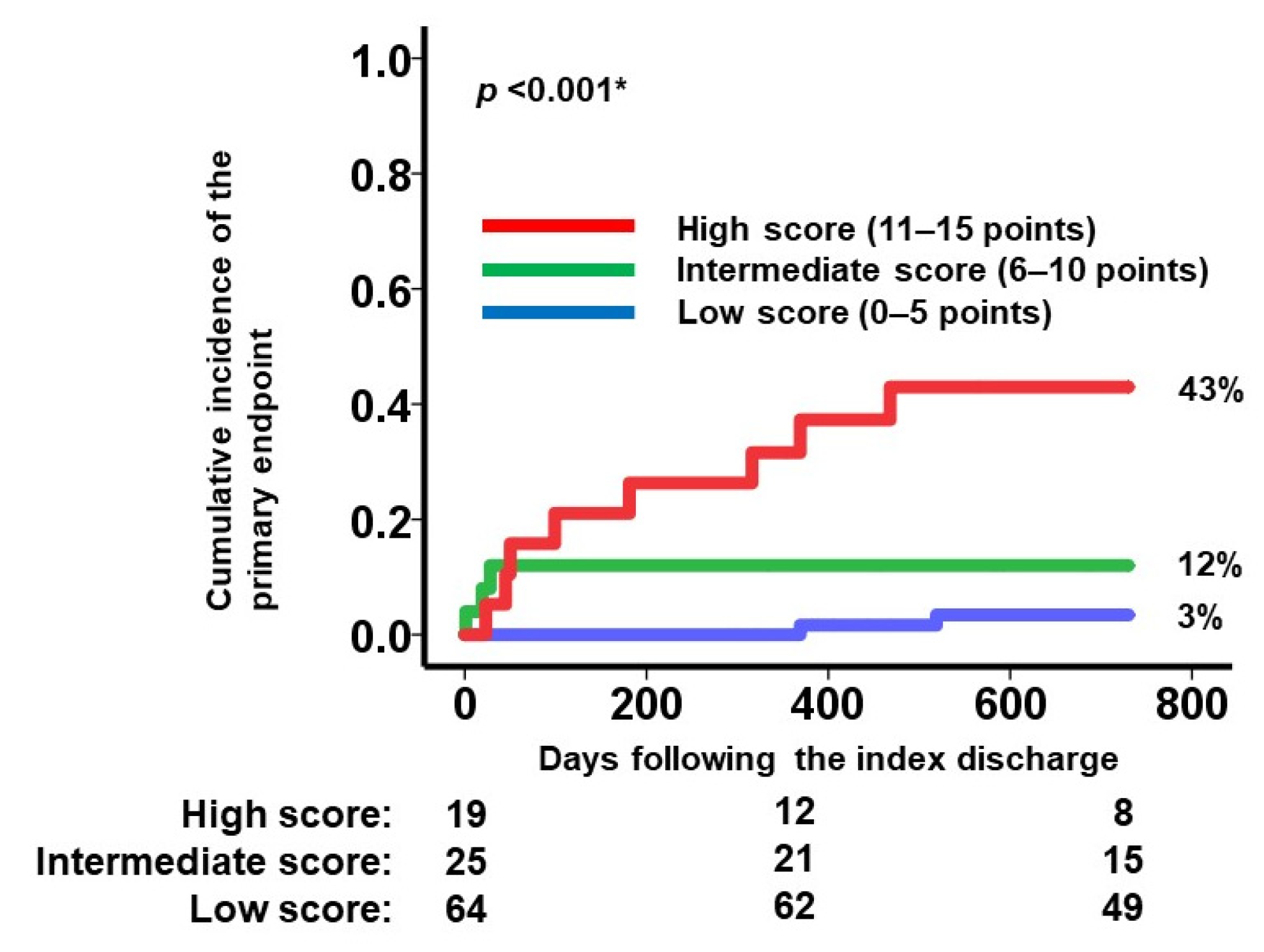
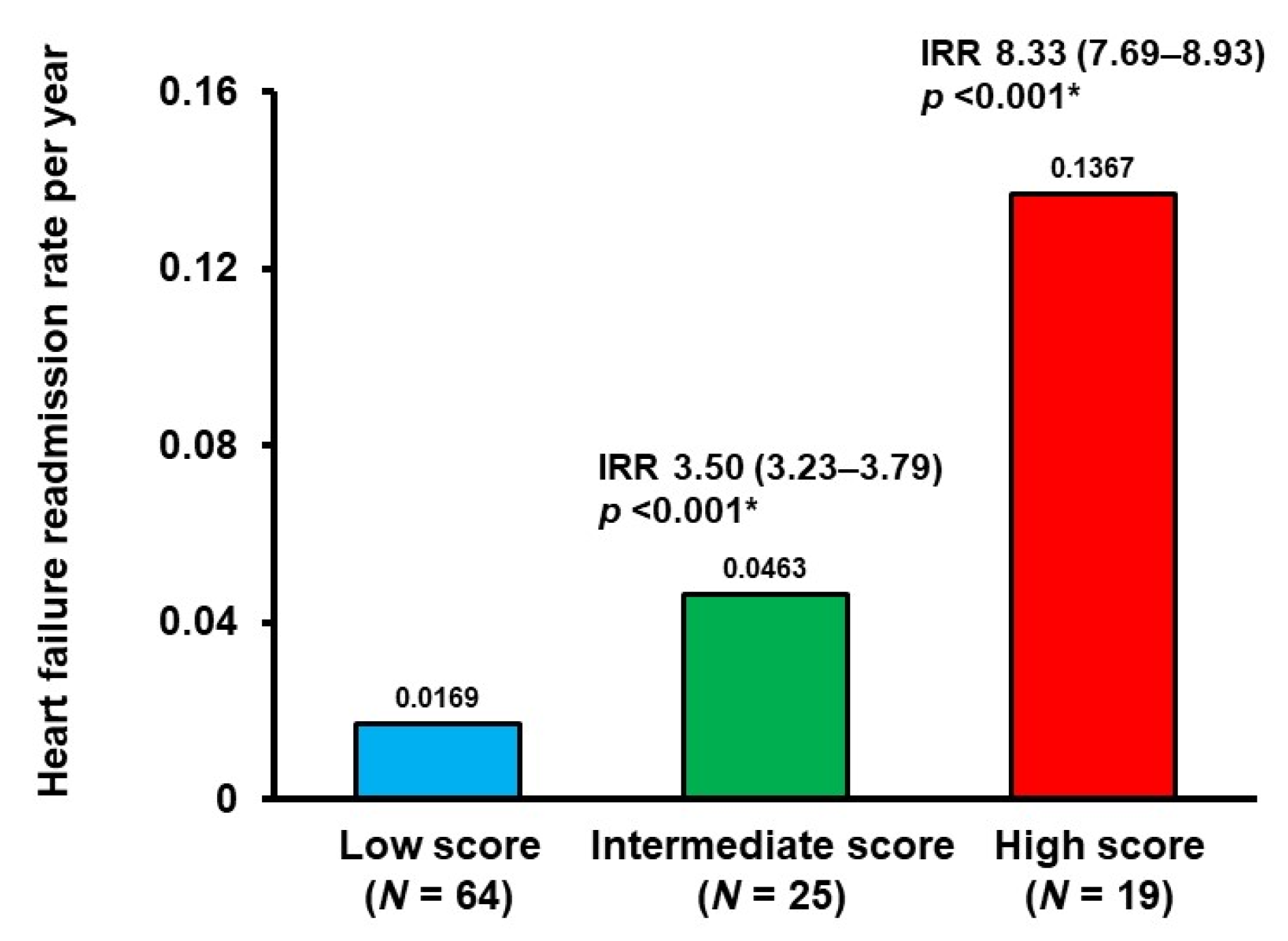
3.3. Validation of the Modified SHFM Score
4. Discussion
4.1. SHFM Score
4.2. Modified SHFM Score
4.3. Clinical Implications of the Modified SHFM Score
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leon, M.B.; Smith, C.R.; Mack, M.J.; Makkar, R.R.; Svensson, L.G.; Kodali, S.K.; Thourani, V.H.; Tuzcu, E.M.; Miller, D.C.; Herrmann, H.C.; et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med. 2016, 374, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter Aortic-Valve Replacement with a Balloon-Expandable Valve in Low-Risk Patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, C.E.; Hermiller, J.B.; Pinto, D.S., Jr.; Chetcuti, S.J.; Arshi, A.; Forrest, J.K.; Huang, J.; Yakubov, S.J. Predictors and Risk Calculator of Early Unplanned Hospital Readmission Following Contemporary Self-Expanding Transcatheter Aortic Valve Replacement from the STS/ACC TVT Registry. Cardiovasc. Revasc Med. 2020, 21, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Hamm, C.W.; Arsalan, M.; Mack, M.J. The future of transcatheter aortic valve implantation. Eur. Heart J. 2016, 37, 803–810. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The Seattle Heart Failure Model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Pamboukian, S.V.; Tallaj, J.A.; Brown, R.N.; Nielsen, T.; George, J.F.; Kirklin, J.K.; Holman, W.L.; Levy, W. Comparison of observed survival after ventricular assist device placement versus predicted survival without assist device using the Seattle heart failure model. ASAIO J. 2012, 58, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, Y.; Kohsaka, S.; Nagai, T.; Goda, A.; Mizuno, A.; Nagatomo, Y.; Sujino, Y.; Fukuoka, R.; Sawano, M.; Kohno, T.; et al. Validation and Recalibration of Seattle Heart Failure Model in Japanese Acute Heart Failure Patients. J. Card. Fail. 2019, 25, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.B.; Mentz, R.J.; Stevens, S.R.; Felker, G.M.; Lombardi, C.; Metra, M.; Stevenson, L.W.; O’Connor, C.M.; Milano, C.A.; Patel, C.B.; et al. Hemodynamic Predictors of Heart Failure Morbidity and Mortality: Fluid or Flow? J. Card. Fail. 2016, 22, 182–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Samad, Z.; Bloomfield, G.S.; Douglas, P.S. A critical review of hemodynamic changes and left ventricular remodeling after surgical aortic valve replacement and percutaneous aortic valve replacement. Am. Heart J. 2014, 168, 150–159.e1-7. [Google Scholar] [CrossRef] [PubMed]
- Shimura, T.; Yamamoto, M.; Yamaguchi, R.; Adachi, Y.; Sago, M.; Tsunaki, T.; Kagase, A.; Koyama, Y.; Otsuka, T.; Yashima, F.; et al. Calculated plasma volume status and outcomes in patients undergoing transcatheter aortic valve replacement. ESC Heart Fail. 2021, 8, 1990–2001. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Narang, N.; Sobajima, M.; Tanaka, S.; Ushijima, R.; Fukuda, N.; Ueno, H.; Kinugawa, K. Inadequate Cardiac Unloading Following Transcatheter Aortic Valve Replacement. Circ. Rep. 2021, 3, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; Codner, P.; Landes, U.; Barbanti, M.; Valvo, R.; De Backer, O.; Ooms, J.F.; Sievert, K.; El Sabbagh, A.; Jimenez-Quevedo, P.; et al. Transcatheter Treatment of Residual Significant Mitral Regurgitation Following TAVR: A Multicenter Registry. JACC Cardiovasc. Interv. 2020, 13, 2782–2791. [Google Scholar] [CrossRef] [PubMed]
- Sathananthan, J.; Murdoch, D.J.; Lindman, B.R.; Zajarias, A.; Jaber, W.A.; Cremer, P.; Wood, D.; Moss, R.; Cheung, A.; Ye, J.; et al. Implications of Concomitant Tricuspid Regurgitation in Patients Undergoing Transcatheter Aortic Valve Replacement for Degenerated Surgical Aortic Bioprosthesis: Insights From the PARTNER 2 Aortic Valve-in-Valve Registry. JACC Cardiovasc. Interv. 2018, 11, 1154–1160. [Google Scholar] [CrossRef]
- Otto, C.M. Informed Shared Decisions for Patients with Aortic Stenosis. N. Engl. J. Med. 2019, 380, 1769–1770. [Google Scholar] [CrossRef]
- Mantovani, F.; Clavel, M.A.; Potenza, A.; Leuzzi, C.; Grimaldi, T.; Vignali, L.; Navazio, A.; Guiducci, V. Balloon aortic valvuloplasty as a palliative treatment in patients with severe aortic stenosis and limited life expectancy: A single center experience. Aging 2020, 12, 16597–16608. [Google Scholar] [CrossRef]
| Total (N = 217) | Derivation Cohort (N = 108) | Validation Cohort (N = 109) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 86 (83, 88) | 86 (83, 88) | 85 (82, 88) | 0.56 |
| Men | 64 (29%) | 33 (31%) | 31 (28%) | 0.49 |
| Body surface area, m2 | 1.38 (1.28, 1.52) | 1.38 (1.26, 1.49) | 1.39 (1.30, 1.53) | 0.65 |
| Systolic blood pressure, mmHg | 114 (103, 125) | 111 (101, 123) | 115 (106, 126) | 0.10 |
| Pulse rate, bpm | 69 (61, 78) | 68 (61, 75) | 70 (61, 79) | 0.34 |
| Comorbidity | ||||
| Atrial fibrillation | 26 (12%) | 10 (9%) | 16 (15%) | 0.22 |
| Diabetes mellitus | 40 (18%) | 23 (21%) | 17 (16%) | 0.17 |
| Ischemic heart disease | 57 (26%) | 25 (23%) | 32 (29%) | 0.21 |
| History of stroke | 36 (17%) | 17 (16%) | 19 (17%) | 0.45 |
| History of heart failure admission | 91 (42%) | 49 (45%) | 42 (39%) | 0.17 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 11.0 (10.0, 12.1) | 10.9 (10.0, 12.3) | 11.0 (9.8, 11.9) | 0.45 |
| Serum albumin, g/dL | 3.8 (3.5, 4.0) | 3.8 (3.6, 4.0) | 3.8 (3.5, 4.1) | 0.60 |
| Serum sodium, mEq/L | 141 (139, 142) | 140 (139, 142) | 141 (139, 142) | 0.66 |
| Serum potassium, mEq/L | 4.4 (4.1, 4.6) | 4.4 (4.1, 4.7) | 4.3 (4.0, 4.6) | 0.34 |
| Estimated glomerular filtration ratio, mL/min/m2 | 50 (37, 62) | 50 (36, 61) | 50 (40, 62) | 0.52 |
| Plasma B-type natriuretic peptide, pg/mL | 271 (125, 514) | 230 (127, 455) | 294 (123, 596) | 0.31 |
| Echocardiography | ||||
| Left ventricular end diastolic diameter, mm | 46 (42, 51) | 46 (42, 50) | 46 (42, 52) | 0.93 |
| Left ventricular ejection fraction, % | 65 (54, 70) | 64 (56, 69) | 65 (53, 70) | 0.80 |
| Peak velocity at aortic valve, m/s | 4.5 (4.0, 4.9) | 4.5 (4.0, 4.9) | 4.4 (4.1, 4.8) | 0.98 |
| Mean pressure gradient at aortic valve, mmHg | 47 (38, 57) | 46 (38, 58) | 47 (39, 57) | 0.91 |
| Hemodynamics | ||||
| Mean right atrial pressure, mmHg | 5 (3, 7) | 5 (3, 7) | 6 (3, 8) | 0.65 |
| Mean pulmonary artery pressure, mmHg | 19 (16, 23) | 18 (15, 23) | 19 (16, 24) | 0.27 |
| Pulmonary capillary wedge pressure, mmHg | 12 (9, 16) | 11 (8, 15) | 13 (9, 16) | 0.29 |
| Cardiac index, L/min/m2 | 2.7 (2.4, 3.0) | 2.6 (2.3, 3.1) | 2.7 (2.4, 3.0) | 0.70 |
| Medication | ||||
| Beta-blocker | 71 (33%) | 39 (36%) | 32 (29%) | 0.16 |
| Angiotensin converting enzyme II inhibitor | 37 (17%) | 16 (15%) | 21 (19%) | 0.27 |
| Mineralocorticoid receptor antagonist | 59 (27%) | 33 (31%) | 26 (24%) | 0.15 |
| Loop diuretics | 123 (57%) | 63 (58%) | 60 (55%) | 0.31 |
| Univariate Analyses | Multivariate Analyses | |||
|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | p Value | Hazard Ratio (95% Confidence Interval) | p Value | |
| Continuous variables | ||||
| Original SHFM score | 0.95 (0.89–1.02) | 0.098 | ||
| Mean right atrial pressure, mmHg | 1.06 (0.86–1.30) | 0.57 | ||
| Mean pulmonary artery pressure, mmHg | 1.05 (0.97–1.13) | 0.27 | ||
| Pulmonary capillary wedge pressure, mmHg | 1.07 (0.99–1.15) | 0.052 | ||
| Cardiac index, L/min/m2 | 0.19 (0.07–0.52) | 0.001 * | ||
| Dichotomized variables | ||||
| Original SHFM score < 88.1% | 7.99 (1.73–37.0) | 0.008 * | 5.90 (1.27–27.4) | 0.024 * |
| Mean capillary wedge pressure > 14 mmHg | 7.83 (2.08–29.6) | 0.002 * | 4.70 (1.24–17.9) | 0.023 * |
| Cardiac index < 2.26 L/min/m2 | 6.61 (2.02–21.7) | 0.002 * | 4.49 (1.36–14.8) | 0.014 * |
| Novel combination variable | ||||
| Modified SHFM score | 1.38 (1.18–1.61) | <0.001 * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imamura, T.; Narang, N.; Onoda, H.; Tanaka, S.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Prognostic Implications of a Modified Seattle Heart Failure Model Score Following Transcatheter Aortic Valve Replacement. J. Clin. Med. 2021, 10, 5807. https://doi.org/10.3390/jcm10245807
Imamura T, Narang N, Onoda H, Tanaka S, Ushijima R, Sobajima M, Fukuda N, Ueno H, Kinugawa K. Prognostic Implications of a Modified Seattle Heart Failure Model Score Following Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2021; 10(24):5807. https://doi.org/10.3390/jcm10245807
Chicago/Turabian StyleImamura, Teruhiko, Nikhil Narang, Hiroshi Onoda, Shuhei Tanaka, Ryuichi Ushijima, Mitsuo Sobajima, Nobuyuki Fukuda, Hiroshi Ueno, and Koichiro Kinugawa. 2021. "Prognostic Implications of a Modified Seattle Heart Failure Model Score Following Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 10, no. 24: 5807. https://doi.org/10.3390/jcm10245807
APA StyleImamura, T., Narang, N., Onoda, H., Tanaka, S., Ushijima, R., Sobajima, M., Fukuda, N., Ueno, H., & Kinugawa, K. (2021). Prognostic Implications of a Modified Seattle Heart Failure Model Score Following Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 10(24), 5807. https://doi.org/10.3390/jcm10245807







