Osteopathic Manipulation of the Sphenopalatine Ganglia Versus Sham Manipulation, in Obstructive Sleep Apnoea Syndrom: A Randomised Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
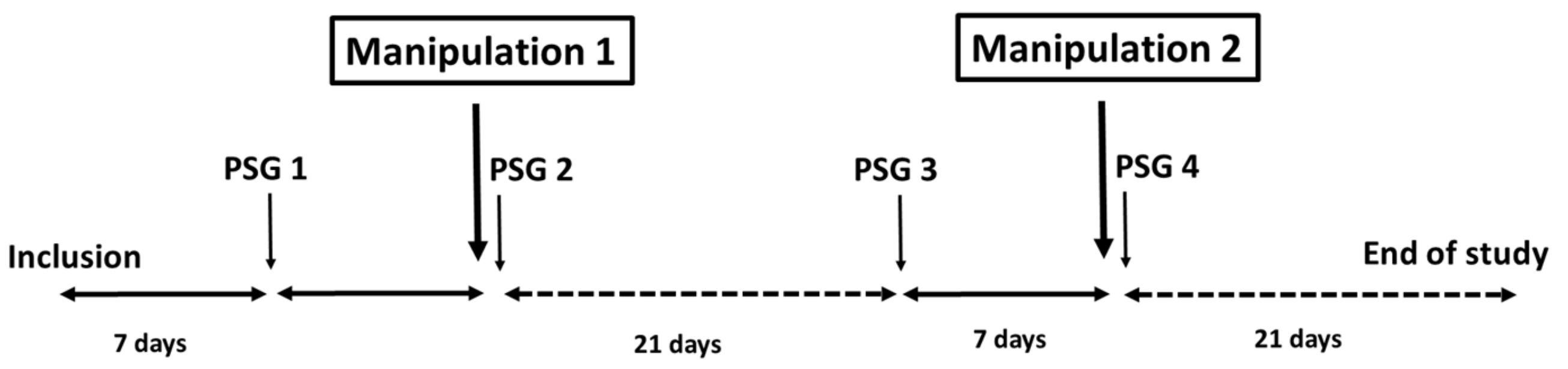
2.1. Study Design and Participants
2.2. Study Procedures
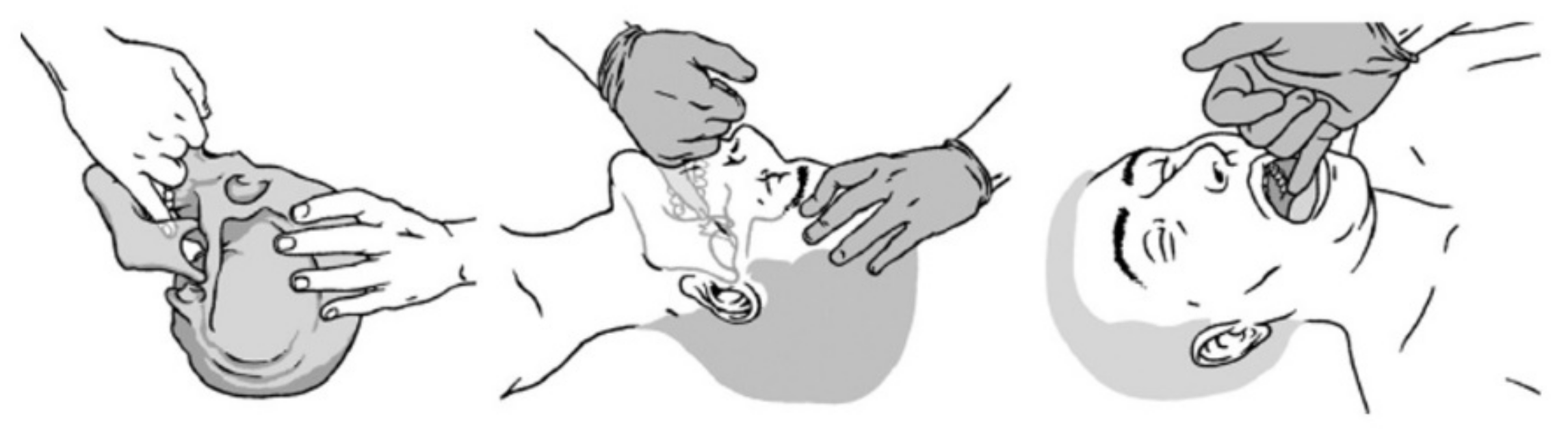
2.3. Interventions
2.4. Outcomes
2.4.1. Primary Endpoint
2.4.2. Secondary Endpoints
2.5. Randomisation and Masking
2.6. Statistical Analysis
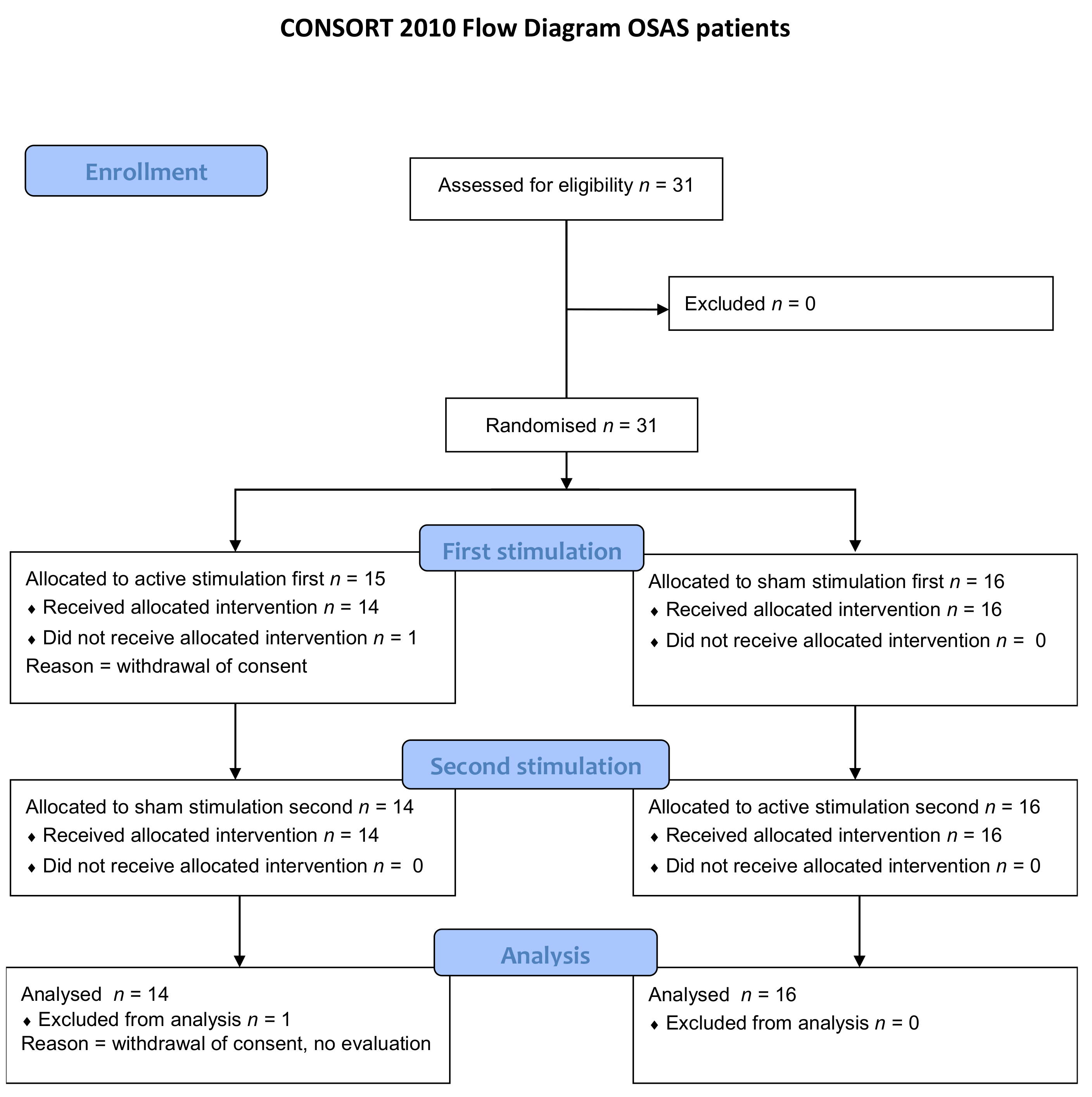
3. Results
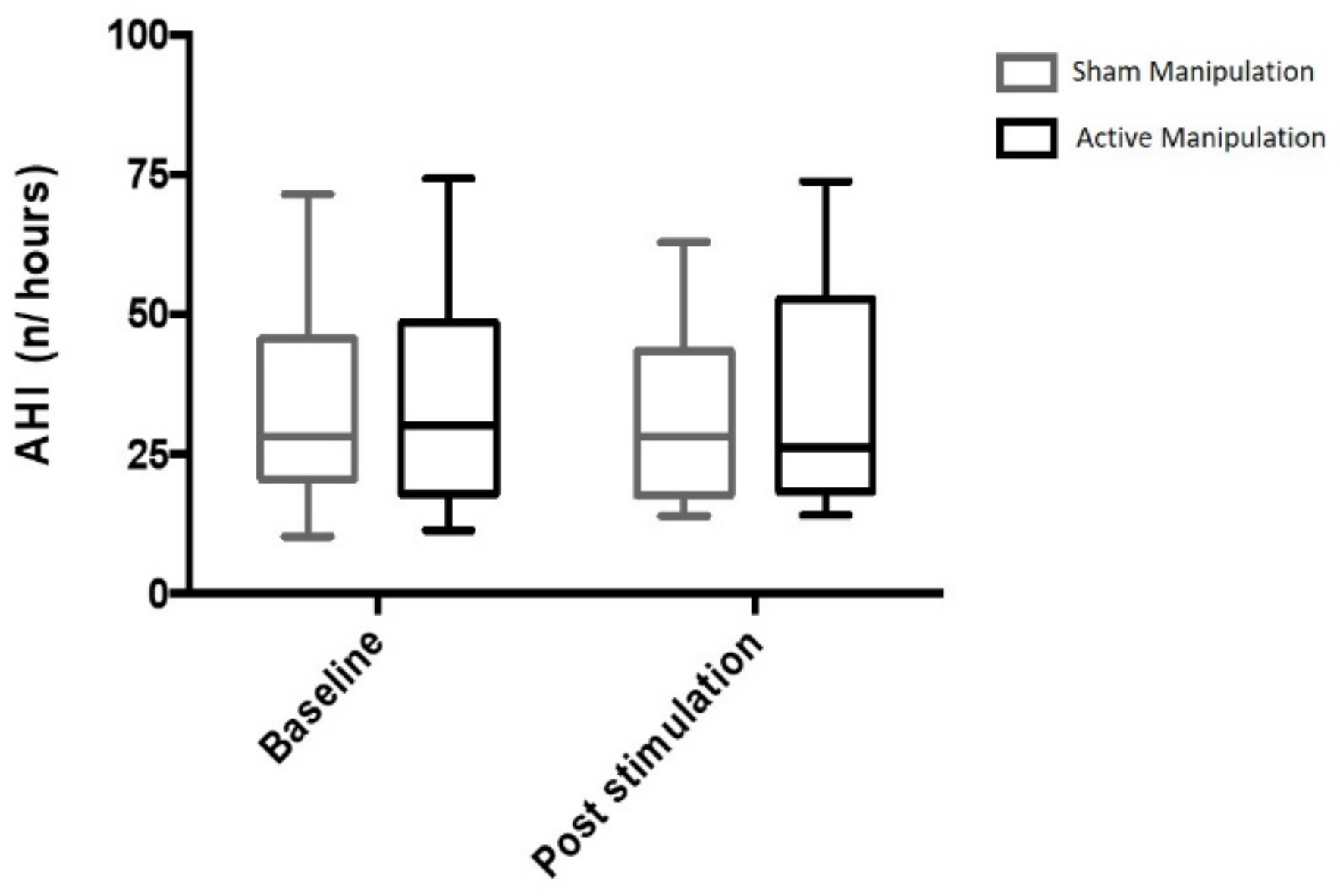
3.1. Primary Endpoint
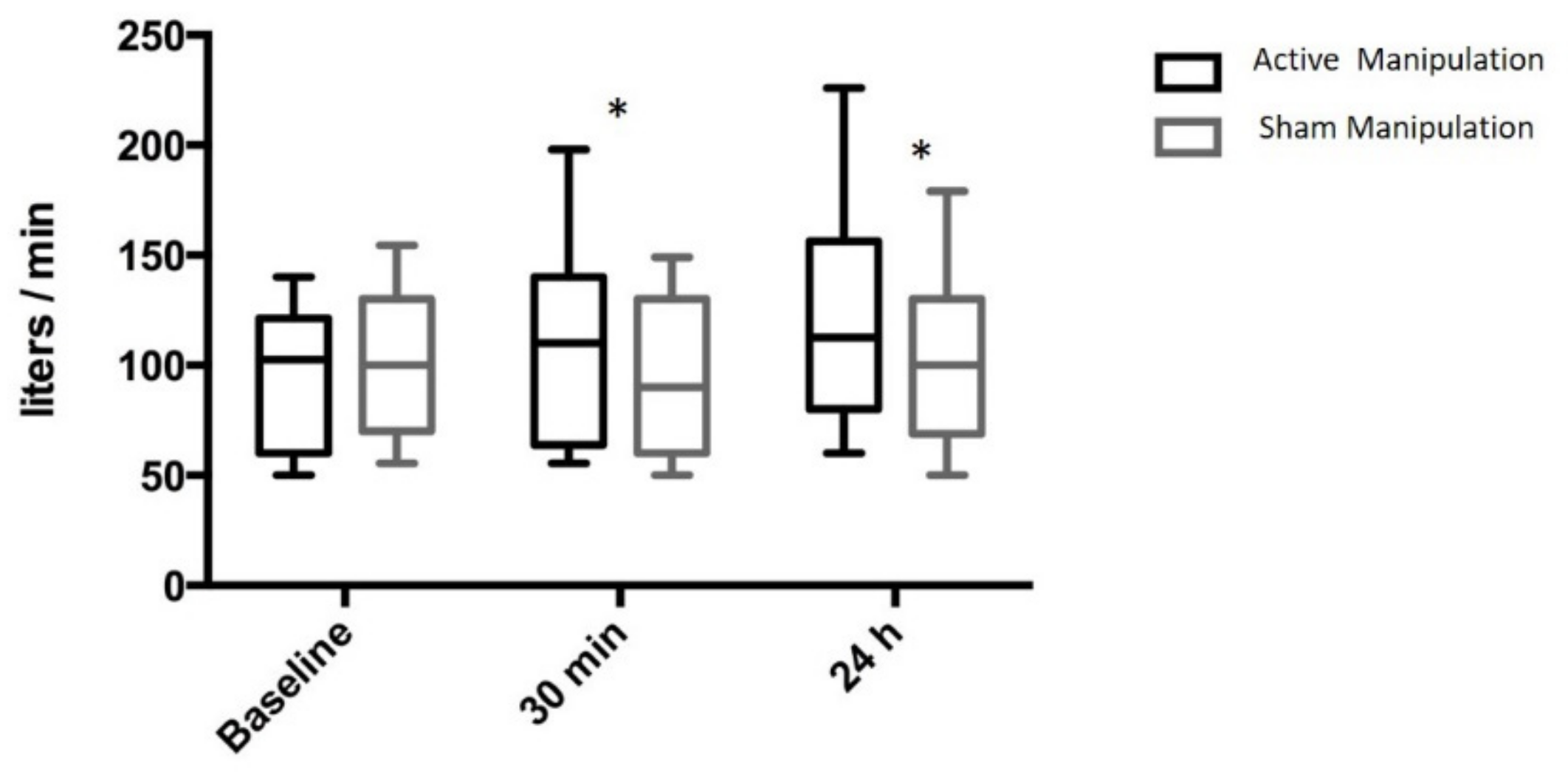
3.2. Secondary Endpoints
3.3. Adverse Effects
4. Discussion
4.1. Effects of AM on the Upper Airways and OSAS
4.2. Mechanism of Action of AM
4.3. Methodological Considerations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, P.; Kohler, M.; McNicholas, W.T.; Barbe, F.; McEvoy, R.D.; Somers, V.K.; Lavie, L.; Pepin, J.L. Obstructive sleep apnoea syndrome. Nat. Rev. Dis. Primers 2015, 1, 15015. [Google Scholar] [CrossRef] [PubMed]
- Clavel, L.; Remy-Neris, S.; Skalli, W.; Rouch, P.; Lespert, Y.; Similowski, T.; Sandoz, B.; Attali, V. Cervical Spine Hyperextension and Altered Posturo-Respiratory Coupling in Patients With Obstructive Sleep Apnea Syndrome. Front. Med. 2020, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Heinzer, R.; Marti-Soler, H.; Haba-Rubio, J. Prevalence of sleep apnoea syndrome in the middle to old age general population. Lancet Respir. Med. 2016, 4, e5–e6. [Google Scholar] [CrossRef]
- Wolkove, N.; Baltzan, M.; Kamel, H.; Dabrusin, R.; Palayew, M. Long-term compliance with continuous positive airway pressure in patients with obstructive sleep apnea. Can. Respir. J. 2008, 15, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Eckert, D.J.; White, D.P.; Jordan, A.S.; Malhotra, A.; Wellman, A. Defining phenotypic causes of obstructive sleep apnea. Identification of novel therapeutic targets. Am. J. Respir. Crit. Care Med. 2013, 188, 996–1004. [Google Scholar] [CrossRef]
- Launois, C.; Attali, V.; Georges, M.; Raux, M.; Morawiec, E.; Rivals, I.; Arnulf, I.; Similowski, T. Cortical Drive to Breathe during Wakefulness in Patients with Obstructive Sleep Apnea Syndrome. Sleep 2015, 38, 1743–1749. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Barbé, F.; Gozal, D.; Agustí, A. Precision medicine in obstructive sleep apnoea. Lancet Respir. Med. 2019, 7, 456–464. [Google Scholar] [CrossRef]
- Messineo, L.; Eckert, D.J.; Lim, R.; Chiang, A.; Azarbarzin, A.; Carter, S.G.; Carberry, J.C. Zolpidem increases sleep efficiency and the respiratory arousal threshold without changing sleep apnoea severity and pharyngeal muscle activity. J. Physiol. 2020, 598, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Messineo, L.; Magri, R.; Corda, L.; Pini, L.; Taranto-Montemurro, L.; Tantucci, C. Phenotyping-based treatment improves obstructive sleep apnea symptoms and severity: A pilot study. Sleep Breath 2017, 21, 861–868. [Google Scholar] [CrossRef]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef]
- Jacq, O.; Arnulf, I.; Similowski, T.; Attali, V. Upper airway stabilization by osteopathic manipulation of the sphenopalatine ganglion versus sham manipulation in OSAS patients: A proof-of-concept, randomized, crossover, double-blind, controlled study. BMC Complement. Altern. Med. 2017, 17, 546. [Google Scholar] [CrossRef] [PubMed]
- Robbins, M.S.; Robertson, C.E.; Kaplan, E.; Ailani, J.; Charleston, L.t.; Kuruvilla, D.; Blumenfeld, A.; Berliner, R.; Rosen, N.L.; Duarte, R.; et al. The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. Headache 2016, 56, 240–258. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Sahai-Srivastava, S.; Kezirian, E.J.; Calhoun, A.H.; Matthews, D.C.; McAllister, P.J.; Costantino, P.D.; Friedman, D.I.; Zuniga, J.R.; Mechtler, L.L.; et al. Safety and efficacy of sphenopalatine ganglion stimulation for chronic cluster headache: A double-blind, randomised controlled trial. Lancet Neurol. 2019, 18, 1081–1090. [Google Scholar] [CrossRef]
- Kalamir, A.; Graham, P.L.; Vitiello, A.L.; Bonello, R.; Pollard, H. Intra-oral myofascial therapy versus education and self-care in the treatment of chronic, myogenous temporomandibular disorder: A randomised, clinical trial. Chiropr. Man. Ther. 2013, 21, 17. [Google Scholar] [CrossRef] [PubMed]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Zeev, M.S.; Miller, D.D.; Latkany, R. Diagnosis of dry eye disease and emerging technologies. Clin. Ophthalmol. 2014, 8, 581–590. [Google Scholar] [CrossRef]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef]
- Cho, S.I.; Hauser, R.; Christiani, D.C. Reproducibility of nasal peak inspiratory flow among healthy adults: Assessment of epidemiologic utility. Chest 1997, 112, 1547–1553. [Google Scholar] [CrossRef]
- Ottaviano, G.; Scadding, G.K.; Coles, S.; Lund, V.J. Peak nasal inspiratory flow; normal range in adult population. Rhinology 2006, 44, 32–35. [Google Scholar]
- Kjaergaard, T.; Cvancarova, M.; Steinsvag, S.K. Does nasal obstruction mean that the nose is obstructed? Laryngoscope 2008, 118, 1476–1481. [Google Scholar] [CrossRef]
- Gleeson, M.J.; Youlten, L.J.; Shelton, D.M.; Siodlak, M.Z.; Eiser, N.M.; Wengraf, C.L. Assessment of nasal airway patency: A comparison of four methods. Clin. Otolaryngol. Allied Sci. 1986, 11, 99–107. [Google Scholar] [CrossRef]
- Su, M.C.; Chiu, K.L.; Ruttanaumpawan, P.; Shiota, S.; Yumino, D.; Redolfi, S.; Haight, J.S.; Bradley, T.D. Lower body positive pressure increases upper airway collapsibility in healthy subjects. Respir. Physiol. Neurobiol. 2008, 161, 306–312. [Google Scholar] [CrossRef]
- Attali, V.; Collet, J.M.; Jacq, O.; Souchet, S.; Arnulf, I.; Rivals, I.; Kerbrat, J.B.; Goudot, P.; Morelot-Panzini, C.; Similowski, T. Mandibular advancement reveals long-term suppression of breathing discomfort in patients with obstructive sleep apnea syndrome. Respir. Physiol. Neurobiol. 2019, 263, 47–54. [Google Scholar] [CrossRef]
- Ottaviano, G.; Fokkens, W.J. Measurements of nasal airflow and patency: A critical review with emphasis on the use of peak nasal inspiratory flow in daily practice. Allergy 2016, 71, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Tsounis, M.; Swart, K.M.; Georgalas, C.; Markou, K.; Menger, D.J. The clinical value of peak nasal inspiratory flow, peak oral inspiratory flow, and the nasal patency index. Laryngoscope 2014, 124, 2665–2669. [Google Scholar] [CrossRef]
- McNicholas, W.T. The nose and OSA: Variable nasal obstruction may be more important in pathophysiology than fixed obstruction. Eur. Respir. J. 2008, 32, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Young, T.; Finn, L.; Kim, H. Nasal obstruction as a risk factor for sleepdisordered breathing. J. Allergy Clin. Immunol. 1997, 99, S757–S762. [Google Scholar] [CrossRef]
- Hudgel, D.W. Variable site of airway narrowing among obstructive sleep apnea patients. J. Appl. Physiol. 1986, 61, 1403–1409. [Google Scholar] [CrossRef]
- Series, F.; St Pierre, S.; Carrier, G. Surgical correction of nasal obstruction in the treatment of mild sleep apnoea: Importance of cephalometry in predicting outcome. Thorax 1993, 48, 360–363. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jankowski, R.; Klossek, J.M.; Attali, V.; Coste, A.; Serrano, E. Long-term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposis. Allergy 2009, 64, 944–950. [Google Scholar] [CrossRef] [PubMed]
- Kiely, J.L.; Nolan, P.; McNicholas, W.T. Intranasal corticosteroid therapy for obstructive sleep apnoea in patients with co-existing rhinitis. Thorax 2004, 59, 50–55. [Google Scholar]
- McLean, H.A.; Urton, A.M.; Driver, H.S.; Tan, A.K.; Day, A.G.; Munt, P.W.; Fitzpatrick, M.F. Effect of treating severe nasal obstruction on the severity of obstructive sleep apnoea. Eur. Respir. J. 2005, 25, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Georgalas, C. The role of the nose in snoring and obstructive sleep apnoea: An update. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 1365–1373. [Google Scholar] [CrossRef]
- Camacho, M.; Riaz, M.; Capasso, R.; Ruoff, C.M.; Guilleminault, C.; Kushida, C.A.; Certal, V. The effect of nasal surgery on continuous positive airway pressure device use and therapeutic treatment pressures: A systematic review and meta-analysis. Sleep 2015, 38, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Ng, A.T.; Qian, J.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Influence of nasal resistance on oral appliance treatment outcome in obstructive sleep apnea. Sleep 2008, 31, 543–547. [Google Scholar] [CrossRef]
- Saver, J.L.; Kharaishvili, N.; Janelidze, T.; Beridze, M.; Zarqua, N.; Solberg, Y.; Bornstein, N.M. Refined Sphenopalatine Ganglion Stimulator Placement and Intensity Setting to Augment Blood Flow and Neurologic Function. Stroke 2019, 50, 3512–3518. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.B.; Saver, J.L. Mechanisms of action of acute and subacute sphenopalatine ganglion stimulation for ischemic stroke. Int. J. Stroke 2020, 15, 839–848. [Google Scholar] [CrossRef]
- Schytz, H.W.; Barlose, M.; Guo, S.; Selb, J.; Caparso, A.; Jensen, R.; Ashina, M. Experimental activation of the sphenopalatine ganglion provokes cluster-like attacks in humans. Cephalalgia 2013, 33, 831–841. [Google Scholar] [CrossRef]
- Schoenen, J.; Jensen, R.H.; Lanteri-Minet, M.; Lainez, M.J.; Gaul, C.; Goodman, A.M.; Caparso, A.; May, A. Stimulation of the sphenopalatine ganglion (SPG) for cluster headache treatment. Pathway CH-1: A randomized, sham-controlled study. Cephalalgia 2013, 33, 816–830. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMC Med. 2010, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Lebret, M.; Arnol, N.; Contal, O.; Martinot, J.B.; Tamisier, R.; Pepin, J.L.; Borel, J.C. Nasal obstruction and male gender contribute to the persistence of mouth opening during sleep in CPAP-treated obstructive sleep apnoea. Respirology 2015, 20, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
| OSAS Patients (n = 30) | ||
|---|---|---|
| Age (Years) | 57 [33; 64] | |
| Gender (Male/Female; n) | 24/6 | |
| Before AM | Before SM | |
| Weight (kg) | 89 [78; 103] | 90 [78; 102] |
| BMI (kg/m2) | 28.3 [27.1; 34,4] | 29.1 [27; 35] |
| ESS (/24) | 7 [4; 13] | 9 [5; 12] |
| PNIF (L/min) | 103 [60; 120] | 100 [70; 130] |
| Awake Pcrit | −19.6 [−31.7; −12.9] | −25.6 [−39.2; −16.5] |
| Polysomnography | ||
| TST (min) | 443 [388; 489] | 452 [391; 499] |
| Sleep efficiency (%) | 88 [79; 93] | 85 [78; 91] |
| LSO (min) | 24 [14; 40] | 16 [9; 25] |
| Arousal Index (n/h) | 26 [15; 36] | 26 [19; 40] |
| N1 sleep (min) | 6 [4; 10] | 5 [3; 11] |
| N2 sleep (min) | 250 [219; 289] | 251 [187; 300] |
| N3 sleep (min) | 81 [58; 108] | 70 [53; 102] |
| REM sleep (min) | 98 [78; 117] | 100 [77; 139] |
| AHI (n/h) | 30 [18; 48] | 28 [21; 44] |
| AHI ≥ 30 (% patients) | 50 | 47 |
| SpO2 < 90% (%TST) | 7 [1; 15] | 4 [1; 14] |
| After AM | After SM | p | |
|---|---|---|---|
| Total Sleep Time (min) | 19 [−25; 59] | 12 [−31; 52] | 0.68 |
| N1 sleep (min) | 0 [−3; 6] | −1 [−4; 4] | 0.57 |
| N2 sleep (min) | −6 [−23; 40] | −10 [−35; 18] | 0.21 |
| N3 sleep (min) | 1 [−17; 20] | 10 [−24; 42] | 0.42 |
| REM sleep (min) | 14 [−3; 23] | 13 [−8; 46] | 0.54 |
| Arousal Index (n/h) | 2 [−4; 5] | −3 [−7; 4] | 0.37 |
| Apnea-Hypopnea Index (%) | −2.3 [−24.9; 22.6] | 0.9 [−27.8; 21.1] | 0.67 |
| Desaturation Index (%) | 0.0 [−19.6; 38.7] | 0.0 [−13.5; 21.0] | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Attali, V.; Jacq, O.; Martin, K.; Arnulf, I.; Similowski, T. Osteopathic Manipulation of the Sphenopalatine Ganglia Versus Sham Manipulation, in Obstructive Sleep Apnoea Syndrom: A Randomised Controlled Trial. J. Clin. Med. 2022, 11, 99. https://doi.org/10.3390/jcm11010099
Attali V, Jacq O, Martin K, Arnulf I, Similowski T. Osteopathic Manipulation of the Sphenopalatine Ganglia Versus Sham Manipulation, in Obstructive Sleep Apnoea Syndrom: A Randomised Controlled Trial. Journal of Clinical Medicine. 2022; 11(1):99. https://doi.org/10.3390/jcm11010099
Chicago/Turabian StyleAttali, Valérie, Olivier Jacq, Karine Martin, Isabelle Arnulf, and Thomas Similowski. 2022. "Osteopathic Manipulation of the Sphenopalatine Ganglia Versus Sham Manipulation, in Obstructive Sleep Apnoea Syndrom: A Randomised Controlled Trial" Journal of Clinical Medicine 11, no. 1: 99. https://doi.org/10.3390/jcm11010099
APA StyleAttali, V., Jacq, O., Martin, K., Arnulf, I., & Similowski, T. (2022). Osteopathic Manipulation of the Sphenopalatine Ganglia Versus Sham Manipulation, in Obstructive Sleep Apnoea Syndrom: A Randomised Controlled Trial. Journal of Clinical Medicine, 11(1), 99. https://doi.org/10.3390/jcm11010099






