Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Methods
2.2.1. Assessment of Clinical Risk Factors
2.2.2. Ultrasonography of Carotid Arteries
2.2.3. Laboratory Investigations
2.3. Statistical Analysis
3. Results
3.1. Clinical Characteristics
3.2. Laboratory Parameters
3.3. Risk Factors for CA and CAS in Univariate Analysis
3.4. Risk Factors for CA and CAS in Multivariate Analysis
3.5. Combinations of the Risk Factors for CA and CAS
4. Discussion
4.1. General Risk Factors for CA and CAS in Subjects with T2D
4.2. Diabetes-Related Risk Factors for CA and CAS
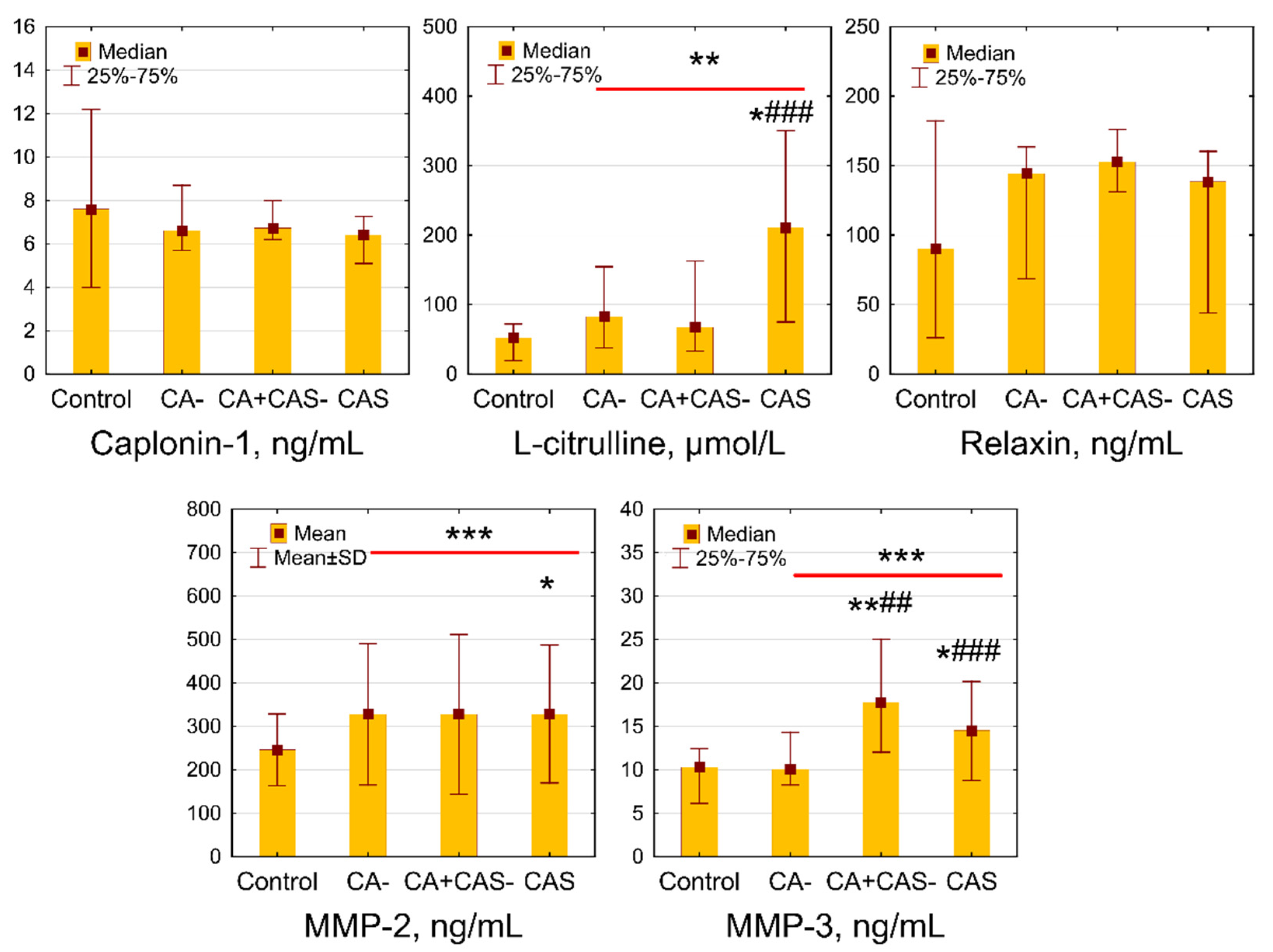
4.3. The Search for Biomarkers of CA and CAS in Subjects with T2D
4.4. Limitations and Future Remarks
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Cardiovascular Diseases (CVDs) Fact Sheet; World Health Organization: Geneva, Switzerland, 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 7 November 2021).
- Kabłak-Ziembicka, A.; Przewłocki, T. Clinical Significance of Carotid Intima-Media Complex and Carotid Plaque Assessment by Ultrasound for the Prediction of Adverse Cardiovascular Events in Primary and Secondary Care Patients. J. Clin. Med. 2021, 10, 4628. [Google Scholar] [CrossRef] [PubMed]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Carotid Plaque, Compared with Carotid Intima-Media Thickness, More Accurately Predicts Coronary Artery Disease Events: A Meta-Analysis. Atherosclerosis 2012, 220, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Krawisz, A.K.; Carroll, B.J.; Secemsky, E.A. Risk Stratification and Management of Extracranial Carotid Artery Disease. Cardiol. Clin. 2021, 39, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; He, J.; Sun, X.; Zhao, Y.-M.; Lou, H.-Y.; Ji, X.; Pang, X.-H.; Shan, L.-Z.; Kang, Y.-X.; Xu, J.; et al. Carotid Atherosclerosis and Its Relationship to Coronary Heart Disease and Stroke Risk in Patients with Type 2 Diabetes Mellitus. Medicine (Baltimore) 2017, 96, e8151. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, C.R.L.; Salles, G.C.; Leite, N.C.; Salles, G.F. Prognostic Impact of Carotid Intima-Media Thickness and Carotid Plaques on the Development of Micro- and Macrovascular Complications in Individuals with Type 2 Diabetes: The Rio de Janeiro Type 2 Diabetes Cohort Study. Cardiovasc. Diabetol. 2019, 18, 2. [Google Scholar] [CrossRef]
- Hoke, M.; Schillinger, M.; Minar, E.; Goliasch, G.; Binder, C.J.; Mayer, F.J. Carotid Ultrasound Investigation as a Prognostic Tool for Patients with Diabetes Mellitus. Cardiovasc. Diabetol. 2019, 18, 90. [Google Scholar] [CrossRef]
- Brohall, G.; Oden, A.; Fagerberg, B. Carotid Artery Intima-Media Thickness in Patients with Type 2 Diabetes Mellitus and Impaired Glucose Tolerance: A Systematic Review. Diabet. Med. 2006, 23, 609–616. [Google Scholar] [CrossRef]
- Wu, T.-W.; Chou, C.-L.; Cheng, C.-F.; Lu, S.-X.; Wang, L.-Y. Prevalences of Diabetes Mellitus and Carotid Atherosclerosis and Their Relationships in Middle-Aged Adults and Elders: A Community-Based Study. J. Formos. Med. Assoc. 2021, S0929664621004770. [Google Scholar] [CrossRef]
- Cardoso, C.R.L.; Marques, C.E.C.; Leite, N.C.; Salles, G.F. Factors Associated with Carotid Intima-Media Thickness and Carotid Plaques in Type 2 Diabetic Patients. J. Hypertens. 2012, 30, 940–947. [Google Scholar] [CrossRef]
- Yuan, C.; Lai, C.W.K.; Chan, L.W.C.; Chow, M.; Law, H.K.W.; Ying, M. Cumulative Effects of Hypertension, Dyslipidemia, and Chronic Kidney Disease on Carotid Atherosclerosis in Chinese Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2014, 2014, 179686. [Google Scholar] [CrossRef]
- Sjöblom, P.; Nystrom, F.H.; Länne, T.; Engvall, J.; Östgren, C.J. Microalbuminuria, but Not Reduced EGFR, Is Associated with Cardiovascular Subclinical Organ Damage in Type 2 Diabetes. Diabetes Metab. 2014, 40, 49–55. [Google Scholar] [CrossRef]
- Zhang, K.; Lin, Q.; Zhang, T.; Guo, D.; Cao, L. Contemporary Prevalence and Risk Factors of Carotid Artery Stenosis in Asymptomatic Low-Income Chinese Individuals: A Population-Based Study. Postgrad. Med. 2020, 132, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-Y.; Qiu, H.-M.; Yang, Y.; Han, Y.-Y. Analysis of Risk Factors for Carotid Intima-Media Thickness in Patients with Type 2 Diabetes Mellitus in Western China Assessed by Logistic Regression Combined with a Decision Tree Model. Diabetol. Metab. Syndr. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Mostaza, J.M.; Lahoz, C.; Salinero-Fort, M.A.; de Burgos-Lunar, C.; Laguna, F.; Estirado, E.; García-Iglesias, F.; González-Alegre, T.; Cornejo-Del-Río, V.; Sabín, C.; et al. Carotid Atherosclerosis Severity in Relation to Glycemic Status: A Cross-Sectional Population Study. Atherosclerosis 2015, 242, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Yubero-Serrano, E.M.; Delgado-Lista, J.; Alcala-Diaz, J.F.; Garcia-Rios, A.; Perez-Caballero, A.I.; Blanco-Rojo, R.; Gomez-Delgado, F.; Marin, C.; Tinahones, F.J.; Caballero, J.; et al. A Dysregulation of Glucose Metabolism Control Is Associated with Carotid Atherosclerosis in Patients with Coronary Heart Disease (CORDIOPREV-DIAB Study). Atherosclerosis 2016, 253, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Martinez, E.; Martorell, J.; Riambau, V. Review of Serum Biomarkers in Carotid Atherosclerosis. J. Vasc. Surg. 2020, 71, 329–341. [Google Scholar] [CrossRef]
- Puig, N.; Jiménez-Xarrié, E.; Camps-Renom, P.; Benitez, S. Search for Reliable Circulating Biomarkers to Predict Carotid Plaque Vulnerability. Int. J. Mol. Sci. 2020, 21, 8236. [Google Scholar] [CrossRef]
- Liu, R.; Jin, J.-P. Calponin Isoforms CNN 1, CNN 2 and CNN 3: Regulators for Actin Cytoskeleton Functions in Smooth Muscle and Non-Muscle Cells. Gene 2016, 585, 143–153. [Google Scholar] [CrossRef]
- Teichman, S.L.; Unemori, E.; Teerlink, J.R.; Cotter, G.; Metra, M. Relaxin: Review of Biology and Potential Role in Treating Heart Failure. Curr. Heart Fail. Rep. 2010, 7, 75–82. [Google Scholar] [CrossRef]
- Allerton, T.; Proctor, D.; Stephens, J.; Dugas, T.; Spielmann, G.; Irving, B. L-Citrulline Supplementation: Impact on Cardiometabolic Health. Nutrients 2018, 10, 921. [Google Scholar] [CrossRef]
- Vacek, T.; Rahman, S.; Yu, S.; Neamtu, D.; Givimani, S.; Tyagi, S. Matrix Metalloproteinases in Atherosclerosis: Role of Nitric Oxide, Hydrogen Sulfide, Homocysteine, and Polymorphisms. Vasc. Health Risk Manag. 2015, 11, 173. [Google Scholar] [CrossRef]
- World Health Organization. Definition, Diagnosis and Classification of Diabetes Mellitus and Its Complications: Report of a WHO Consultation. Part 1. Diagnosis and Classification of Diabetes Mellitus; Document number WHO/NCD/NCS/99.2; World Health Organization: Geneva, Switzerland, 1999. [Google Scholar]
- World Health Organization. Definition and Diagnosis of Diabetes Mellitus and Intermediate Hyperglycemia: Report of a WHO/IDF Consultation; WHO Document Production Services: Geneva, Switzerland, 2006; ISBN 92-4-159493-4. [Google Scholar]
- Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 2013, 3, 1–150.
- European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO) EASL–EASD–EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Diabetologia 2016, 59, 1121–1140. [CrossRef]
- Touboul, P.-J.; Grobbee, D.E.; den Ruijter, H. Assessment of Subclinical Atherosclerosis by Carotid Intima Media Thickness: Technical Issues. Eur. J. Prev. Cardiolog. 2012, 19, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.H.; Korcarz, C.E.; Hurst, R.T.; Lonn, E.; Kendall, C.B.; Mohler, E.R.; Najjar, S.S.; Rembold, C.M.; Post, W.S. Use of Carotid Ultrasound to Identify Subclinical Vascular Disease and Evaluate Cardiovascular Disease Risk: A Consensus Statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force Endorsed by the Society for Vascular Medicine. J. Am. Soc. Echocardiogr. 2008, 21, 93–111. [Google Scholar] [CrossRef]
- Grant, E.G.; Benson, C.B.; Moneta, G.L.; Alexandrov, A.V.; Baker, J.D.; Bluth, E.I.; Carroll, B.A.; Eliasziw, M.; Gocke, J.; Hertzberg, B.S.; et al. Carotid Artery Stenosis: Gray-Scale and Doppler US Diagnosis—Society of Radiologists in Ultrasound Consensus Conference. Radiology 2003, 229, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Brott, T.G.; Halperin, J.L.; Abbara, S.; Bacharach, J.M.; Barr, J.D.; Bush, R.L.; Cates, C.U.; Creager, M.A.; Fowler, S.B.; Friday, G.; et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS Guideline on the Management of Patients with Extracranial Carotid and Vertebral Artery Disease: Executive Summary: A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Associ. Cathet. Cardiovasc. Intervent. 2013, 81, E76–E123. [Google Scholar] [CrossRef]
- Hill, N.R.; Oliver, N.S.; Choudhary, P.; Levy, J.C.; Hindmarsh, P.; Matthews, D.R. Normal Reference Range for Mean Tissue Glucose and Glycemic Variability Derived from Continuous Glucose Monitoring for Subjects without Diabetes in Different Ethnic Groups. Diabetes Technol. Ther. 2011, 13, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Catalan, M.; Herreras, Z.; Pinyol, M.; Sala-Vila, A.; Amor, A.J.; de Groot, E.; Gilabert, R.; Ros, E.; Ortega, E. Prevalence by Sex of Preclinical Carotid Atherosclerosis in Newly Diagnosed Type 2 Diabetes. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 742–748. [Google Scholar] [CrossRef]
- Bouchi, R.; Takeuchi, T.; Akihisa, M.; Ohara, N.; Nakano, Y.; Nishitani, R.; Murakami, M.; Fukuda, T.; Fujita, M.; Minami, I.; et al. High Visceral Fat with Low Subcutaneous Fat Accumulation as a Determinant of Atherosclerosis in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2015, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Lee, Y.; Suh, Y.J.; Ahn, S.H.; Hong, S.; Choi, Y.J.; Huh, B.W.; Park, S.W.; Lee, E.; Kim, S.H. Low Muscle Mass Is Associated with Carotid Atherosclerosis in Patients with Type 2 Diabetes. Atherosclerosis 2020, 305, 19–25. [Google Scholar] [CrossRef]
- Kim, B.-J.; Cho, S.-H.; Cho, K.-I.; Kim, H.-S.; Heo, J.-H.; Cha, T.-J. The Combined Impact of Neutrophil-to-Lymphocyte Ratio and Type 2 Diabetic Mellitus on Significant Coronary Artery Disease and Carotid Artery Atherosclerosis. J. Cardiovasc. Ultrasound 2016, 24, 115. [Google Scholar] [CrossRef]
- La Sala, L.; Prattichizzo, F.; Ceriello, A. The Link between Diabetes and Atherosclerosis. Eur. J. Prev. Cardiolog. 2019, 26, 15–24. [Google Scholar] [CrossRef]
- Senior, P.A. Glucose as a Modifiable Cause of Atherosclerotic Cardiovascular Disease: Insights from Type 1 Diabetes and Transplantation. Atherosclerosis 2021, 335, 16–22. [Google Scholar] [CrossRef]
- Beks, P.H.J.; Mackaay, A.J.C.; de Vries, H.; de Neeling, J.N.D.; Bouter, L.M.; Heine, R.J. Carotid Artery Stenosis Is Related to Blood Glucose Level in an Elderly Caucasian Population: The Hoorn Study. Diabetologia 1997, 40, 290–298. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taya, N.; Katakami, N.; Mita, T.; Okada, Y.; Wakasugi, S.; Yoshii, H.; Shiraiwa, T.; Otsuka, A.; Umayahara, Y.; Ryomoto, K.; et al. Associations of Continuous Glucose Monitoring-Assessed Glucose Variability with Intima-Media Thickness and Ultrasonic Tissue Characteristics of the Carotid Arteries: A Cross-Sectional Analysis in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2021, 20, 95. [Google Scholar] [CrossRef] [PubMed]
- Eto, F.; Washida, K.; Matsubara, M.; Makino, H.; Takahashi, A.; Noda, K.; Hattori, Y.; Nakaoku, Y.; Nishimura, K.; Hosoda, K.; et al. Glucose Fluctuation and Severe Internal Carotid Artery Siphon Stenosis in Type 2 Diabetes Patients. Nutrients 2021, 13, 2379. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Kameyama, T.; Inoue, H. Association of Reduced Levels of Serum 1,5-Anhydro-d-Glucitol with Carotid Atherosclerosis in Patients with Type 2 Diabetes. J. Diabetes Complicat. 2014, 28, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Saik, O.V.; Klimontov, V.V. Bioinformatic Reconstruction and Analysis of Gene Networks Related to Glucose Variability in Diabetes and Its Complications. Int. J. Mol. Sci. 2020, 21, 8691. [Google Scholar] [CrossRef] [PubMed]
- Klimontov, V.V.; Saik, O.V.; Korbut, A.I. Glucose Variability: How Does It Work? Int. J. Mol. Sci. 2021, 22, 7783. [Google Scholar] [CrossRef]
- Mori, H.; Okada, Y.; Kurozumi, A.; Narisawa, M.; Tanaka, Y. Factors Influencing Inter-Day Glycemic Variability in Diabetic Outpatients Receiving Insulin Therapy. J. Diabetes Investig. 2017, 8, 69–74. [Google Scholar] [CrossRef]
- Herman, M.E.; O’Keefe, J.H.; Bell, D.S.H.; Schwartz, S.S. Insulin Therapy Increases Cardiovascular Risk in Type 2 Diabetes. Prog. Cardiovasc. Dis. 2017, 60, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Ke, J.-F.; Wang, J.-W.; Zhang, Z.-H.; Chen, M.-Y.; Lu, J.-X.; Li, L.-X. Insulin Therapy Is Associated with an Increased Risk of Carotid Plaque in Type 2 Diabetes: A Real-World Study. Front. Cardiovasc. Med. 2021, 8, 599545. [Google Scholar] [CrossRef] [PubMed]
- Valdivielso, J.M.; Rodríguez-Puyol, D.; Pascual, J.; Barrios, C.; Bermúdez-López, M.; Sánchez-Niño, M.D.; Pérez-Fernández, M.; Ortiz, A. Atherosclerosis in Chronic Kidney Disease: More, Less, or Just Different? Arterioscler. Thromb. Vasc. Biol. 2019, 39, 1938–1966. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Shen, M.; Samuel, C.S.; Schlossmann, J.; Bennett, R.G. Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways. Mol. Cell Endocrinol. 2019, 487, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hien, T.T.; Turczyńska, K.M.; Dahan, D.; Ekman, M.; Grossi, M.; Sjögren, J.; Nilsson, J.; Braun, T.; Boettger, T.; Garcia-Vaz, E.; et al. Elevated Glucose Levels Promote Contractile and Cytoskeletal Gene Expression in Vascular Smooth Muscle via Rho/Protein Kinase C and Actin Polymerization. J. Biol. Chem. 2016, 291, 3552–3568. [Google Scholar] [CrossRef] [PubMed]
- Chettimada, S.; Ata, H.; Rawat, D.K.; Gulati, S.; Kahn, A.G.; Edwards, J.G.; Gupte, S.A. Contractile Protein Expression Is Upregulated by Reactive Oxygen Species in Aorta of Goto-Kakizaki Rat. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H214–H224. [Google Scholar] [CrossRef]
- Chawla, V.; Simionescu, A.; Langan, E.M.; LaBerge, M. Influence of Clinically Relevant Mechanical Forces on Vascular Smooth Muscle Cells Under Chronic High Glucose: An In Vitro Dynamic Disease Model. Ann. Vasc. Surg. 2016, 34, 212–226. [Google Scholar] [CrossRef] [PubMed]
- Ng, H.H.; Leo, C.H.; Parry, L.J.; Ritchie, R.H. Relaxin as a Therapeutic Target for the Cardiovascular Complications of Diabetes. Front. Pharmacol. 2018, 9, 501. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, M.; Zhao, M.; Chen, W.; Fu, Y.; Liu, Y.; Liu, W.; Zhang, B.; Yin, X.; Bai, B. The Plasma Levels of Relaxin-2 and Relaxin-3 in Patients with Diabetes. Clin. Biochem. 2013, 46, 1713–1716. [Google Scholar] [CrossRef]
- Gao, X.; Li, H.; Wang, P.; Chen, H. Decreased Serum Relaxin-2 Is Correlated with Impaired Islet β-Cell Function in Patients with Unstable Angina and Abnormal Glucose Metabolism. Int. Heart J. 2018, 59, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Kapelouzou, A.; Tsilimigras, D.I.; Patelis, N.; Kouvelos, G.; Schizas, D.; Karavokyros, I.; Georgopoulos, S. Associations between Serum Relaxin 2, Aneurysm Formation/Size and Severity of Atherosclerosis: A Preliminary Prospective Analysis. Acta Pharmacol. Sin. 2018, 39, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, K.; Kapelouzou, A.; Georgiopoulos, G.; Kontogiannis, C.; Kourek, C.; Mylonas, K.S.; Patelis, N.; Cokkinos, D.V.; Karavokyros, I.; Georgopoulos, S. Tissue-Specific Relaxin-2 Is Differentially Associated with the Presence/Size of an Arterial Aneurysm and the Severity of Atherosclerotic Disease in Humans. Acta Pharmacol. Sin. 2020, 41, 745–752. [Google Scholar] [CrossRef]
- Szepietowska, B.; Gorska, M.; Szelachowska, M. Plasma Relaxin Concentration Is Related to Beta-Cell Function and Insulin Sensitivity in Women with Type 2 Diabetes Mellitus. Diabetes Res. Clin. Pract. 2008, 79, e1–e3. [Google Scholar] [CrossRef]
- Verdam, F.J.; Greve, J.W.M.; Roosta, S.; van Eijk, H.; Bouvy, N.; Buurman, W.A.; Rensen, S.S. Small Intestinal Alterations in Severely Obese Hyperglycemic Subjects. J. Clin. Endocrinol. Metab. 2011, 96, E379–E383. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Zamora, S.; Méndez-Rodríguez, M.L.; Olguín-Martínez, M.; Sánchez-Sevilla, L.; Quintana-Quintana, M.; García-García, N.; Hernández-Muñoz, R. Increased Erythrocytes By-Products of Arginine Catabolism Are Associated with Hyperglycemia and Could Be Involved in the Pathogenesis of Type 2 Diabetes Mellitus. PLoS ONE 2013, 8, e66823. [Google Scholar] [CrossRef] [PubMed]
- Sailer, M.; Dahlhoff, C.; Giesbertz, P.; Eidens, M.K.; de Wit, N.; Rubio-Aliaga, I.; Boekschoten, M.V.; Müller, M.; Daniel, H. Increased Plasma Citrulline in Mice Marks Diet-Induced Obesity and May Predict the Development of the Metabolic Syndrome. PLoS ONE 2013, 8, e63950. [Google Scholar] [CrossRef]
- Assumpo, C.R.; Brunini, T.M.C.; Matsuura, C.; Resende, A.C.; Mendes-Ribeiro, A.C. Impact of the L-Arginine-Nitric Oxide Pathway and Oxidative Stress on the Pathogenesis of the Metabolic Syndrome. Open Biochem. J. 2008, 2, 108–115. [Google Scholar] [CrossRef]
- Sumarriva, K.; Uppal, K.; Ma, C.; Herren, D.J.; Wang, Y.; Chocron, I.M.; Warden, C.; Mitchell, S.L.; Burgess, L.G.; Goodale, M.P.; et al. Arginine and Carnitine Metabolites Are Altered in Diabetic Retinopathy. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3119. [Google Scholar] [CrossRef]
- Magnusson, M.; Wang, T.; Hedblad, B.; Engstrom, G.; Ostling, G.; Gerszten, R.; Melander, O. High Levels of Arginine, Citrulline and ADMA Are Independent Predictors of Cardiovascular Disease. Eur. Heart J. 2013, 34, P5687. [Google Scholar] [CrossRef]
- Aguayo, E.; Martínez-Sánchez, A.; Fernández-Lobato, B.; Alacid, F. L-Citrulline: A Non-Essential Amino Acid with Important Roles in Human Health. Appl. Sci. 2021, 11, 3293. [Google Scholar] [CrossRef]
- Shatanawi, A.; Momani, M.S.; Al-Aqtash, R.; Hamdan, M.H.; Gharaibeh, M.N. L-Citrulline Supplementation Increases Plasma Nitric Oxide Levels and Reduces Arginase Activity in Patients With Type 2 Diabetes. Front. Pharmacol. 2020, 11, 584669. [Google Scholar] [CrossRef] [PubMed]
- Azizi, S.; Mahdavi, R.; Mobasseri, M.; Aliasgharzadeh, S.; Abbaszadeh, F.; Ebrahimi-Mameghani, M. The Impact of L-citrulline Supplementation on Glucose Homeostasis, Lipid Profile, and Some Inflammatory Factors in Overweight and Obese Patients with Type 2 Diabetes: A Double-blind Randomized Placebo-controlled Trial. Phytother. Res. 2021, 35, 3157–3166. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadeh, F.; Azizi, S.; Mobasseri, M.; Ebrahimi-Mameghani, M. The Effects of Citrulline Supplementation on Meta-Inflammation and Insulin Sensitivity in Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetol. Metab. Syndr 2021, 13, 52. [Google Scholar] [CrossRef]
- Prado, A.F.; Batista, R.I.M.; Tanus-Santos, J.E.; Gerlach, R.F. Matrix Metalloproteinases and Arterial Hypertension: Role of Oxidative Stress and Nitric Oxide in Vascular Functional and Structural Alterations. Biomolecules 2021, 11, 585. [Google Scholar] [CrossRef]
- Wang, X.; Khalil, R.A. Matrix Metalloproteinases, Vascular Remodeling, and Vascular Disease. Adv. Pharmacol. 2018, 81, 241–330. [Google Scholar] [CrossRef] [PubMed]
- Cui, N.; Hu, M.; Khalil, R.A. Biochemical and Biological Attributes of Matrix Metalloproteinases. Prog. Mol. Biol. Transl. Sci. 2017, 147, 1–73. [Google Scholar] [CrossRef]
- Ma, Y.-D.; Thiyagarajan, V.; Tsai, M.-J.; Lue, S.-I.; Chia, Y.-C.; Shyue, S.-K.; Weng, C.-F. Pyrogallol Abates VSMC Migration via Modulation of Caveolin-1, Matrix Metalloproteinase and Intima Hyperplasia in Carotid Ligation Mouse. Environ. Toxicol. Pharmacol. 2016, 48, 63–75. [Google Scholar] [CrossRef]
- Ruddy, J.M.; Ikonomidis, J.S.; Jones, J.A. Multidimensional Contribution of Matrix Metalloproteinases to Atherosclerotic Plaque Vulnerability: Multiple Mechanisms of Inhibition to Promote Stability. J. Vasc. Res. 2016, 53, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lien, L.-M.; Hsieh, Y.-C.; Bai, C.-H.; Chen, W.-H.; Chiu, H.-C.; Hsieh, F.-I.; Shyu, K.-G.; Chiou, H.-Y.; Hsu, C.Y. Association of Blood Active Matrix Metalloproteinase-3 with Carotid Plaque Score from a Community Population in Taiwan. Atherosclerosis 2010, 212, 595–600. [Google Scholar] [CrossRef]
- Gruszka, K.; Rajzer, M.; Drożdż, T.; Wojciechowska, W.; Pizoń, T.; Migacz-Gruszka, K.; Czarnecka, D. Selected Matrix Metalloproteinases Activity and Hypertension-Mediated Organ Damage in Relation to Uric Acid Serum Level. Cardiol. J. 2019. [Google Scholar] [CrossRef]
- Hu, W.; Wei, R.; Wang, L.; Lu, J.; Liu, H.; Zhang, W. Correlations of MMP-1, MMP-3, and MMP-12 with the Degree of Atherosclerosis, Plaque Stability and Cardiovascular and Cerebrovascular Events. Exp. Ther. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Straface, G.; Bertoletti, G.; Vincenzoni, C.; Snider, F.; Arena, V.; Landolfi, R.; Flex, A. Identification of a Potential Proinflammatory Genetic Profile Influencing Carotid Plaque Vulnerability. J. Vasc. Surg. 2015, 61, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Pleskovič, A.; Letonja, M.Š.; Vujkovac, A.C.; Starčević, J.N.; Caprnda, M.; Curilla, E.; Mozos, I.; Kruzliak, P.; Prosecky, R.; Petrovič, D. Matrix Metalloproteinase-3 Gene Polymorphism (Rs3025058) Affects Markers Atherosclerosis in Type 2 Diabetes Mellitus. Vasa 2017, 46, 363–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tripolt, N.J.; Narath, S.H.; Eder, M.; Pieber, T.R.; Wascher, T.C.; Sourij, H. Multiple risk factor intervention reduces carotid atherosclerosis in patients with type 2 diabetes. Cardiovasc. Diabetol. 2014, 13, 95. [Google Scholar] [CrossRef]
| Parameter | CA− n = 54 | CA+ CAS− n = 201 | CAS n = 134 |
|---|---|---|---|
| General parameters | |||
| Sex, m/f, n (%) | 9/45 (16.7/83.3) | 61/140 (30.3/69.7) * | 42/92 (31.3/68.7) * |
| Age, years | 58 (51–63) | 64 (58–70) *** | 68 (63–71) ***,### |
| Weight, kg | 93.5 (83–120) | 88 (76–102) * | 86 (74–94) *** |
| BMI, kg/m2 | 36.8 (31.6–42.5) | 32.9 (28.9–37.5) *** | 31.9 (29.3–36.4) *** |
| Waist circumference, cm | 115 ± 17 | 109 ± 13 | 112 ± 14 |
| Hip circumference, cm | 120 (105.5–130.5) | 112 (100–120) | 110 (104.5–117.5) |
| WHR | 0.93 (0.91–1.01) | 0.97 (0.91–1.04) | 0.98 (0.94–1.07) |
| Current smoking, n (%) | 7 (13.0) | 17 (8.5) | 20 (14.9) |
| Diabetes duration since diagnosis, years | 8 (5–14) | 12 (8–17) * | 15 (8–22) ***,# |
| Diabetes complications and associated diseases | |||
| Diabetic retinopathy, n (%) | 20 (37.0) | 95 (47.3) | 85 (63.4) **,## |
| CKD, n (%) | 34 (63.0) | 156 (77.6) * | 121 (90.3) ***,## |
| Hypertension, n (%) | 50 (92.5) | 195 (97.0) | 132 (98.5) * |
| Hypertension duration, years | 14 (9–20) | 15 (8–22) | 20 (10–30) *,## |
| CAD, n (%) | 9 (16.6) | 67 (33.3) * | 75 (56.0) ***,### |
| Myocardial infarction, n (%) | 2 (3.7) | 19 (9.5) * | 30 (22.4) ***,### |
| PAD, n (%) | 15 (27.7) | 90 (44.8) * | 90 (67.7) ***,### |
| Revascularization surgery, n (%) | 2 (3.7) | 21 (10.4) | 30 (22.4) **,## |
| Stroke, n (%) | 2 (3.7) | 20 (9.95) | 18 (13.4) * |
| NAFLD, n (%) | 39 (72.2) | 125 (63.2) | 77 (57.5) |
| Treatment | |||
| Metformin, n (%) | 39 (72.2) | 145 (72.1) | 88 (65.7) |
| Sulfonylurea, n (%) | 15 (27.8) | 76 (37.8) | 35 (26.1) # |
| DPP4 inhibitors, n (%) | 4 (7.4) | 23 (11.4) | 14 (10.4) |
| SGLT2 inhibitors, n (%) | 6 (11.1) | 20 (10.0) | 12 (9.0) |
| GLP-1 receptor agonists, n (%) | 2 (3.7) | 4 (2.0) | 2 (1.5) |
| Insulin, n (%) | 28 (51.9) | 121 (60.2) | 91 (67.9) |
| Duration of insulin therapy, years | 2 (0.1–6) | 5 (1–10) * | 7 (3–11) *** |
| Daily insulin dose, IU/kg | 0.5 (0.3–0.8) | 0.5 (0.3–0.7) | 0.6 (0.4–0.8) |
| RAS blockers, n (%) | 41 (75.9) | 160 (79.6) | 105 (78.4) |
| Diuretics, n (%) | 23 (42.5) | 94 (46.7) | 71 (53.0) |
| Beta blockers, n (%) | 21 (38.9) | 90 (44.8) | 67 (50.0) |
| Calcium channel blockers, n (%) | 16 (29.6) | 65 (32.3) | 56 (41.8) |
| Antiplatelet agents, n (%) | 18 (33.3) | 116 (57.7) ** | 93 (69.4) ***,# |
| Statins, n (%) | 15 (27.8) | 86 (42.8) * | 72 (53.7) ** |
| Parameter | CA− n = 54 | CA+ CAS− n = 201 | CAS n = 134 |
|---|---|---|---|
| Biochemical parameters | |||
| HbA1c, % | 7.38 (6.30–9.90) | 8.36 (7.23–10.0) | 8.17 (7.05–9.55) |
| MAGE, mmol/L | 2.96 (2.22–4.08) | 3.67 (2.6–4.77) | 4.03 (2.73–5.25) *** |
| LBGI, a.u. | 0.02 (0–0.39) | 0.14 (0–0.94) | 0.18 (0–1.15) |
| HBGI, a.u. | 8.22 (2.12–15.5) | 7.24 (3.71–14.6) | 7.68 (4.03–15.4) |
| Total cholesterol, mmol/L | 4.99 (4.4–5.97) | 5.10 (4.30–6.06) | 4.92 (4.12–6.10) |
| LDL cholesterol, mmol/L | 3.23 (2.74–3.95) | 3.21 (2.59–3.97) | 3.14 (2.36–4.03) |
| HDL cholesterol, mmol/L | 1.15 (0.98–1.39) | 1.17 (0.98–1.40) | 1.14 (0.97–1.33) |
| Triglycerides, mmol/L | 2.22 (1.62–3.14) | 1.97 (1.30–2.96) | 1.93 (1.30–2.71) |
| Uric acid, µmol/L | 342 ± 86 | 326 ± 94 | 339 ± 94 |
| Renal tests | |||
| Serum creatinine, µmol/L | 85.6 (73.0–95.3) | 86 (74.2–97) | 89.3 (77.0–106) |
| eGFR, mL/min/1.73 m2 | 69 (60–84) | 68 (58–84) | 62 (53–75) **,## |
| UACR, mg/mmol | 0.95 (0.40–1.70) | 1.05 (0.50–4.00) | 1.50 (0.50–7.00) * |
| Hematology and coagulation | |||
| Hemoglobin, g/L | 137 (127–150) | 138 (127–146) | 137 (125–146) |
| RBCs, ×1012/L | 4.67 (4.44–5.09) | 4.69 (4.37–5.01) | 4.64 (4.34–4.96) |
| WBCs, ×109/L | 6.63 (5.83–8.22) | 6.52 (5.37–8.00) | 6.70 (5.64–7.86) |
| Neutrophils, ×109/L | 3.82 (3.12–5.33) | 3.96 (3.20–5.03) | 3.96 (3.00–5.15) |
| Lymphocytes, ×109/L | 1.97 (1.64–2.44) | 2.0 (1.64–2.43) | 1.97 (1.57–2.52) |
| Neutrophil-to-lymphocyte ratio | 1.98 (1.53–2.27) | 1.96 (1.55–2.52) | 2.11 (1.48–2.76) |
| Monocytes, ×109/L | 0.29 (0.20–0.37) | 0.27 (0.21–0.36) | 0.29 (0.21–0.38) |
| Eosinophils, ×109/L | 0.13 (0.10–0.20) | 0.15 (0.11–0.22) | 0.15 (0.09–0.21) |
| Platelets, ×109/L | 246 ± 63 | 246 ± 61 | 241 ± 60 |
| Fibrinogen, mmol/L | 4.0 (3.4–4.5) | 4.3 (3.6–5.0) | 4.3 (3.7–5.3) * |
| SFMC, mg/dL | 8.0 (3.5–14.5) | 10.0 (4.3–16.0) | 13.0 (5.5–21.0) * |
| D-dimer, ng/mL | 266 (228–349) | 273 (237–321) | 274 (247–330) |
| Parameter | Cutoff Point | AUC ± SE (95% CI), p-Value | Se | Sp | OR (95% CI), p-Value |
|---|---|---|---|---|---|
| Parameters associated with CA | |||||
| Age | ≥62 years | 0.761 ± 0.033, (0.696–0.826), p < 0.001 | 0.70 | 0.67 | 4.70 (2.55–8.67), p < 0.001 |
| BMI | ≤34.5 kg/m2 | 0.677 ± 0.040, (0.598–0.756), p < 0.001 | 0.61 | 0.61 | 2.47 (1.37–4.45), p = 0.003 |
| Diabetes duration | ≥11 years | 0.649 ± 0.041, (0.570–0.729), p < 0.001 | 0.60 | 0.61 | 2.36 (1.31–4.25), p = 0.004 |
| MAGE | ≥3.38 mmol/L | 0.619 ± 0.041, (0.538–0.700), p = 0.005 | 0.60 | 0.59 | 2.16 (1.20–3.87), p = 0.01 |
| Log MMP-3 | ≥1.12 | 0.733 ± 0.063 (0.61–0.856), p = 0.001 | 0.69 | 0.70 | 4.45 (1.65–12.0), p = 0.003 |
| Parameters associated with CAS | |||||
| Age | ≥66 years | 0.662 ± 0.028, (0.607–0.717), p < 0.001 | 0.62 | 0.62 | 2.61 (1.70–4.01), p < 0.001 |
| BMI | ≤32.5 kg/m2 | 0.571 ± 0.030, (0.512–0.629), p = 0.02 | 0.55 | 0.56 | 1.55 (1.02–2.37), p = 0.04 |
| Diabetes duration | ≥13 years | 0.610 ± 0.030, (0.500–0.670), p < 0.001 | 0.59 | 0.56 | 1.83 (1.20–2.80), p = 0.005 |
| Hypertension duration | ≥18 years | 0.616 ± 0.031, (0.555–0.677), p < 0.001 | 0.57 | 0.60 | 1.95 (1.26–3.04), p = 0.003 |
| Daily insulin dose, IU/kg | ≥0.59 IU/kg | 0.580 ± 0.037, (0.508–0.651), p = 0.03 | 0.58 | 0.59 | 1.91 (1.14–3.20), p = 0.01 |
| eGFR | ≤65.5 mL/min/1.73 m2 | 0.608 ± 0.030, (0.548–0.667), p = 0.001 | 0.56 | 0.55 | 1.53 (1.003–2.33), p = 0.048 |
| Log L-citrulline | ≥2.10 | 0.675 ± 0.078 (0,523–0.827), p = 0.03 | 0.68 | 0.74 | 4.83 (1.47–15.9), p = 0.01 |
| Log MMP-3 | ≥1.10 | 0.649 ± 0.054 (0.543–0.756), p = 0.01 | 0.63 | 0.65 | 2.78 (1.23–6.28), p = 0.01 |
| Parameter | OR | 95% CI | p-Value |
|---|---|---|---|
| Parameters associated with CA | |||
| Age ≥ 62 years | 4.70 | 2.55–8.67 | 0.001 |
| Male sex | 2.22 | 1.05–4.72 | 0.04 |
| BMI ≤ 34.5 kg/m2 | 2.47 | 1.37–4.45 | 0.003 |
| Diabetes duration ≥ 11 years | 2.47 | 1.37–4.45 | 0.003 |
| MAGE ≥ 3.38 mmol/L | 2.16 | 1.20–3.87 | 0.01 |
| log MMP-3 ≥ 1.12 | 4.45 | 1.65–12.0 | 0.003 |
| Diabetic retinopathy | 1.97 | 1.09–3.57 | 0.02 |
| CKD | 2.81 | 1.51–5.23 | 0.001 |
| CAD | 3.68 | 1.74–7.77 | 0.001 |
| Myocardial infarction | 4.47 | 1.05–18.9 | 0.04 |
| PAD | 3.04 | 1.61–5.72 | 0.001 |
| Parameters associated with CAS | |||
| Age ≥ 66 years | 3.36 | 2.04–5.42 | <0.001 |
| BMI ≤ 32.5 kg/m2 | 1.55 | 1.02–2.37 | 0.04 |
| Diabetes duration ≥ 13 years | 1.83 | 1.20–2.80 | 0.005 |
| Duration of hypertension ≥ 18 years | 1.95 | 1.26–3.04 | 0.003 |
| Daily insulin dose ≥ 0.59 IU/kg | 1.91 | 1.14–3.20 | 0.01 |
| eGFR ≤ 65.5 mL/min/1.73 m2 | 1.53 | 1.003–2.33 | 0.048 |
| log L-citrulline ≥ 2.1 | 4.83 | 1.47–15.9 | 0.01 |
| Diabetic retinopathy | 2.11 | 1.37–3.25 | 0.001 |
| CKD | 3.18 | 1.68–6.02 | 0.0004 |
| CAD | 2.99 | 1.94–4.62 | <0.0001 |
| Myocardial infarction | 3.20 | 1.75–5.83 | 0.0002 |
| PAD | 2.99 | 1.92–4.65 | <0.0001 |
| Parameter | Adjusted OR | 95% CI | p-Value |
|---|---|---|---|
| Parameters associated with CA1 | |||
| Age, years | 1.30 | 1.09–1.55 | 0.003 |
| BMI, kg/m2 | 0.84 | 0.72–0.97 | 0.02 |
| Male sex | 2.91 | 1.08–7.81 | 0.03 |
| Diabetes duration, years | 0.99 | 0.87–1.12 | 0.83 |
| MAGE, mmol/L | 2.38 | 1.16–4.86 | 0.02 |
| eGFR, mL/min/1.73 m2 | 1.06 | 1.003–1.13 | 0.04 |
| MMP-3, ng/mL | 1.09 | 0.9996–1.18 | 0.05 |
| Parameters associated with CAS2 | |||
| Age, years | 1.19 | 0.96–1.49 | 0.12 |
| BMI, kg/m2 | 0.60 | 0.39–0.90 | 0.01 |
| Male sex | 4.83 | 1.20–19.5 | 0.03 |
| Diabetes duration, years | 1.21 | 1.02–1.44 | 0.03 |
| eGFR, mL/min/1.73 m2 | 1.11 | 1.01–1.21 | 0.03 |
| L-citrulline, 10 μmol/L | 1.08 | 1.01–1.16 | 0.03 |
| Parameter | OR | 95% CI | p-Value | Se | Sp |
|---|---|---|---|---|---|
| Combinations associated with CA | |||||
| Age ≥ 62 years AND male sex | 3.20 | 0.96–10.6 | 0.06 | 0.16 | 0.94 |
| Age ≥ 62 years AND male sex AND duration of diabetes ≥ 11 years | 5.60 | 0.75–41.8 | 0.09 | 0.10 | 0.98 |
| Age ≥ 62 years AND male sex AND BMI ≤ 34.5 kg/m2 | 7.39 | 1.00–54.9 | 0.05 | 0.12 | 0.98 |
| (Age ≥ 62 years OR duration of diabetes ≥ 11 years) AND diabetic retinopathy | 2.38 | 1.26–4.48 | 0.007 | 0.48 | 0.72 |
| (Age ≥ 62 years OR duration of diabetes ≥ 11 years) AND CKD | 3.48 | 1.92–6.26 | 0.00004 | 0.70 | 0.59 |
| Male sex AND (age ≥ 62 years OR duration of diabetes ≥ 11 years) AND macrovascular disease (CAD OR PAD) | 4.33 | 1.31–14.3 | 0.02 | 0.20 | 0.94 |
| (Age ≥ 62 years OR duration of diabetes ≥ 11 years) AND macrovascular disease (CAD OR PAD) | 3.70 | 2.05–6.68 | 0.00001 | 0.76 | 0.54 |
| Age ≥ 62 years AND Duration of diabetes ≥ 11 years AND MAGE ≥ 3.38 mmol/L | 6.11 | 2.15–17.4 | 0.001 | 0.33 | 0.93 |
| Age ≥ 62 years AND Log MMP-3 ≥ 1.12 | 4.11 | 1.20–14.1 | 0.02 | 0.21 | 0.94 |
| (Age ≥ 62 years OR duration of diabetes ≥ 11 years) AND Log MMP-3 ≥ 1.12 | 3.87 | 1.43–10.5 | 0.008 | 0.33 | 0.89 |
| Combinations associated with CAS | |||||
| Age ≥ 66 years AND male sex | 2.60 | 1.32–5.12 | 0.006 | 0.16 | 0.93 |
| Age ≥ 66 years AND BMI ≤ 32.5 kg/m2 | 2.01 | 1.27–3.18 | 0.003 | 0.36 | 0.78 |
| Age ≥ 66 years AND duration of diabetes ≥ 13 years | 1.93 | 1.24–3.02 | 0.004 | 0.40 | 0.74 |
| Age ≥ 66 years AND (duration of diabetes ≥ 13 years OR duration of hypertension ≥ 18 years) | 2.22 | 1.44–3.42 | 0.0003 | 0.50 | 0.69 |
| Age ≥ 66 years AND (duration of diabetes ≥ 13 years OR insulin dosage ≥ 0.59) AND diabetic retinopathy | 2.24 | 1.39–3.61 | 0.001 | 0.35 | 0.80 |
| Age ≥ 66 years AND (duration of diabetes ≥ 13 years OR insulin dosage ≥ 0.59) AND CKD | 2.49 | 1.57–3.96 | 0.0001 | 0.47 | 0.74 |
| (Age ≥ 66 years OR duration of diabetes ≥ 13 years) AND eGFR ≤ 65.5 mL/min/1.73 m2 | 2.06 | 1.34–3.18 | 0.001 | 0.48 | 0.69 |
| Age ≥ 66 years AND (duration of diabetes ≥ 13 years OR insulin dosage ≥ 0.59) AND macrovascular disease (CAD OR PAD) | 3.18 | 2.03–4.97 | <0.00001 | 0.49 | 0.77 |
| (Age ≥ 66 years OR duration of diabetes ≥ 13 years) AND myocardial infarction | 3.67 | 1.86–7.24 | 0.0002 | 0.19 | 0.94 |
| Age ≥ 66 years AND log L-citrulline ≥ 2.10 | 7.27 | 1.81–29.1 | 0.005 | 0.12 | 0.98 |
| (Age ≥ 66 years OR duration of diabetes ≥ 13 years) AND log L-citrulline ≥ 2.10 | 3.86 | 1.50–9.94 | 0.005 | 0.26 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klimontov, V.V.; Koroleva, E.A.; Khapaev, R.S.; Korbut, A.I.; Lykov, A.P. Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers. J. Clin. Med. 2022, 11, 72. https://doi.org/10.3390/jcm11010072
Klimontov VV, Koroleva EA, Khapaev RS, Korbut AI, Lykov AP. Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers. Journal of Clinical Medicine. 2022; 11(1):72. https://doi.org/10.3390/jcm11010072
Chicago/Turabian StyleKlimontov, Vadim V., Elena A. Koroleva, Rustam S. Khapaev, Anton I. Korbut, and Alexander P. Lykov. 2022. "Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers" Journal of Clinical Medicine 11, no. 1: 72. https://doi.org/10.3390/jcm11010072
APA StyleKlimontov, V. V., Koroleva, E. A., Khapaev, R. S., Korbut, A. I., & Lykov, A. P. (2022). Carotid Artery Disease in Subjects with Type 2 Diabetes: Risk Factors and Biomarkers. Journal of Clinical Medicine, 11(1), 72. https://doi.org/10.3390/jcm11010072








