Acute and Long-Term Effects of an Internet-Based, Self-Help Comprehensive Behavioral Intervention for Children and Teens with Tic Disorders with Comorbid Attention Deficit Hyperactivity Disorder, or Obsessive Compulsive Disorder: A Reanalysis of Data from a Randomized Controlled Trial
Abstract
1. Introduction
2. Method
2.1. Assessment
2.2. Measures
2.3. Secondary Outcomes
2.4. Intervention
2.5. Statistical Analysis
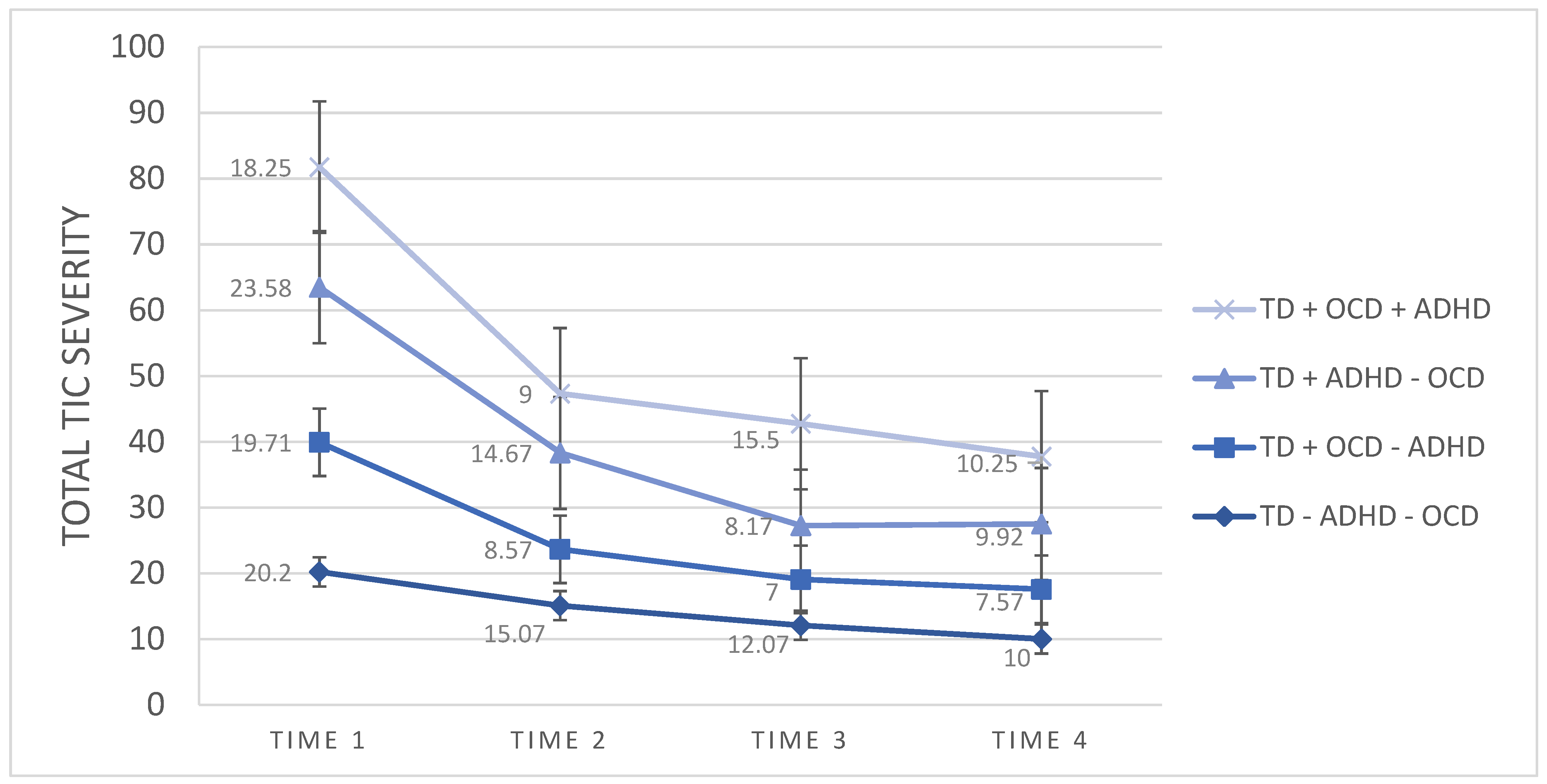
3. Results
3.1. TD + ADHD Group vs. TD − ADHD Group
3.2. TD + OCD Group vs. TD − OCD Group
4. Discussion
5. Clinical Implications
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Ethical Approval
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5; American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Bloch, M.H.; Leckman, J.F.; Zhu, H.; Peterson, B.S. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology 2005, 65, 1253–1258. [Google Scholar] [CrossRef] [PubMed]
- Scahill, L.; Specht, M.; Page, C. The prevalence of tic disorders and clinical characteristics in children. J. Obs.-Compuls. Relat. Disord. 2014, 3, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Hirschtritt, M.E.; Lee, P.C.; Pauls, D.L.; Dion, Y.; Grados, M.; Illmann, C.; King, R.A.; Sandor, P.; McMahon, W.M.; Lyon, G.J.; et al. Lifetime Prevalence, Age of Risk, and Genetic Relationships of Comorbid Psychiatric Disorders in Tourette Syndrome. JAMA Psychiatry 2015, 72, 325–333. [Google Scholar] [CrossRef]
- Andrén, P.; Jakubovski, E.; Murphy, T.L.; Woitecki, K.; Tarnok, Z.; Zimmerman-Brenner, S.; van de Griendt, J.; Debes, N.M.; Viefhaus, P.; Robinson, S.; et al. European clinical guidelines for Tourette syndrome and other tic disorders—Version 2.0. Part II: Psychological interventions. Eur. Child Adolesc. Psychiatry 2021, 1–21. [Google Scholar] [CrossRef]
- Ueda, K.; Kim, S.; Greene, D.J.; Black, K.J. Correlates and Clinical Implications of Tic Suppressibility. Curr. Dev. Disord. Rep. 2021, 8, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Sambrani, T.; Jakubovski, E.; Müller-Vahl, K. New Insights into Clinical Characteristics of Gilles de la Tourette Syndrome: Findings in 1032 Patients from a Single German Center. Front. Neurosci. 2016, 10, 415. [Google Scholar] [CrossRef]
- Coffey, B.J. Complexities for Assessment and Treatment of Co-Occurring ADHD and Tics. Curr. Dev. Disord. Rep. 2015, 2, 293–299. [Google Scholar] [CrossRef][Green Version]
- Deckersbach, T.; Chou, T.; Britton, J.C.; Carlson, L.E.; Reese, H.; Siev, J.; Scahill, L.; Piacentini, J.C.; Woods, D.W.; Walkup, J.T.; et al. Neural correlates of behavior therapy for Tourette׳s disorder. Psychiatry Res. Neuroimaging 2014, 224, 269–274. [Google Scholar] [CrossRef][Green Version]
- Sukhodolsky, D.G.; Woods, D.W.; Piacentini, J.; Wilhelm, S.; Peterson, A.L.; Katsovich, L.; Dziura, J.; Walkup, J.T.; Scahill, L. Moderators and predictors of response to behavior therapy for tics in Tourette syndrome. Neurology 2017, 88, 1029–1036. [Google Scholar] [CrossRef]
- Takács, Á.; Shilon, Y.; Janacsek, K.; Kóbor, A.; Tremblay, A.; Németh, D.; Ullman, M.T. Procedural learning in Tourette syndrome, ADHD, and comorbid Tourette-ADHD: Evidence from a probabilistic sequence learning task. Brain Cogn. 2017, 117, 33–40. [Google Scholar] [CrossRef]
- Roessner, V.; Becker, A.; Banaschewski, T.; Rothenberger, A. Executive functions in children with chronic tic disorders with/without ADHD: New insights. Eur. Child Adolesc. Psychiatry 2007, 16, 36–44. [Google Scholar] [CrossRef]
- Openneer, T.J.C.; van der Meer, D.; Marsman, J.-B.C.; Forde, N.J.; Akkermans, S.E.A.; Naaijen, J.; Buitelaar, J.K.; Hoekstra, P.J.; Dietrich, A. Impaired response inhibition during a stop-signal task in children with Tourette syndrome is related to ADHD symptoms: A functional magnetic resonance imaging study. World J. Biol. Psychiatry 2020, 22, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Shephard, E.; Jackson, G.M.; Groom, M.J. The effects of co-occurring ADHD symptoms on electrophysiological correlates of cognitive control in young people with Tourette syndrome. J. Neuropsychol. 2015, 10, 223–238. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; Grot, S.; Lavoie, J.; Leclerc, J.B.; Luck, D.; Lavoie, M.E. The puzzling question of inhibitory control in Tourette syndrome: A meta-analysis. Neurosci. Biobehav. Rev. 2017, 80, 240–262. [Google Scholar] [CrossRef] [PubMed]
- Erenberg, G. The Relationship Between Tourette Syndrome, Attention Deficit Hyperactivity Disorder, and Stimulant Medication: A Critical Review. Semin. Pediatr. Neurol. 2005, 12, 217–221. [Google Scholar] [CrossRef]
- McGuire, J.F.; Piacentini, J.; Brennan, E.A.; Lewin, A.B.; Murphy, T.K.; Small, B.; Storch, E.A. A meta-analysis of behavior therapy for Tourette Syndrome. J. Psychiatr. Res. 2014, 50, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman-Brenner, S.; Pilowsky-Peleg, T.; Rachamim, L.; Ben-Zvi, A.; Gur, N.; Murphy, T.; Fattal-Valevski, A.; Rotstein, M. Group behavioral interventions for tics and comorbid symptoms in children with chronic tic disorders. Eur. Child Adolesc. Psychiatry 2021, 1–12. [Google Scholar] [CrossRef]
- Conelea, C.A.; Wellen, B.; Woods, D.W.; Greene, D.; Black, K.; Specht, M.; Himle, M.B.; Lee, H.-J.; Capriotti, M. Patterns and Predictors of Tic Suppressibility in Youth With Tic Disorders. Front. Psychiatry 2018, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Lyon, G.J.; Samar, S.M.; Conelea, C.; Trujillo, M.R.; Lipinski, C.M.; Bauer, C.C.; Brandt, B.C.; Kemp, J.J.; Lawrence, Z.; Howard, J.; et al. Testing Tic Suppression: Comparing the Effects of Dexmethylphenidate to No Medication in Children and Adolescents with Attention-Deficit/Hyperactivity Disorder and Tourette’s Disorder. J. Child Adolesc. Psychopharmacol. 2010, 20, 283–289. [Google Scholar] [CrossRef]
- Hollis, C.; Hall, C.L.; Jones, R.; Marston, L.; Le Novere, M.; Hunter, R.; Brown, B.J.; Sanderson, C.; Andrén, P.; Bennett, S.D.; et al. Therapist-supported online remote behavioural intervention for tics in children and adolescents in England (ORBIT): A multicentre, parallel group, single-blind, randomised controlled trial. Lancet Psychiatry 2021, 8, 871–882. [Google Scholar] [CrossRef]
- Ganos, C.; Martino, D.; Pringsheim, T. Tics in the Pediatric Population: Pragmatic Management. Mov. Disord. Clin. Pract. 2016, 4, 160–172. [Google Scholar] [CrossRef]
- Woods, D.W.; Piacentini, J.; Chang, S.; Deckersbach, T.; Ginsburg, G.; Peterson, A.; Wilhelm, S. Managing Tourette Syndrome: A Behavioral Intervention for Children and Adult’s Therapist Guide; Oxford University Press: New York, NY, USA, 2008. [Google Scholar]
- Piacentini, J.; Woods, D.W.; Scahill, L.; Wilhelm, S.; Peterson, A.L.; Chang, S.; Ginsburg, G.S.; Deckersbach, T.; Dziura, J.; Levi-Pearl, S.; et al. Behavior Therapy for Children With Tourette Disorder. JAMA 2010, 303, 1929–1937. [Google Scholar] [CrossRef]
- Rachamim, L.; Zimmerman-Brenner, S.; Rachamim, O.; Mualem, H.; Zingboim, N.; Rotstein, M. Internet-based guided self-help comprehensive behavioral intervention for tics (ICBIT) for youth with tic disorders: A feasibility and effectiveness study with 6 month-follow-up. Eur. Child Adolesc. Psychiatry 2020, 1–13. [Google Scholar] [CrossRef]
- Woods, D.W.; Piacentini, J.C.; Scahill, L.; Peterson, A.L.; Wilhelm, S.; Chang, S.; Deckersbach, T.; McGuire, J.; Specht, M.; Conelea, C.A.; et al. Behavior Therapy for Tics in Children: Acute and Long-Term Effects on Psychiatric and Psychosocial Functioning. J. Child Neurol. 2011, 26, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Hollis, C.; Pennant, M.; Cuenca, J.; Glazebrook, C.; Kendall, T.; Whittington, C.; Stockton, S.; Larsson, L.; Bunton, P.; Dobson, S.; et al. Clinical effectiveness and patient perspectives of different treatment strategies for tics in children and adolescents with Tourette syndrome: A systematic review and qualitative analysis. Health Technol. Assess. 2016, 20, 1–450. [Google Scholar] [CrossRef] [PubMed]
- Woods, D.W.; Conelea, C.A.; Himle, M.B. Behavior therapy for Tourette’s disorder: Utilization in a community sample and an emerging area of practice for psychologists. Prof. Psychol. Res. Pract. 2010, 41, 518–525. [Google Scholar] [CrossRef]
- Leckman, J.F.; Riddle, M.A.; Hardin, M.T.; Ort, S.I.; Swartz, K.L.; Stevenson, J.; Cohen, D.J. The Yale Global Tic Severity Scale: Initial Testing of a Clinician-Rated Scale of Tic Severity. J. Am. Acad. Child Adolesc. Psychiatry 1989, 28, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Silverman, W.K.; Albano, A.M. The Anxiety Disorders Interview Schedule for DSM–IV—Child and Parent Versions; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Guy, W. Clinical Global Impressions, ECDEU Assessment Manual for Psychopharmacology; National Institute for Mental Health: Rockville, MD, USA, 1976; pp. 218–222.
- Shaffer, D.; Gould, M.S.; Brasic, J.; Ambrosini, P.; Fisher, P.; Bird, H.; Aluwahlia, S. A Children’s Global Assessment Scale (CGAS). Arch. Gen. Psychiatry 1983, 40, 1228–1231. [Google Scholar] [CrossRef]
- Goyette, C.H.; Conners, C.K.; Ulrich, R.F. Normative data on Revised Conners Parent and Teacher Rating Scales. J. Abnorm. Child Psychol. 1978, 6, 221–236. [Google Scholar] [CrossRef] [PubMed]
- Foa, E.B.; Coles, M.; Huppert, J.; Pasupuleti, R.V.; Franklin, M.E.; March, J. Development and Validation of a Child Version of the Obsessive Compulsive Inventory. Behav. Ther. 2010, 41, 121–132. [Google Scholar] [CrossRef]
- Lavoie, M.E.; Imbriglio, T.V.; Stip, E.; O’Connor, K.P. Neurocognitive Changes Following Cognitive-Behavioral Treatment in Tourette Syndrome and Chronic Tic Disorder. Int. J. Cogn. Ther. 2011, 4, 34–50. [Google Scholar] [CrossRef]
- Morand-Beaulieu, S.; Leclerc, J.B.; Valois, P.; Lavoie, M.E.; O’Connor, K.P.; Gauthier, B. A Review of the Neuropsychological Dimensions of Tourette Syndrome. Brain Sci. 2017, 7, 106. [Google Scholar] [CrossRef] [PubMed]
- Fosco, W.D.; Hawk, L.W.; Rosch, K.S.; Bubnik, M.G. Evaluating cognitive and motivational accounts of greater reinforcement effects among children with attention-deficit/hyperactivity disorder. Behav. Brain Funct. 2015, 11, 20. [Google Scholar] [CrossRef] [PubMed]
| Measure | TD + OCD (n = 11) | TD − OCD (n = 27) | Statistic | p | TD + ADHD (n = 16) | TD − ADHD (n = 22) | Statistic | p | |
|---|---|---|---|---|---|---|---|---|---|
| Age, mean (SD) | 12.00 (2.37) | 10.97 (1.72) | t(36) = −1.61 | 0.11 | 11.84 (2.47) | 10.90 (1.43) | t(36) = −1.48 | 0.14 | |
| Gender, n (%) | Males | 7 (63.63%) | 19 (70.37%) | χ2(1) = 0.16 | 0.68 | 15 (93.75%) | 11 (50.00%) | χ2(1) = 8.26 | 0.00 |
| Females | 4 (36.36%) | 8 (29.62%) | 1 (6.25%) | 11 (50.00%) | |||||
| Current medication use, n (%) | No | 7 (63.63%) | 22 (81.48%) | χ2(6) = 8.53 | 0.20 | 9 (56.25%) | 20 (90.90%) | χ2(6) = 8.43 | 0.20 |
| Past psycho-therapy experience, n (%) | No | 6 (54.54%) | 7 (25.92%) | χ2(5) = 4.22 | 0.51 | 3 (18.75%) | 10 (45.45%) | χ2(5) = 7.54 | 0.18 |
| Tic disorder, n (%) | CTD | 3 (27.27%) | 6 (22.22%) | t(7) = 0.00 | 0.38 | 3 (18.75%) | 6 (27.27%) | t(7) = 0.79 | 0.45 |
| TS | 8 (72.72%) | 21 (77.77%) | t(27) = 1.25 | 0.58 | 13 (81.25%) | 16 (72.72%) | t(27) = −1.21 | 0.23 | |
| SAD, n (%) | 3 (27.27%) | 3 (11.11%) | χ2(1) = 2.54 | 0.11 | 2 (12.5%) | 4 (18.18%) | χ2(1) = 0.11 | 0.73 | |
| GAD, n (%) | 5 (45.45%) | 10 (37.03%) | χ2(1) = 2.78 | 0.42 | 7 (43.75%) | 8 (36.36%) | χ2(1) = 1.25 | 0.53 | |
| SPD, n (%) | 2 (18.18%) | 7 (25.92%) | χ2(1) = 0.25 | 0.61 | 7 (43.75%) | 2 (9.09%) | χ2(1) = 6.15 | 0.01 | |
| SP, n (%) | 1 (9.09%) | 3 (11.11%) | χ2(1) = 0.07 | 0.78 | - | 4 (18.18%) | χ2(1) = 3.17 | 0.07 | |
| OCD, n (%) | 4 (36.36%) | 7 (25.92%) | χ2(1) = 2.66 | 0.44 | 4 (25.00%) | 7 (31.81%) | χ2(1) = 2.66 | 0.44 | |
| Dysthymia, n (%) | 1 (9.09%) | 2 (7.40%) | χ2(1) = 0.37 | 0.53 | 1 (6.25%) | 2 (9.09%) | χ2(1) = 0.06 | 0.80 | |
| Enuresis, n (%) | 1 (9.09%) | 1 (3.70%) | χ2(1) = 1.04 | 0.30 | 1 (6.25%) | 1 (4.54%) | χ2(1) = 0.10 | 0.74 | |
| Encopresis, n (%) | - | 1 (3.70%) | χ2(1) = 0.41 | 0.51 | 1 (6.25%) | - | χ2(1) = 1.41 | 0.23 | |
| LD, n (%) | 2 (18.18%) | 4 (14.81%) | χ2(1) = 0.07 | 0.79 | 5 (31.25%) | 1 (4.54%) | χ2(1) = 4.96 | 0.02 | |
| SMD, n (%) | 1 (9.09%) | 5 (18.51%) | χ2(1) = 0.52 | 0.47 | 4 (25.00%) | 2 (9.09%) | χ2(1) = 1.76 | 0.18 | |
| YGTSSMTS, mean (SD) | 19.28 (4.85) | 21.63 (6.22) | t(36) = 1.16 | 0.25 | 15.63 (2.36) | 15.18 (3.66) | t(36) = −0.42 | 0.67 | |
| YGTSSVTS, mean (SD) | 13.73 (2.61) | 16.04 (3.14) | t(36) = 2.14 | 0.38 | 6.06 (4.38) | 4.77 (5.26) | t(36) = −0.79 | 0.43 | |
| YGTSS TTS, mean (SD) | 5.45 (5.76) | 2.26 (4.61) | t(36) = −0.11 | 0.91 | 22.25 (5.67) | 19.95 (6.00) | t(36) = −1.19 | 0.24 | |
| YGTSS IS, mean (SD) | 31.82 (14.70) | 28.89 (15.27) | t(36) = −0.54 | 0.59 | 31.88 (16.41) | 28.18 (14.01) | t(36) = −0.74 | 0.46 | |
| OCI-CV (parent), mean (SD) | 4.82 (4.57) | 4.48 (4.24) | t(36) = −0.21 | 0.83 | - | - | - | - | |
| OCI-CV, mean (SD) | 13.73 (5.40) | 10.74 (6.54) | t(36) = −1.33 | 0.19 | - | - | - | - | |
| CPRS-R, mean (SD) | 41.75 (16.05) | 21.73 (14.42) | t(36) = −4.02 | 0.00 | |||||
| Inattentive, mean (SD) | 6.19 (2.58) | 2.45 (2.52) | t(36) = −4.45 | 0.00 | |||||
| Hyperactivity, mean (SD) | 9.50 (3.68) | 5.95 (3.98) | t(36) = −2.79 | 0.00 | |||||
| Anxiety, mean (SD) | 6.94 (2.74) | 3.64 (2.96) | t(36) = −3.49 | 0.00 | |||||
| Disruptive behavior, mean (SD) | 15.31 (11.14) | 6.55 (6.02) | t(36) = −3.12 | 0.00 | |||||
| Psychosomatic, mean (SD) | 3.81 (2.90) | 3.14 (2.90) | t(36) = −0.73 | 0.46 | |||||
| CGAS, mean (SD) | 66.64 (10.49) | 69.15 (12.78) | t(36) = 0.57 | 0.56 | 62.00 (10.12) | 73.09 (11.38) | t(36) = 3.10 | 0.00 |
| TD + ADHD (n = 16) | TD − ADHD (n = 22) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Time 4 Mean (SD) | Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Time 4 Mean (SD) | Time Effect (F Value, p) | Interaction (F Value, p) | |
| YGTSS MTS | 15.61 (0.97) | 9.55 (1.17) | 7.42 (1.10) | 7.86 (0.99) | 15.95 (0.86) | 10.71 (1.02) | 7.83 (0.98) | 6.47 (0.88) | F = 81.27, p = 0.00 | F = 0.96, p = 0.42 |
| YGTSS VTS | 7.55 (1.30) | 4.51 (1.00) | 3.04 (0.91) | 2.36 (0.68) | 4.78 (1.15) | 3.05 (0.87) | 2.25 (0.80) | 1.93 (0.60) | F = 7.94, p = 0.00 | F = 0.65, p = 0.58 |
| YGTSS TTS | 23.66 (1.61) | 15.66 (2.02) | 11.50 (1.95) | 11.36 (1.75) | 20.82 (1.43) | 14.08 (1.79) | 11.23 (1.69) | 9.86 (1.75) | F = 56.02, p = 0.00 | F = 0.47, p = 0.70 |
| YGTSS IS | 35.00 (3.27) | 12.10 (2.36) | 6.43 (2.35) | 2.73 (1.70) | 27.82 (3.27) | 12.10 (2.36) | 3.89 (2.09) | 4.95 (1.51) | F = 40.61, p = 0.00 | F = 1.62, p = 0.20 |
| CPRS-R | 44.27 (3.86) | 35.38 (3.93) | 31.61 (3.77) | 29.25 (3.65) | 23.08 (3.41) | 17.52 (3.48) | 18.82 (3.33) | 15.29 (3.65) | F = 14.96, p = 0.00 | F = 1.96, p = 0.13 |
| Inattentive | 6.44 (0.63) | 5.66 (0.70) | 5.61 (0.72) | 5.00 (0.62) | 2.47 (0.56) | 1.91 (0.62) | 2.30 (0.64) | 1.41 (0.54) | F = 4.27, p = 0.01 | F = 0.20 p = 0.89 |
| Hyperactivity | 10.00 (0.90) | 7.83 (0.83) | 7.33 (0.89) | 6.10 (0.87) | 6.04 (0.80) | 4.47 (0.73) | 4.39 (0.79) | 3.95 (0.76) | F = 14.51, p = 0.00 | F = 1.09, p = 0.36 |
| Anxiety | 7.16 (0.58) | 5.16 (0.63) | 4.38 (0.66) | 3.26 (0.53) | 3.60 (0.58) | 2.52 (0.56) | 2.82 (0.58) | 1.87 (0.53) | F = 12.40, p = 0.00 | F = 2.31, p = 0.09 |
| Disruptive Behavior | 16.33 (2.30) | 13.94 (2.27) | 11.50 (2.07) | 12.14 (2.15) | 7.91 (2.04) | 6.47 (2.01) | 6.95 (1.83) | 5.98 (1.89) | F = 5.34, p = 0.00 | F = 1.64, p = 0.19 |
| Psychosomatic | 4.33 (0.71) | 2.77 (0.67) | 2.77 (0.74) | 1.84 (0.54) | 3.04 (0.63) | 2.13 (0.59) | 2.34 (0.65) | 1.84 (0.54) | F = 6.03, p = 0.00 | F = 0.42, p = 0.73 |
| CGAS | 61.27 (2.54) | 67.23 (2.73) | 71.49 (2.78) | 74.13 (2.94) | 72.51 (2.24) | 79.73 (2.37) | 82.82 (2.44) | 84.00 (2.56) | F = 18.08, p = 0.00 | F = 0.50, p = 0.68 |
| CGI-I TD | - | 1.93 (0.19) | 1.56 (0.21) | 1.37 (0.15) | - | 1.86 (0.16) | 1.55 (0.19) | 1.45 (0.13) | F = 91.87, p = 0.00 | F = 0.28, p = 0.83 |
| CGI-I ADHD | - | - | - | - | - | 3.50 (0.19) | 3.18 (0.20) | 3.12 (0.22) | F = 1.69, p = 0.19 | - |
| TD + OCD (n = 11) | TD − OCD (n = 27) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Time 4 Mean (SD) | Time 1 Mean (SD) | Time 2 Mean (SD) | Time 3 Mean (SD) | Time 4 Mean (SD) | Time Effect (F Value, p) | Interaction (F Value, p) | |
| YGTSS MTS | 15.46 (1.15) | 7.15 (0.75) | 8.03 (1.36) | 7.09 (1.25) | 15.96 (0.78) | 11.35 (0.75) | 7.62 (0.90) | 7.17 (0.83) | F = 69.78, p = 0.00 | F = 6.74, p = 0.00 |
| YGTSS VTS | 6.38 (1.58) | 3.24 (1.21) | 3.16 (1.09) | 2.42 (0.81) | 5.82 (1.07) | 3.86 (0.80) | 2.33 (0.71) | 1.98 (0.54) | F = 6.74, p = 0.00 | F = 0.99, p = 0.40 |
| YGTSS TTS | 21.84 (1.94) | 13.00 (2.37) | 13.56 (2.55) | 10.43 (1.45) | 22.17 (1.32) | 15.60 (1.61) | 10.97 (1.70) | 10.43 (1.45) | F = 44.02, p = 0.00 | F = 3.69, p = 0.02 |
| YGTSS IS | 33.07 (4.45) | 11.53 (3.26) | 6.43 (2.85) | 3.070 (2.09) | 30.00 (3.03) | 14.25 (2.15) | 4.33 (1.87) | 3.98 (1.37) | F = 35.31, p = 0.00 | F = 1.46, p = 0.24 |
| CGAS | 64.92 (2.28) | 75.05 (3.62) | 77.54 (3.66) | 81.14 (3.72) | 68.82 (2.28) | 73.98 (2.45) | 77.98 (2.46) | 79.06 (3.72) | F = 22.29, P = 0.00 | F = 2.26, p = 0.09 |
| OCI-CV | 14.07 (1.67) | 9.15 (1.70) | 9.23 (1.84) | 5.47 (1.67) | 10.71 (1.13) | 6.67 (1.16) | 6.89 (1.25) | 4.86 (1.11) | F = 10.80, P = 0.00 | F = 0.35, p = 0.78 |
| CGI-I TD | - | 1.36 (0.20) | 1.72 (0.25) | 1.36 (0.18) | - | 2.11 (0.13) | 1.48 (0.17) | 1.44 (0.12) | F = 78.99, p = 0.00 | F = 2.50, p = 0.06 |
| CGI-I OCD | - | - | - | - | - | 1.80 (0.24) | 1.80 (2.00) | 1.60 (0.26) | F = 1.79, p = 0.18 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rachamim, L.; Mualem-Taylor, H.; Rachamim, O.; Rotstein, M.; Zimmerman-Brenner, S. Acute and Long-Term Effects of an Internet-Based, Self-Help Comprehensive Behavioral Intervention for Children and Teens with Tic Disorders with Comorbid Attention Deficit Hyperactivity Disorder, or Obsessive Compulsive Disorder: A Reanalysis of Data from a Randomized Controlled Trial. J. Clin. Med. 2022, 11, 45. https://doi.org/10.3390/jcm11010045
Rachamim L, Mualem-Taylor H, Rachamim O, Rotstein M, Zimmerman-Brenner S. Acute and Long-Term Effects of an Internet-Based, Self-Help Comprehensive Behavioral Intervention for Children and Teens with Tic Disorders with Comorbid Attention Deficit Hyperactivity Disorder, or Obsessive Compulsive Disorder: A Reanalysis of Data from a Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(1):45. https://doi.org/10.3390/jcm11010045
Chicago/Turabian StyleRachamim, Lilach, Hila Mualem-Taylor, Osnat Rachamim, Michael Rotstein, and Sharon Zimmerman-Brenner. 2022. "Acute and Long-Term Effects of an Internet-Based, Self-Help Comprehensive Behavioral Intervention for Children and Teens with Tic Disorders with Comorbid Attention Deficit Hyperactivity Disorder, or Obsessive Compulsive Disorder: A Reanalysis of Data from a Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 1: 45. https://doi.org/10.3390/jcm11010045
APA StyleRachamim, L., Mualem-Taylor, H., Rachamim, O., Rotstein, M., & Zimmerman-Brenner, S. (2022). Acute and Long-Term Effects of an Internet-Based, Self-Help Comprehensive Behavioral Intervention for Children and Teens with Tic Disorders with Comorbid Attention Deficit Hyperactivity Disorder, or Obsessive Compulsive Disorder: A Reanalysis of Data from a Randomized Controlled Trial. Journal of Clinical Medicine, 11(1), 45. https://doi.org/10.3390/jcm11010045





