Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. TAVR Procedure
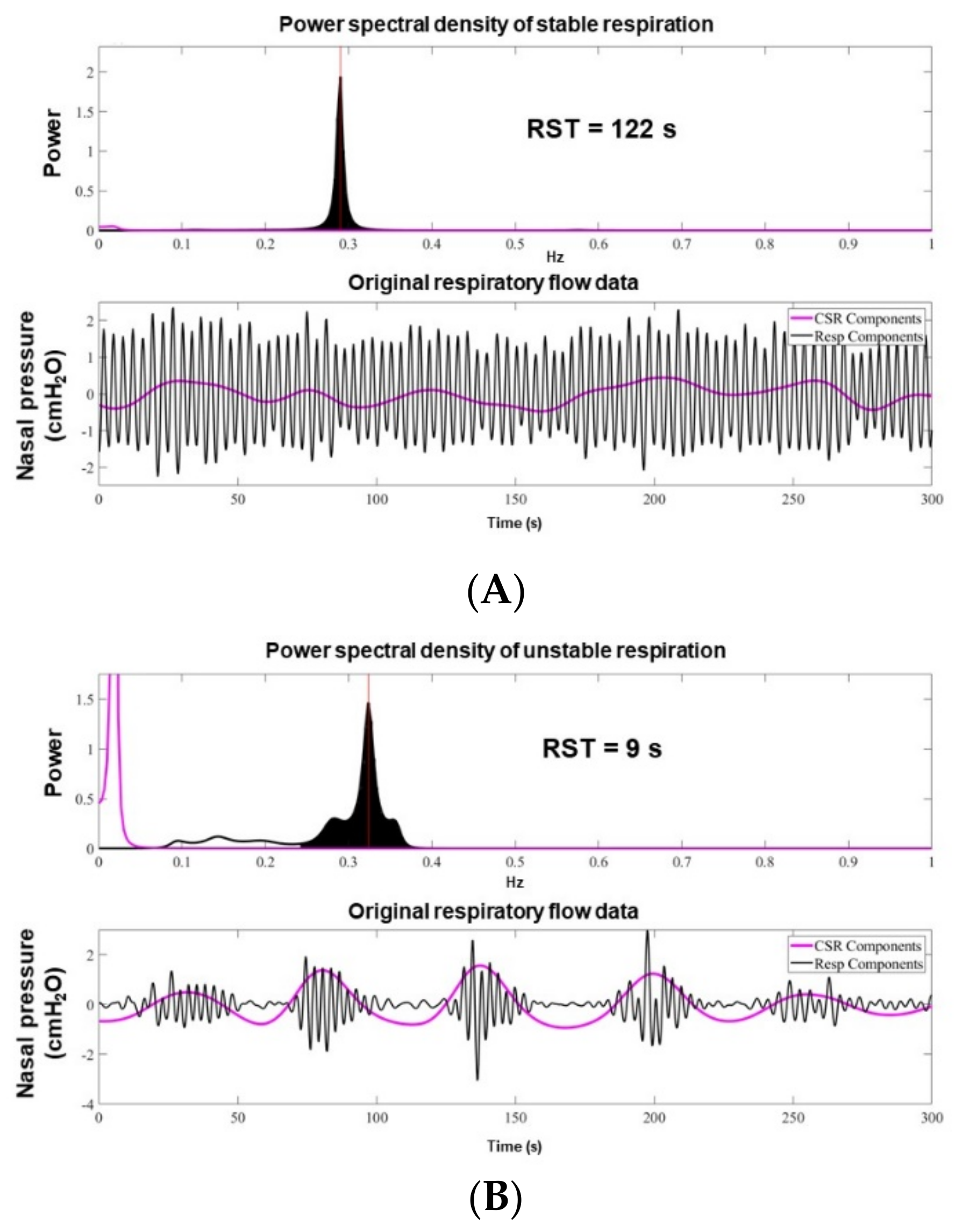
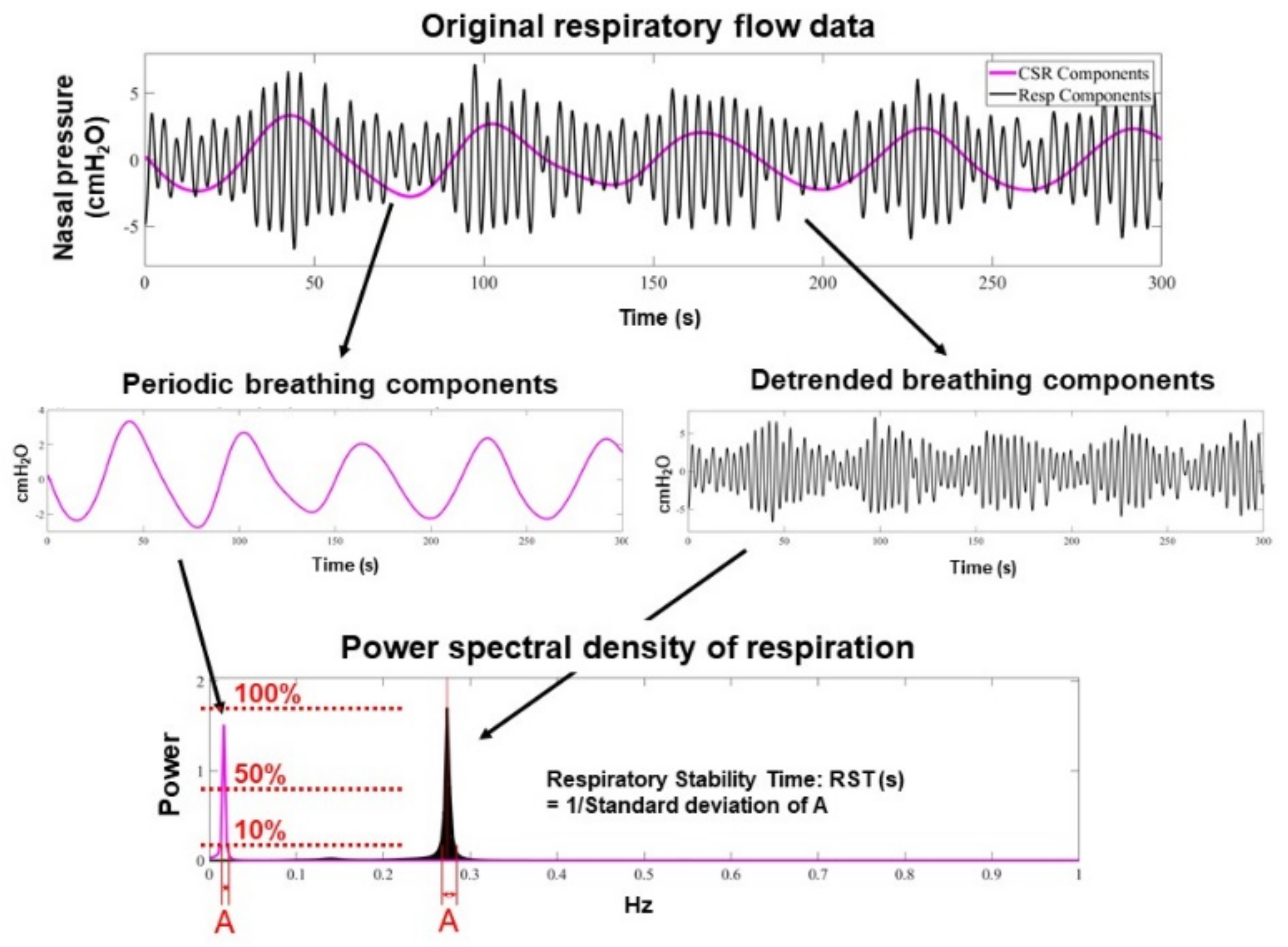
2.3. RST Measurement
2.4. Clinical Variables
2.5. Statistical Analyses
3. Results
3.1. Baseline Characteristics
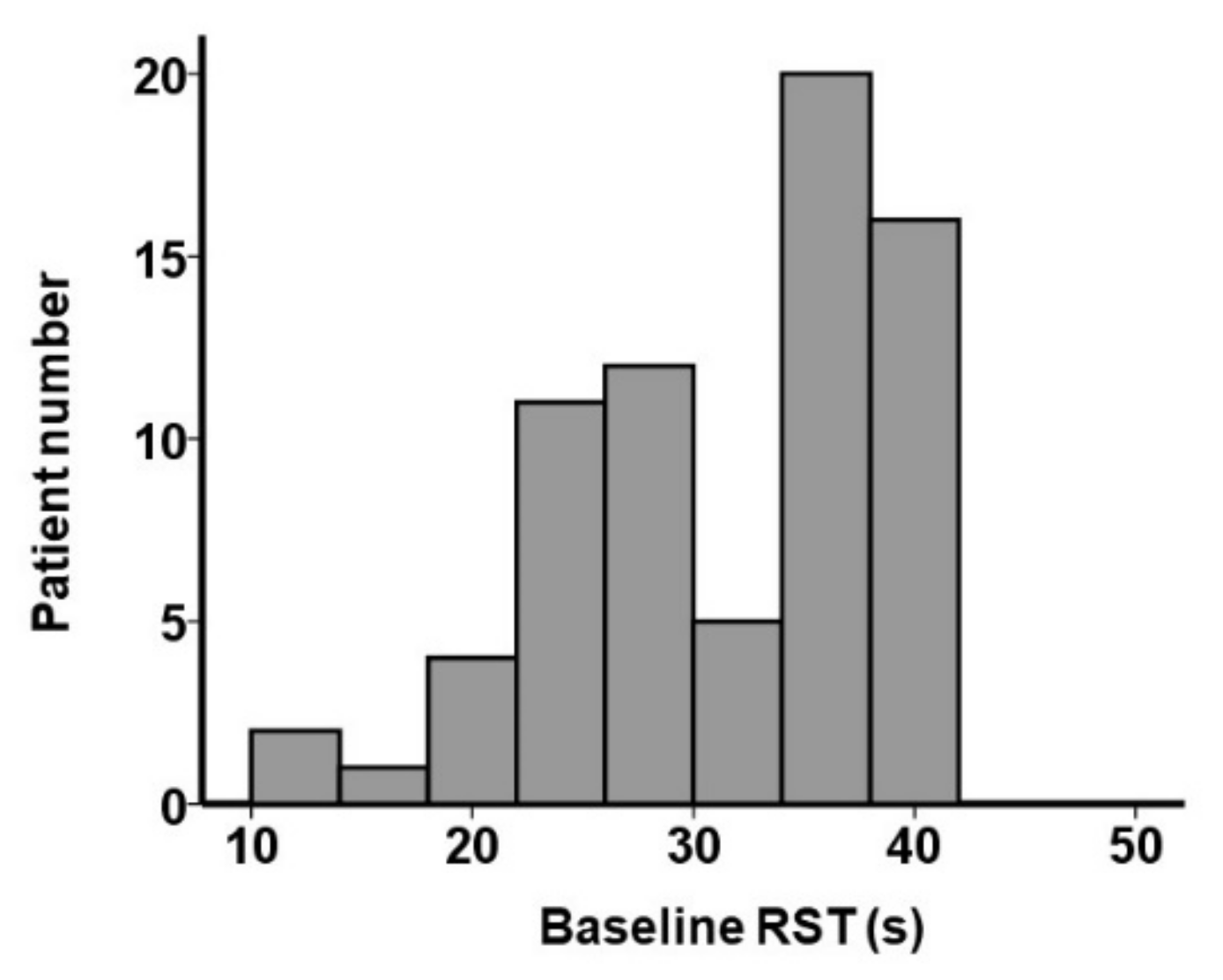
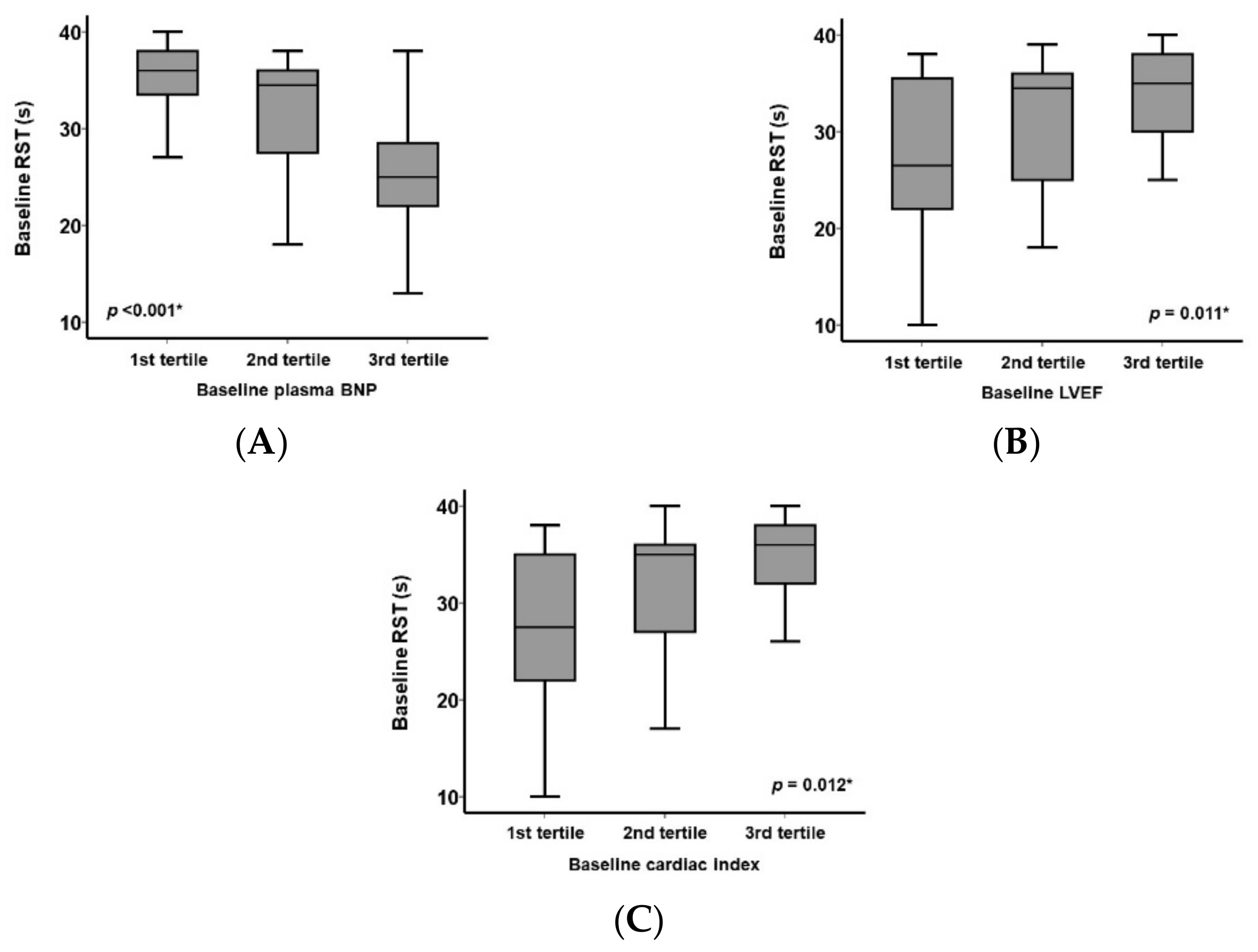
3.2. Baseline RST and Other Clinical Parameters
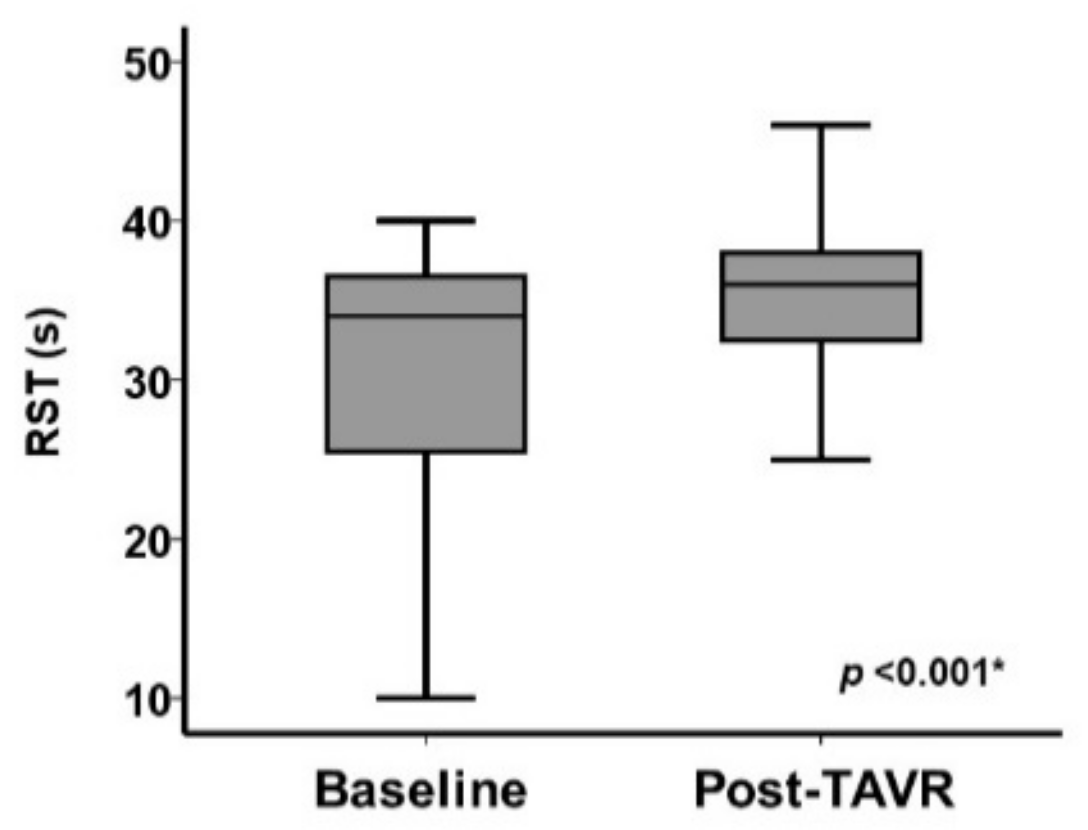
3.3. Change in Clinical Parameters Including RST Following TAVR
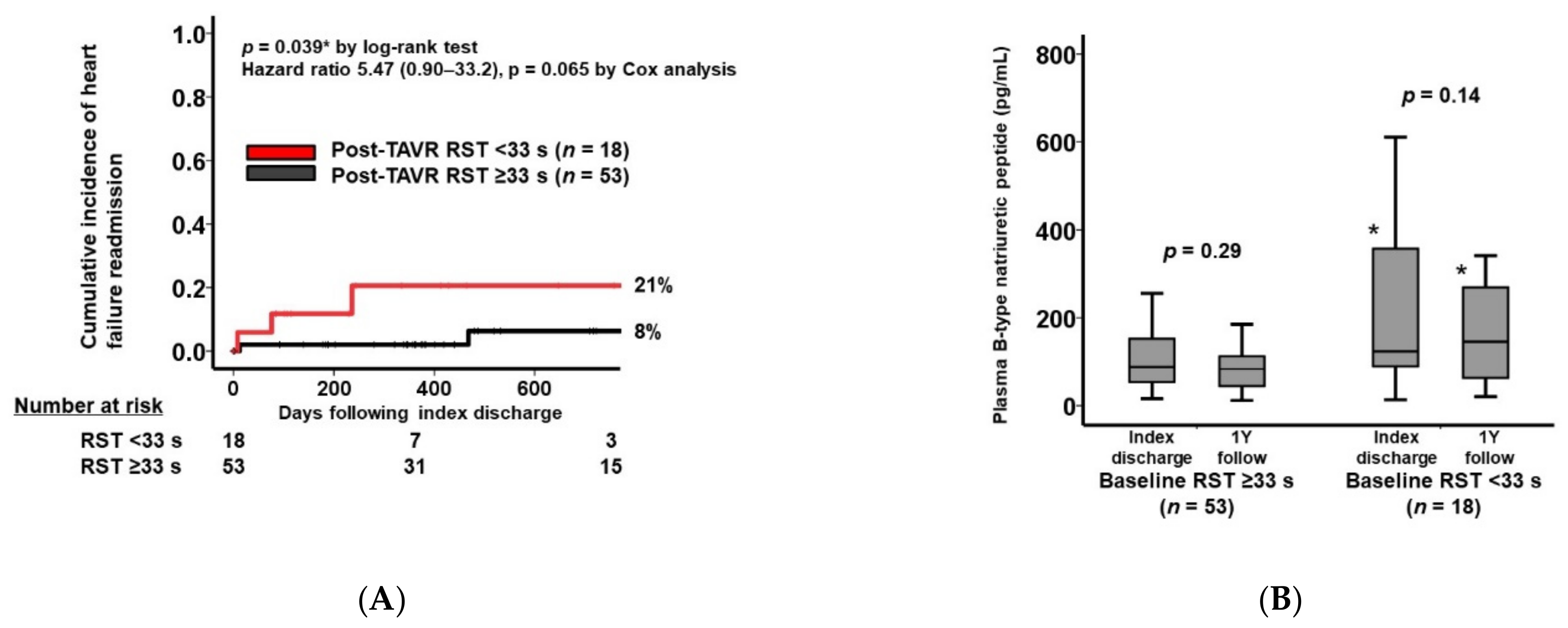
3.4. Clinical Outcomes
4. Discussion
4.1. Respiratory Instability and AS
4.2. Impact of TAVR on Respiratory Instability
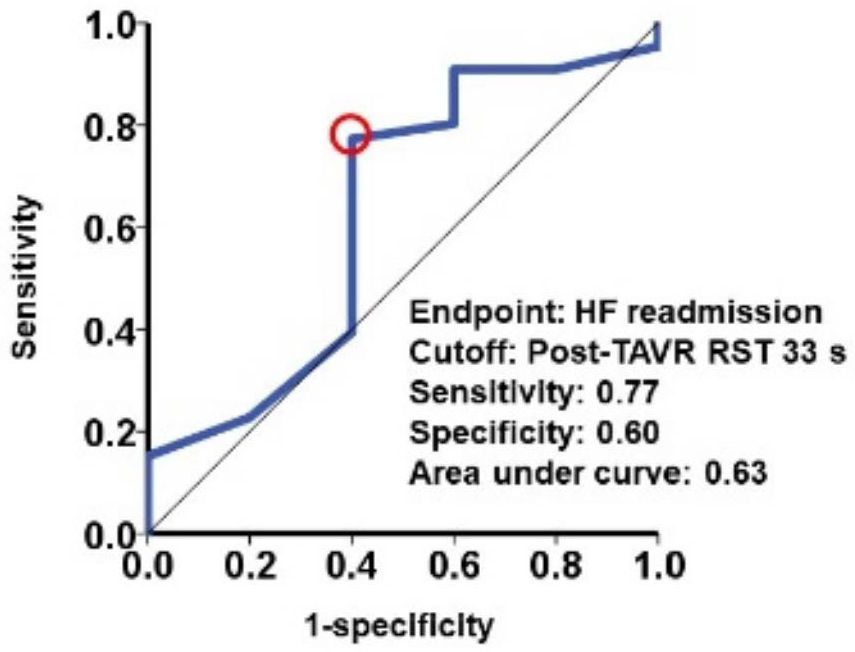
4.3. Respiratory Instability Following TAVR and Heart Failure Recurrence
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Leon, M.B.; Smith, C.R.; Mack, M.; Miller, D.C.; Moses, J.W.; Svensson, L.G.; Tuzcu, E.M.; Webb, J.G.; Fontana, G.P.; Makkar, R.R.; et al. Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N. Engl. J. Med. 2010, 363, 1597–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. PARTNER 3 investigators. transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Kishi, T. Deep and future insight into neuromodulation therapies for heart failure. J. Cardiol. 2016, 68, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Croix, C.M.; Satoh, M.; Morgan, B.J.; Skatrud, J.B.; Dempsey, J.A. Role of respiratory output in within-breath modulation of muscle sympathetic nerve activity in humans. Circ. Res. 1999, 85, 457–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goso, Y.; Asanoi, H.; Ishise, H.; Kameyama, T.; Hirai, T.; Nozawa, T.; Takashima, S.; Umeno, K.; Inoue, H. Respiratory modulation of muscle sympathetic nerve activity in patients with chronic heart failure. Circulation 2001, 104, 418–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueno, H.; Asanoi, H.; Yamada, K.; Oda, Y.; Takagawa, J.; Kameyama, T.; Hirai, T.; Nozawa, T.; Takashima, S.; Inoue, H. Attenuated respiratory modulation of chemoreflex- mediated sympathoexcitation in patients with chronic heart failure. J. Cardiac. Fail. 2004, 10, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Asanoi, H.; Harada, D.; Oda, Y.; Ueno, H.; Takagawa, J.; Ishise, H.; Goso, Y.; Joho, S.; Inoue, H. Independent prognostic importance of respiratory instability and sympathetic nerve activity in patients with chronic heart failure. J. Cardiol. 2017, 70, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Takagawa, J.; Asanoi, H.; Tobushi, T.; Kumagai, N.; Kadokami, T.; Dohi, K.; Joho, S.; Wada, O.; Koyama, T.; Haruki, N.; et al. Multicenter, prospective study on respiratory stability during recovery from deterioration of chronic heart failure. Circ. J. 2019, 83, 164–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linhart, M.; Sinning, J.M.; Ghanem, A.; Kozhuppakalam, F.J.; Fistéra, R.; Hammerstingl, C.; Pizarro, C.; Grube, E.; Werner, N.; Nickenig, G.; et al. Prevalence and impact of sleep disordered breathing in patients with severe aortic stenosis. PLoS ONE 2015, 10, e0133176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumonteil, N.; Vaccaro, A.; Despas, F.; Labrunee, M.; Marcheix, B.; Lambert, E.; Esler, M.; Carrie, D.; Senard, J.M.; Galinier, M.; et al. Transcatheter aortic valve implantation reduces sympathetic activity and normalizes arterial spontaneous baroreflex in patients with aortic stenosis. JACC Cardiovasc. Interv. 2013, 6, 1195–1202. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Asanoi, H.; Ueno, H.; Joho, S.; Takagawa, J.; Kameyama, T.; Hirai, T.; Nozawa, T.; Inoue, H. Role of sympathoexcitation in enhanced hypercapnic chemosensitivity in patients with heart failure. Am. Heart J. 2004, 148, 964–970. [Google Scholar] [CrossRef] [PubMed]
- Khoo, M.C.K.; Kronauer, R.E.; Strohl, K.P.; Slutsky, A.S. Factors inducing periodic breathing in humans: A general model. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982, 53, 644–659. [Google Scholar] [CrossRef] [PubMed]
- Bruce, E.N.; Daubenspeck, J.A. Mechanisms and analysis of ventilator stability. In Regulation of Breathing; Dempsey, J.A., Pack, A.I., Eds.; Marcel Dekker: New York, NY, USA, 1995; pp. 285–313. [Google Scholar]
- Roberts, A.M.; Bhattacharya, J.; Schultz, H.D.; Coleridge, H.M.; Coleridge, J.C. Stimulation of pulmonary vagal afferent C-fibers by lung edema in dogs. Circ. Res. 1986, 58, 512–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobajima, M.; Ueno, H.; Onoda, H.; Kuwahara, H.; Tanaka, S.; Ushijima, R.; Fukuda, N.; Yokoyama, S.; Nagura, S.; Doi, T.; et al. Transcatheter aortic valve implantation improves cardiac sympathetic nerve activity on 123 i-metaiodobenzylguanidine myocardial scintigraphy in severe aortic valve stenosis. Circ. J. 2018, 82, 579–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Total (n = 71) | Post-TAVR RST < 33 s (n = 18) | Post-TAVR RST ≥ 33 s (n = 53) | p Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 86 (83, 88) | 86 (83, 88) | 85 (82, 88) | 0.56 |
| Men | 25 (35%) | 6 (33%) | 19 (36%) | 0.54 |
| Body surface area, m2 | 1.45 (1.32, 1.57) | 1.38 (1.30, 1.61) | 1.47 (1.35, 1.58) | 0.65 |
| New York Heart Association (I/II/III/IV) | 1/34/35/1 | 0/6/12/0 | 1/27/21/1 | 0.32 |
| Comorbidity | ||||
| Diabetes mellitus | 20 (28%) | 4 (22%) | 16 (30%) | 0.37 |
| Atrial fibrillation | 5 (7%) | 1 (6%) | 4 (8%) | 0.77 |
| Ischemic heart disease | 20 (28%) | 7 (39%) | 13 (25%) | 0.19 |
| Chronic obstructive pulmonary disease | 4 (6%) | 1 (6%) | 3 (6%) | 0.73 |
| History of cardiac surgery | 3 (4%) | 2 (11%) | 1 (2%) | 0.16 |
| Dyslipidemia | 27 (38%) | 6 (33%) | 21 (40%) | 0.41 |
| Laboratory data | ||||
| Hemoglobin, g/dL | 11.4 (10.4, 12.3) | 11.5 (9.9, 12.4) | 11.4 (10.4, 12.2) | 0.99 |
| Serum albumin, g/dL | 3.8 (3.5, 3.9) | 3.8 (3.6, 3.9) | 3.7 (3.5, 3.9) | 0.96 |
| eGFR, mL/min/1.73 m2 | 51.2 (38.0, 60.2) | 52.8 (36.8, 64.1) | 51.2 (41.0, 60.2) | 0.63 |
| Serum sodium, mEq/L | 141(139, 143) | 140 (139, 141) | 141 (139, 143) | 0.12 |
| Plasma B-type natriuretic peptide, pg/mL | 245 (108, 495) | 344 (191, 665) | 258 (100, 476) | 0.043 * |
| Aortic valve parameter | ||||
| Maximum velocity, m/s | 4.41 (3.99, 4.77) | 4.49 (3.83, 4.91) | 4.31 (4.00, 4.73) | 0.63 |
| Mean pressure gradient, mmHg | 46 (36, 55) | 46 (34, 57) | 45 (36, 53) | 1.0 |
| Valve area, cm2 | 0.59 (0.48, 0.69) | 0.60 (0.52, 0.70) | 0.59 (0.48, 0.67) | 0.38 |
| Echocardiography | ||||
| Left ventricular end-diastolic diameter, mm | 44 (40, 50) | 47 (44, 51) | 48 (41, 54) | 0.92 |
| Left ventricular ejection fraction, % | 66 (53, 73) | 55 (53, 68) | 65 (51, 73) | 0.49 |
| Moderate or greater mitral regurgitation | 11 (15%) | 5 (28%) | 6 (11%) | 0.095 |
| Moderate or greater tricuspid regurgitation | 1 (1%) | 0 (0%) | 1 (2%) | 1.0 |
| Hemodynamics | ||||
| Mean right atrial pressure, mmHg | 6 (4, 8) | 5 (4, 7) | 6 (4, 8) | 0.59 |
| Pulmonary capillary wedge pressure, mmHg | 13 (10, 16) | 13 (10, 15) | 13 (10, 16) | 0.98 |
| Cardiac index, L/min/m2 | 2.5 (2.3, 2.5) | 2.5 (2.3, 2.8) | 2.8 (2.4, 3.0) | 0.11 |
| Systolic blood pressure, mmHg | 128 (118, 150) | 112 (109, 134) | 115 (104, 127) | 0.95 |
| Diastolic blood pressure, mmHg | 63 (55, 76) | 57 (51, 65) | 56 (51, 67) | 0.66 |
| Heart rate, bpm | 65 (60, 76) | 66 (62, 75) | 68 (59, 79) | 0.64 |
| Medication | ||||
| Beta-blocker | 27 (38%) | 9 (50%) | 18 (34%) | 0.18 |
| Renin-angiotensin system inhibitor | 45 (63%) | 12 (67%) | 33 (62%) | 0.49 |
| Scoring | ||||
| STS score | 4.6 (4.0, 6.7) | 5.2 (4.4, 7.3) | 4.6 (4.0, 6.5) | 0.39 |
| EURO II score | 4.6 (3.6, 5.5) | 3.4 (2.3, 7.3) | 4.6 (4.0, 6.5) | 0.79 |
| RST, s | 34 (26, 37) | 28 (25, 30) | 38 (36, 39) | <0.001 * |
| Baseline | After TAVR | p Value | |
|---|---|---|---|
| Laboratory data | |||
| Hemoglobin, g/dL | 11.4 (10.4, 12.3) | 10.4 (9.8, 11.6) | <0.001 * |
| Serum albumin, g/dL | 3.8 (3.5, 3.9) | 3.4 (3.1, 3.6) | <0.001 * |
| eGFR, mL/min/1.73 m2 | 51.2 (38.0, 60.2) | 54.5 (40.6, 64.6) | 0.30 |
| Serum sodium, mEq/L | 141 (139, 143) | 139 (137, 141) | <0.001 * |
| Plasma B-type natriuretic peptide, pg/mL | 244.5 (107.5, 495.1) | 103.4 (55.1, 187.4) | <0.001 * |
| Aortic valve parameter | |||
| Maximum velocity, m/s | 4.41(3.99, 4.77) | 1.98 (1.59, 2.32) | <0.001 * |
| Mean pressure gradient, mmHg | 46 (36, 55) | 8 (5, 11) | <0.001 * |
| Valve area, cm2 | 0.59 (0.48, 0.69) | 1.48 (1.24, 1.65) | <0.001 * |
| Echocardiography | |||
| Left ventricular end-diastolic diameter, mm | 44 (40, 50) | 43 (37, 49) | 0.40 |
| Left ventricular ejection fraction, % | 66 (53, 73) | 64 (55, 77) | 0.81 |
| Post-TAVR RST < 33 s (n = 18) | Post-TAVR RST ≥ 33 s (n = 53) | p Value | |
|---|---|---|---|
| Total major events | 4 (22%) | 8 (15%) | 0.49 |
| Permanent pacemaker implantation | 2 (11%) | 5 (9%) | 0.84 |
| Heart failure | 2 (11%) | 1 (2%) | 0.093 |
| Major bleeding | 0 (0%) | 1 (2%) | 0.56 |
| Stroke | 0 (0%) | 1 (2%) | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ueno, Y.; Imamura, T.; Oshima, A.; Onoda, H.; Ushijima, R.; Sobajima, M.; Fukuda, N.; Ueno, H.; Kinugawa, K. Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement. J. Clin. Med. 2022, 11, 280. https://doi.org/10.3390/jcm11010280
Ueno Y, Imamura T, Oshima A, Onoda H, Ushijima R, Sobajima M, Fukuda N, Ueno H, Kinugawa K. Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine. 2022; 11(1):280. https://doi.org/10.3390/jcm11010280
Chicago/Turabian StyleUeno, Yohei, Teruhiko Imamura, Akira Oshima, Hiroshi Onoda, Ryuichi Ushijima, Mitsuo Sobajima, Nobuyuki Fukuda, Hiroshi Ueno, and Koichiro Kinugawa. 2022. "Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement" Journal of Clinical Medicine 11, no. 1: 280. https://doi.org/10.3390/jcm11010280
APA StyleUeno, Y., Imamura, T., Oshima, A., Onoda, H., Ushijima, R., Sobajima, M., Fukuda, N., Ueno, H., & Kinugawa, K. (2022). Clinical Implications of Changes in Respiratory Instability Following Transcatheter Aortic Valve Replacement. Journal of Clinical Medicine, 11(1), 280. https://doi.org/10.3390/jcm11010280







