Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne)
Abstract
:1. Introduction
2. Materials and Methods
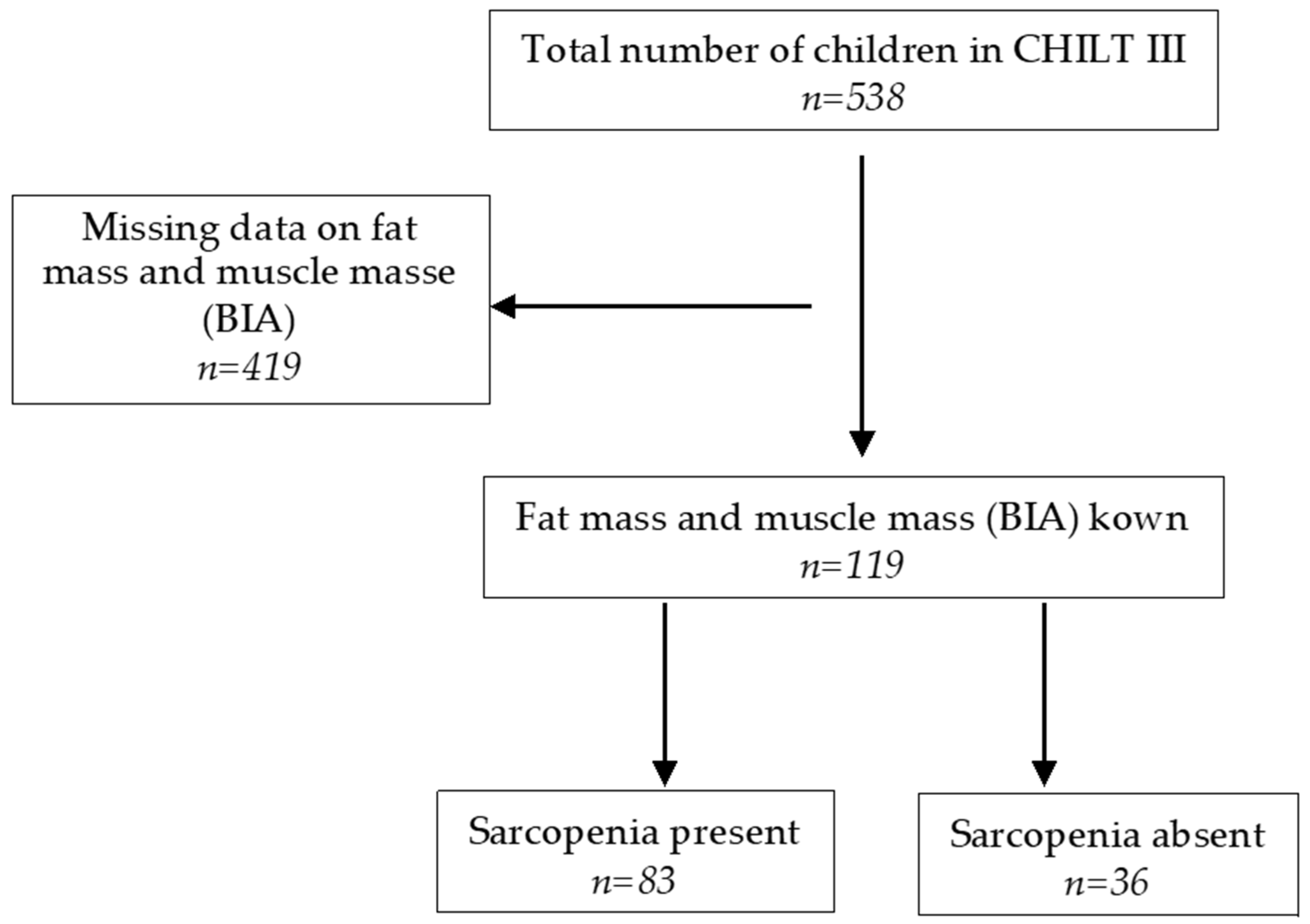
2.1. Sample Description
2.1.1. Anthropometric Data
2.1.2. Bioelectrical Impedance Analysis
2.1.3. Skeletal Muscle Mass, Fat Mass, and Muscle-to-Fat Ratio
2.1.4. Blood Pressure
2.1.5. Laboratory Parameters
2.1.6. Definition of Metabolic Syndrome (MetS)
2.1.7. Cardiorespiratory Fitness/Ergometry
2.1.8. Muscular Fitness/Standing Long Jump
2.2. Statistical Analysis
3. Results
3.1. Anthropometry
3.2. Laboratory Parameters and Blood Pressure
3.3. Muscle Mass and Cardiorespiratory/Muscular Fitness
3.4. Sarcopenia and MetS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Modified IDF Definition [38] for 10–16-Years Old | IDF Definition > 16 Years Old [39] |
|---|---|
| WC ≥ 90. percentile | WC m ≥ 94 cm, f ≥ 80 cm or BMI ≥ 30 kg/m2 |
| SBP ≥ 130 mmHg or DBP ≥ 85 mmHg | SBP ≥ 130 mmHg or DBP ≥ 85 mmHg or treatment of previously diagnosed hypertension |
| Triglycerides ≥ 150 mg/dL (≥1.7 mmol/L) | Triglycerides ≥ 150 mg/dL or specific treatment for this lipid abnormality |
| HDL < 40 mg/dL (<1.03 mmol/L) | HDL m < 40 mg/dL (<1.03 mmol/L), f <50 mg/dL (<1.29 mmol/L) |
| Fasting blood glucose ≥ 100 mg/dL (≥5.6 mmol/L) or DM type 2 | Fasting blood glucose ≥100 mg/dL (≥5.6 mmol/L) or DM type 2 |
| MetS = WC ≥ 90. percentile + 2 criteria | MetS = WC m ≥ 94 cm, f ≥ 80 cm or BMI ≥ 30 kg/m2 + 2 criteria |
| Parameter | Girls | Boys | p-Value |
|---|---|---|---|
| Age (years) | 11.6 ± 2.5 (n = 25) | 12.4 ± 2.2 (n = 58) | p = 0.209 ‡ |
| Height (m) | 1.53 ± 0.13 (n = 25) | 1.58 ± 0.14 (n = 58) | p = 0.105 ‡ |
| Weight (kg) | 72.1 ± 20.8 (n = 25) | 80.7 ± 26.2 (n = 58) | p = 0.288 ‡ |
| BMI (kg/m2) | 30.1 ± 5.0 (n = 25) | 31.4 ± 6.3 (n = 58) | p = 0.481 ‡ |
| BMI-SDS | 2.62 ± 0.44 (n = 25) | 2.57 ± 0.49 (n = 58) | p = 0.627 ‡ |
| Waist circumference (cm) | 92.4 ± 9.7 (n = 25) | 100.9 ± 17.0 (n = 57) | p = 0.042 ‡* |
| Parameter | Girls | Boys | p-Value |
|---|---|---|---|
| Systolic blood pressure (mmHg) | 113.2 ± 11.9 (n = 25) | 116.6 ± 12.3 (n = 58) | p = 0.136 ‡ |
| Diastolic blood pressure (mmHg) | 73.5 ± 7.3 (n = 25) | 71.0 ± 8.4 (n = 58) | p = 0.164 ‡ |
| Fasting blood glucose (mg/dL) | 89.8 ± 5.4 (n = 19) | 93.0 ± 6.8 (n = 46) | p = 0.079 ‡ |
| Insulin (µU/mL) | 22.1 ± 11.0 (n = 17) | 24.5 ± 12.1 (n = 45) | p = 0.390 ‡ |
| HOMA Index | 5.0 ± 2.7 (n = 17) | 5.6 ± 2.7 (n = 45) | p = 0.273 ‡ |
| Total cholesterol (mg/dL) | 171.7 ± 27.2 (n = 19) | 165.8 ± 29.7 (n = 47) | p = 0.332 ‡ |
| HDL cholesterol (mg/dL) | 47.6 ± 6.3 (n = 19) | 45.3 ± 9.4 (n = 47) | p = 0.272 ‡ |
| LDL cholesterol (mg/dL) | 102.9 ± 23.7 (n = 19) | 97.6 ± 27.1 (n = 47) | p = 0.506 ‡ |
| Triglycerides (mg/dL) | 109.8 ± 52.5 (n = 19) | 123.3 ± 85.2 (n = 47) | p = 0.745 ‡ |
| GOT (U/L) | 23.9 ± 8.1 (n = 19) | 30.9 ± 19.3 (n = 46) | p = 0.063 ‡ |
| GPT (U/L) | 21.5 ± 12.4 (n = 19) | 37.7 ± 40.1 (n = 46) | p = 0.006 ‡* |
| GGT (U/L) | 23.4 ± 16.2 (n = 18) | 26.5 ± 15.4 (n = 46) | p = 0.175 ‡ |
| hs-CRP (mg/L) | 3.9 ± 2.5 (n = 7) | 4.4 ± 3.3 (n = 17) | p = 0.852 ‡ |
| Leptin (ng/mL) | 9.1 ± 2.9 (n = 7) | 11.4 ± 4.8 (n = 16) | p = 0.308 ‡ |
| Parameter | Girls | Boys | p-Value |
|---|---|---|---|
| Fat mass (kg), anthropometric | 18.7 ± 6.3 (n = 25) | 21.3 ± 8.1 (n = 58) | p = 0.258 ‡ |
| Fat mass (kg), BIA | 28.5 ± 9.8 (n = 25) | 30.1 ± 12.1 (n = 58) | p = 0.770 ‡ |
| Muscle mass (kg), BIA | 21.0 ± 5.8 (n = 25) | 24.3 ± 8.6 (n = 58) | p = 0.130 ‡ |
| SMM (kg) | 20.2 ± 3.6 (n = 25) | 27.4 ± 5.5 (n = 58) | p < 0.001 ‡* |
| SMI (%) 1 | 29.0 ± 4.1 (n = 25) | 35.3 ± 5.0 (n = 58) | p < 0.001 ‡* |
| SMI (kg/m2) | 8.8 ± 1.4 (n = 25) | 9.4 ± 2.1 (n = 58) | p = 0.202 ‡ |
| MFR | 0.76 ± 0.11 (n = 25) | 0.85 ± 0.19 (n = 58) | p = 0.026 ‡* |
| Relative cardiorespiratory fitness (watts/kg) | 1.6 ± 0.6 (n = 24) | 1.5 ± 0.4 (n = 58) | p = 0.717 ‡ |
| Muscular fitness/standing long jump (cm) | 100.2 ± 9.8 (n = 16) | 107.2 ± 25.7 (n = 41) | p = 0.248 ‡ |
References
- Schienkiewitz, A.; Brettschneider, A.-K.; Damerow, S.; Schaffrath Rosario, A. Übergewicht und Adipositas im Kindes- und Jugendalter in Deutschland—Querschnittergebnisse aus KiGGS Welle 2 und Trends. J. Health Monit. 2018, 3, 16–23. [Google Scholar] [CrossRef]
- Abarca-Gómez, L.; Abdeen, Z.A.; Hamid, Z.A.; Abu-Rmeileh, N.M.; Acosta-Cazares, B.; Acuin, C.; Adams, R.J.; Aekplakorn, W.; Afsana, K.; Aguilar-Salinas, C.A.; et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar] [CrossRef] [Green Version]
- Jia, P.; Zhang, L.; Yu, W.; Yu, B.; Liu, M.; Zhang, D.; Yang, S. Impact of COVID-19 lockdown on activity patterns and weight status among youths in China: The COVID-19 Impact on Lifestyle Change Survey (COINLICS). Int. J. Obes. 2021, 45, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.S.; Mulder, C.; Twisk, J.W.R.; van Mechelen, W.; Chinapaw, M.J.M. Tracking of childhood overweight into adulthood: A systematic review of the literature. Obes. Rev. 2008, 9, 474–488. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Lakomek, M.; Müller, H.; Harz, K.J. Adipositas im Kindesalter: Ursachen und Therapiemöglichkeiten. Monatsschr. Kinderheilkd. 2002, 150, 329–336. [Google Scholar] [CrossRef]
- Knop, C.; Reinehr, T. Adipositas im Kindes- und Jugendalter. Aktuelle Ernährungsmedizin 2015, 40, 109–122. [Google Scholar] [CrossRef]
- Friedemann, C.; Heneghan, C.; Mahtani, K.; Thompson, M.; Perera, R.; Ward, A.M. Cardiovascular disease risk in healthy children and its association with body mass index: Systematic review and meta-analysis. BMJ 2012, 345, e4759. [Google Scholar] [CrossRef] [Green Version]
- Llewellyn, A.; Simmonds, M.; Owen, C.G.; Woolacott, N. Childhood obesity as a predictor of morbidity in adulthood: A systematic review and meta-analysis. Obes. Rev. 2016, 17, 56–67. [Google Scholar] [CrossRef]
- Ebbeling, C.B.; Pawlak, D.B.; Ludwig, D.S. Childhood obesity: Public-health crisis, common sense cure. Lancet 2002, 360, 473–482. [Google Scholar] [CrossRef]
- Reinehr, T.; de Sousa, G.; Toschke, A.M.; Andler, W. Comparison of metabolic syndrome prevalence using eight different definitions: A critical approach. Arch. Dis. Child. 2007, 92, 1067–1072. [Google Scholar] [CrossRef] [Green Version]
- Wabitsch, M.; Moss, A.; Denzer, C.; Fischer-Posovsky, P. Das metabolische Syndrom. Monatsschr. Kinderheilkd. 2012, 160, 277–292. [Google Scholar] [CrossRef]
- Hanefeld, M.; Pistrosch, F. Metabolisches Syndrom und Insulinresistenz. Gastroenterologe 2017, 12, 300–304. [Google Scholar] [CrossRef]
- Park, B.S.; Yoon, J.S. Relative skeletal muscle mass is associated with development of metabolic syndrome. Diabetes Metab. J. 2013, 37, 458–464. [Google Scholar] [CrossRef] [Green Version]
- Cauley, J.A. An Overview of Sarcopenic Obesity. J. Clin. Densitom. 2015, 18, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, R.N. Body composition in healthy aging. Ann. N. Y. Acad. Sci. 2000, 904, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.-P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Steffl, M.; Chrudimsky, J.; Tufano, J.J. Using relative handgrip strength to identify children at risk of sarcopenic obesity. PLoS ONE 2017, 12, e0177006. [Google Scholar] [CrossRef]
- Palacio-Agüero, A.; Díaz-Torrente, X.; Quintiliano Scarpelli Dourado, D. Relative handgrip strength, nutritional status and abdominal obesity in Chilean adolescents. PLoS ONE 2020, 15, e0234316. [Google Scholar] [CrossRef]
- Kim, T.N.; Park, M.S.; Lim, K.I.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; et al. Skeletal muscle mass to visceral fat area ratio is associated with metabolic syndrome and arterial stiffness: The Korean Sarcopenic Obesity Study (KSOS). Diabetes Res. Clin. Pract. 2011, 93, 285–291. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, H.D.; Samani-Radia, D.; Jebb, S.A.; Prentice, A.M. Skeletal muscle mass reference curves for children and adolescents. Pediatr. Obes. 2014, 9, 249–259. [Google Scholar] [CrossRef]
- Lier, L.M.; Breuer, C.; Ferrari, N.; Friesen, D.; Maisonave, F.; Schmidt, N.; Graf, C. Individual Physical Activity Behaviour and Group Composition as Determinants of the Effectiveness of a Childhood Obesity Intervention Program. Obes. Facts 2021, 14, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Hong, S.; Kim, E.Y. Reference Values of Skeletal Muscle Mass for Korean Children and Adolescents Using Data from the Korean National Health and Nutrition Examination Survey 2009–2011. PLoS ONE 2016, 11, e0153383. [Google Scholar] [CrossRef] [PubMed]
- Lier, L.M.; Breuer, C.; Ferrari, N.; Friesen, D.; Maisonave, F.; Schmidt, N.; Graf, C. Cost-effectiveness of a family-based multicomponent outpatient intervention program for children with obesity in Germany. Public Health 2020, 186, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Kromeyer-Hauschild, K.; Wabitsch, M.; Kunze, D.; Geller, F.; Geiß, H.C.; Hesse, V.; von Hippel, A.; Jaeger, U.; Johnsen, D.; Korte, W.; et al. Perzentile für den Body-mass-Index für das Kindes- und Jugendalter unter Heranziehung verschiedener deutscher Stichproben. Monatsschr. Kinderheilkd. 2001, 149, 807–818. [Google Scholar] [CrossRef] [Green Version]
- Wabitsch, M.; Kunze, D. Konsensbasierte AGA S2-Leitlinie zur Diagnostik, Therapie und Prävention von Übergewicht und Adipositas im Kindes- und Jugendalter: Version 15.10.2015. Available online: https://www.awmf.org/uploads/tx_szleitlinien/050-002l_S3_Therapie-Praevention-Adipositas-Kinder-Jugendliche_2019-11.pdf (accessed on 21 November 2021).
- Lohman, T.G.; Roche, A.F.; Martorel, R. Anthropometric Standardization Reference Manual; Human Kinetics Books: Champaign, IL, USA, 1988; ISBN 978-087-322-121-4. [Google Scholar]
- Slaughter, M.H.; Lohman, T.G.; Boileau, R.A.; Horswill, C.A.; Stillman, R.J.; van Loan, M.D.; Bemben, D.A. Skinfold equations for estimation of body fatness in children and youth. Hum. Biol. 1988, 60, 709–723. [Google Scholar] [PubMed]
- Chan, D.F.Y.; Li, A.M.; So, H.K.; Yin, J.; Nelson, E.A.S. New Skinfold-thickness Equation for Predicting Percentage Body Fat in Chinese Obese Children. Hong Kong J. Paediatr. 2009, 14, 96–102. [Google Scholar]
- Rodríguez, G.; Moreno, L.A.; Blay, M.G.; Blay, V.A.; Fleta, J.; Sarría, A.; Bueno, M. Body fat measurement in adolescents: Comparison of skinfold thickness equations with dual-energy X-ray absorptiometry. Eur. J. Clin. Nutr. 2005, 59, 1158–1166. [Google Scholar] [CrossRef]
- Data Input. Nutriguard-MS Gebrauchsanleitung: Version 2.0. 2019. Available online: https://www.data-input.de/media/pdf_deutsch_2018/Gebrauchsanleitung_Nutriguard%20MS_2019.pdf (accessed on 29 November 2021).
- Data Input. Nutri Plus Gebrauchsanleitung: Software zur Bestimmung von Körperzusammensetzung und Ernährungszustand aus BIA-Messungen. Available online: https://data-input.de/media/pdf_deutsch_2018/Data_Input_NutriPlus_Gebrauchsanleitung_DE.pdf (accessed on 21 November 2021).
- Janssen, I.; Heymsfield, S.B.; Baumgartner, R.N.; Ross, R. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J. Appl. Physiol. 2000, 89, 465–471. [Google Scholar] [CrossRef] [Green Version]
- Janssen, I.; Heymsfield, S.B.; Ross, R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J. Am. Geriatr. Soc. 2002, 50, 889–896. [Google Scholar] [CrossRef] [Green Version]
- Hager, A.; Wühl, E.; Bönner, G.; Hulpke-Wette, M.; Läer, S.; Weil, J. S2k Leitlinie Pädiatrische Kardiologie, Pädiatrische Nephrologie und Pädiatrie: Arterielle Hypertonie. Available online: https://www.dgpk.org/fileadmin/user_upload/Leitlinien/LL_Arterielle_Hypertonie.pdf (accessed on 21 November 2021).
- Nauck, M.; Warnick, G.R.; Rifai, N. Methods for Measurement of LDL-Cholesterol: A Critical Assessment of Direct Measurement by Homogeneous Assays versus Calculation. Clin. Chem. 2002, 48, 236–254. [Google Scholar] [CrossRef]
- Landwehr, C. Zusammenhang zwischen dem Mütterlichen Lebensstil, Ausgewählten Gesundheits- und Laborparametern Sowie dem Einfluss auf die Frühkindliche Entwicklung: Die “MAMA”-Studie. Ph.D. Thesis, Deutsche Sporthochschule Köln, Cologne, Germany, 2017. [Google Scholar]
- Matthews, D.R.; Hosker, J.R.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.M.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The metabolic syndrome in children and adolescents—An IDF consensus report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Zimmet, P.; Magliano, D.; Matsuzawa, Y.; Alberti, G.; Shaw, J. The metabolic syndrome: A global public health problem and a new definition. J. Atheroscler. Thromb. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Windhaber, J.; Schober, P.H. Leistungsmedizinische Ergometrie im Kindes- und Jugendalter. Monatsschr. Kinderheilkd. 2014, 162, 216–221. [Google Scholar] [CrossRef]
- Dordel, S.; Koch, B. Basistests zur Erfassung der Motorischen Leistungsfähigkeit von Kindern und Jugendlichen—Dordel-Koch-Test DKT Fitnessolympiade. Available online: https://fitnessolympiade.de/pdfs/manual-dordel-koch-test.pdf (accessed on 21 November 2021).
- Batsis, J.A.; Barre, L.K.; Mackenzie, T.A.; Pratt, S.I.; Lopez-Jimenez, F.; Bartels, S.J. Variation in the Prevalence of Sarcopenia and Sarcopenic Obesity in Older Adults Associated with Different Research Definitions: Dual-Energy X-Ray Absorptiometry Data form the National Health and Nutrition Examination Survey 1999–2004. J. Am. Geriatr. Soc. 2013, 61, 974–980. [Google Scholar] [CrossRef]
- Orkin, S.; Yodoshi, T.; Romantic, E.; Hitchcock, K.; Arce-Clachar, A.C.; Bramlage, K.; Sun, Q.; Fei, L.; Xanthakos, S.A.; Trout, A.T.; et al. Body composition measured by bioelectrical impedance analysis is a viable alternative to magnetic resonance imaging in children with nonalcoholic fatty liver disease. JPEN J. Parenter. Enter. Nutr. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Fuller, N.J.; Fewtrell, M.S.; Dewit, O.; Elia, M.; Wells, J.C.K. Segmental bioelectrical impedance analysis in children aged 8–12 y: 2. The assessment of regional body composition and muscle mass. Int. J. Obes. 2002, 26, 692–700. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Liu, J.; Ma, Y.; Li, Y.; Gao, D.; Chen, L.; Ma, T.; Dong, Y.; Ma, J. Association between Body Fat and Elevated Blood Pressure among Children and Adolescents Aged 7–17 Years: Using Dual-Energy X-ray Absorptiometry (DEXA) and Bioelectrical Impedance Analysis (BIA) from a Cross-Sectional Study in China. Int. J. Environ. Res. Public Health 2021, 18, 9254. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Vélez, R.; Rodrigues-Bezerra, D.; Correa-Bautista, J.E.; Izquierdo, M.; Lobelo, F. Reliability of Health-Related Physical Fitness Tests among Colombian Children and Adolescents: The FUPRECOL Study. PLoS ONE 2015, 10, e0140875. [Google Scholar] [CrossRef] [Green Version]
- Ruiz, J.R.; Castro-Piñero, J.; España-Romero, V.; Artero, E.G.; Ortega, F.B.; Cuenca, M.M.; Jimenez-Pavón, D.; Chillón, P.; Girela-Rejón, M.J.; Mora, J.; et al. Field-based fitness assessment in young people: The ALPHA health-related fitness test battery for children and adolescents. Br. J. Sports Med. 2011, 45, 518–524. [Google Scholar] [CrossRef] [PubMed]
- I’Allemand, D.; Wiegand, S.; Reinehr, T.; Müller, J.; Wabitsch, M.; Widhalm, K.; Holl, R. Cardiovascular risk in 26,008 European overweight children as established by a multicenter database. Obesity 2008, 16, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Total | Sarcopenia Absent | Sarcopenia Present | p-Value |
|---|---|---|---|---|
| Age (years) | 12.2 ± 2.2 (n = 119) | 12.4 ± 1.9 (n = 36) | 12.2 ± 2.3 (n = 83) | p = 0.635 † |
| Height (m) | 1.57 ± 0.12 (n = 118) | 1.58 ± 0.08 (n = 35) | 1.57 ± 0.14 (n = 83) | p = 0.623 † |
| Weight (kg) | 76.6 ± 22.8 (n = 118) | 73.0 ± 16.7 (n = 35) | 78.1 ± 24.9 (n = 83) | p = 0.469 ‡ |
| BMI (kg/m2) | 30.4 ± 5.6 (n = 118) | 29.0 ± 4.3 (n = 35) | 31.0 ± 6.0 (n = 83) | p = 0.083 ‡ |
| BMI-SDS | 2.52 ± 0.48 (n = 118) | 2.36 ± 0.44 (n = 35) | 2.58 ± 0.48 (n = 83) | p = 0.018 †* |
| Waist circumference (cm) | 96.2 ± 14.8 (n = 117) | 91.1 ± 11.2 (n = 35) | 98.3 ± 15.6 (n = 82) | p = 0.024 ‡* |
| Parameter | Total | Sarcopenia Absent | Sarcopenia Present | p-Value |
|---|---|---|---|---|
| Systolic blood pressure (mmHg) | 115.2 ± 12.1 (n = 119) | 114.6 ± 12.0 (n = 36) | 115.5 ± 12.2 (n = 83) | p = 0.720 ‡ |
| Diastolic blood pressure (mmHg) | 70.8 ± 8.1 (n = 119) | 68.5 ± 7.6 (n = 36) | 71.8 ± 8.1 (n = 83) | p = 0.046 ‡* |
| Fasting blood glucose (mg/dL) | 92.2 ± 7.1 (n = 95) | 92.5 ± 8.2 (n = 30) | 92.1 ± 6.5 (n = 65) | p = 0.779 † |
| Insulin (µU/mL) | 24.4 ± 11.7 (n = 86) | 25.7 ± 11.8 (n = 24) | 23.9 ± 11.7 (n = 62) | p = 0.516 ‡ |
| HOMA Index | 5.6 ± 2.7 (n = 86) | 5.9 ± 2.7 (n = 24) | 5.5 ± 2.7 (n = 62) | p = 0.441 ‡ |
| Total cholesterol (mg/dL) | 165.4 ± 26.8 (n = 96) | 160.9 ± 21.3 (n = 30) | 167.5 ± 28.9 (n = 66) | p = 0.268 † |
| HDL cholesterol (mg/dL) | 45.4 ± 8.7 (n = 96) | 44.1 ± 8.9 (n = 30) | 45.9 ± 8.6 (n = 66) | p = 0.354 † |
| LDL cholesterol (mg/dL) | 97.7 ± 24.3 (n = 96) | 94.5 ± 19.8 (n = 30) | 99.1 ± 26.1 (n = 66) | p = 0.388 † |
| Triglycerides (mg/dL) | 119.2 ± 69.9 (n = 96) | 118.6 ± 51.9 (n = 30) | 119.4 ± 77.1 (n = 66) | p = 0.543 ‡ |
| GOT (U/L) | 27.4 ± 14.9 (n = 95) | 24.4 ± 7.9 (n = 30) | 28.8 ± 17.1 (n = 65) | p = 0.207 ‡ |
| GPT (U/L) | 29.2 ± 30.5.(n = 95) | 21.1 ± 14.3 (n = 30) | 33.0 ± 35.1 (n = 65) | p = 0.003 ‡* |
| GGT (U/L) | 23.8 ± 13.5 (n = 93) | 19.7 ± 5.5 (n = 29) | 25.6 ± 15.5 (n = 64) | p = 0.028 ‡* |
| hs-CRP (mg/L) | 3.7 ± 3.0 (n = 30) | 1.4 ± 1.3 (n = 6) | 4.3 ± 3.0 (n = 24) | p = 0.009 ‡* |
| Leptin (ng/mL) | 11.6 ± 6.2 (n = 28) | 15.7 ± 11.2 (n = 5) | 10.7 ± 4.4 (n = 23) | p = 0.684 ‡ |
| Parameter | Total | Sarcopenia Absent | Sarcopenia Present | p-Value |
|---|---|---|---|---|
| Fat mass (kg), anthropometric | 19.8 ± 7.0 (n = 119) | 18.2 ± 4.9 (n = 36) | 20.5 ± 7.7 (n = 83) | p = 0.214 ‡ |
| Fat mass (kg), BIA | 28.1 ± 10.6 (n = 119) | 24.5 ± 7.7 (n = 36) | 29.6 ± 11.4 (n = 83) | p = 0.032 ‡* |
| Muscle mass (kg), BIA | 23.6 ± 7.4 (n = 119) | 24.3 ± 5.8 (n = 36) | 23.3 ± 7.9 (n = 83) | p = 0.163 ‡ |
| SMM (kg) | 24.8 ± 5.6 (n = 118) | 23.7 ± 4.4 (n = 35) | 25.2 ± 6.0 (n = 83) | p = 0.242 ‡ |
| SMI (%) | 33.3 ± 5.2 (n = 118) | 33.1 ± 4.5 (n = 35) | 33.4 ± 5.5 (n = 83) | p = 0.748 ‡* |
| SMI (kg/m2) | 9.4 ± 1.8 (n = 118) | 9.7 ± 1.5 (n = 35) | 9.2 ± 1.9 (n = 83) | p = 0.074 ‡ |
| MFR | 0.89 ± 0.22 (n = 119) | 1.04 ± 0.23 (n = 36) | 0.82 ± 0.18 (n = 83) | p < 0.001 ‡* |
| Relative cardiorespiratory fitness (watts/kg) | 1.6 ± 0.5 (n = 118) | 1.9 ± 0.4 (n = 36) | 1.6 ± 0.5 (n = 82) | p = 0.001 †* |
| Muscular fitness/standing long jump (cm) | 108.8 ± 22.9 (n = 84) | 116.2 ± 22.4 (n = 27) | 105.3 ± 22.5 (n = 57) | p = 0.041 †* |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sack, C.; Ferrari, N.; Friesen, D.; Haas, F.; Klaudius, M.; Schmidt, L.; Torbahn, G.; Wulff, H.; Joisten, C. Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne). J. Clin. Med. 2022, 11, 277. https://doi.org/10.3390/jcm11010277
Sack C, Ferrari N, Friesen D, Haas F, Klaudius M, Schmidt L, Torbahn G, Wulff H, Joisten C. Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne). Journal of Clinical Medicine. 2022; 11(1):277. https://doi.org/10.3390/jcm11010277
Chicago/Turabian StyleSack, Carolin, Nina Ferrari, David Friesen, Fabiola Haas, Marlen Klaudius, Lisa Schmidt, Gabriel Torbahn, Hagen Wulff, and Christine Joisten. 2022. "Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne)" Journal of Clinical Medicine 11, no. 1: 277. https://doi.org/10.3390/jcm11010277
APA StyleSack, C., Ferrari, N., Friesen, D., Haas, F., Klaudius, M., Schmidt, L., Torbahn, G., Wulff, H., & Joisten, C. (2022). Health Risks of Sarcopenic Obesity in Overweight Children and Adolescents: Data from the CHILT III Programme (Cologne). Journal of Clinical Medicine, 11(1), 277. https://doi.org/10.3390/jcm11010277






