The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Study Design
2.2. Study Population
- − The “loss of coverage” group (LoC group) including patients for whom pain coverage was not adequate (<60%).
- − The “SCS tolerance” group (SCStol group), including patients with adequate pain coverage for whom SCS no longer provided pain relief.
2.3. Clinical Strategy and Procedures
2.4. Outcome Measurements
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Pain Related Outcome following Device Adapter
3.2.1. Available Case Analysis
3.2.2. Last Observation Carried Forward Analysis
3.2.3. Worst-Case Analysis
3.3. Comparisons between the “Loss of Coverage” Group and the “SCS Tolerance” Group
3.3.1. Outcome Comparison
3.3.2. Waveform Comparison
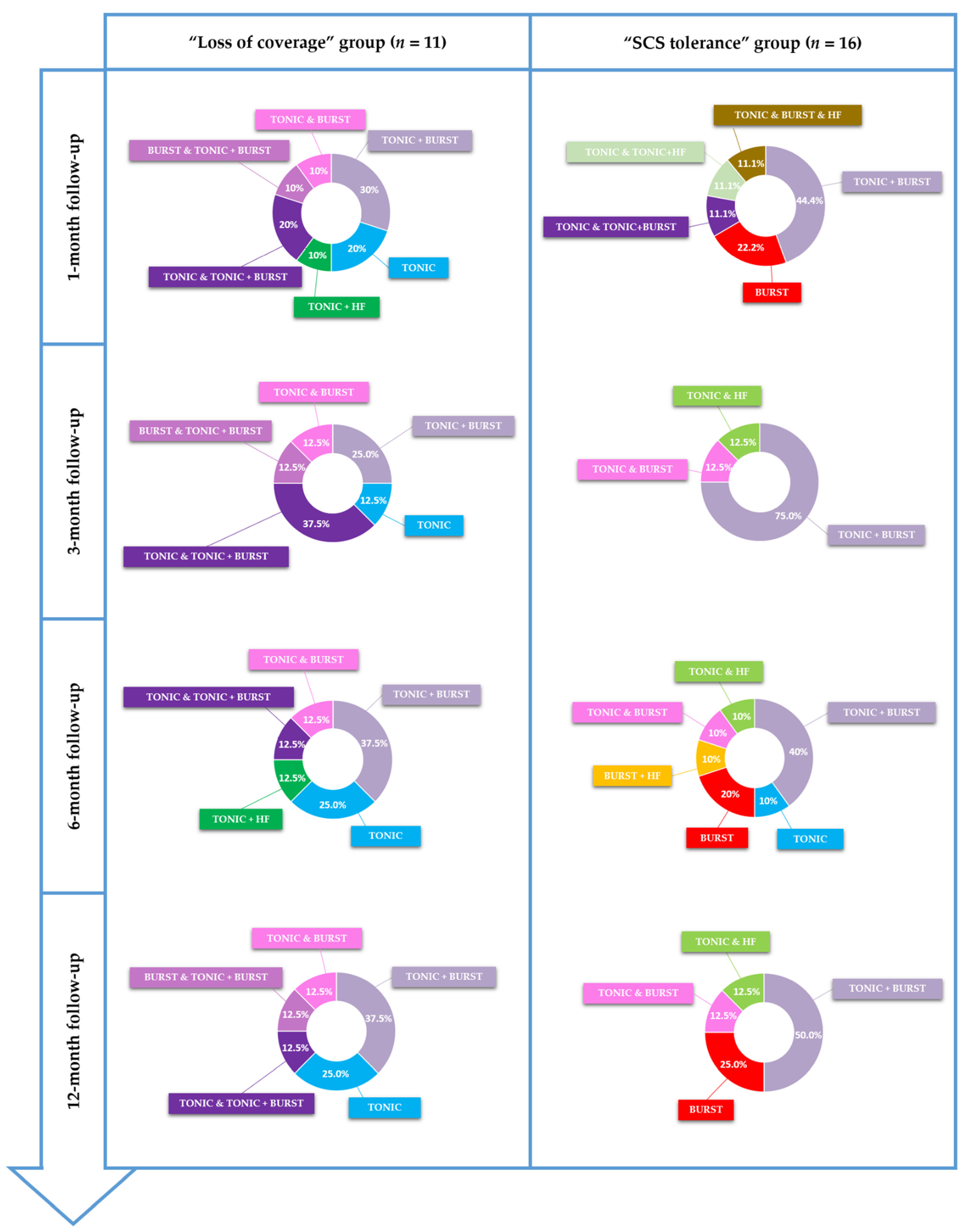
4. Discussion
4.1. Clinical Potential Added Value of Adapters in Case of FSCSS
4.2. Two Patient Profiles to Consider According to FSCSS Pathophysiology, Two Directions to Re-Explore Neural Retargeting but One Philosophy to Delineate, Aiming to Salvage SCS Failure
- − The first group includes patients facing LoC (n = 11), which potentially leads to LoE, and, surprisingly, once again, independently of the waveform used. This lack/loss of coverage might also result from neural plasticity, but the fact that some neurons were adequately depolarized to create an analgesic effect initially, but not longer, could correspond to the notion of “SCS resistance” rather than “SCS tolerance”. There appears to be a physical, architectural change in the localization of the targeted fibers (the so-called “sweet spot”), and the fact that we were able to spatially recapture all patient sweet spots with adapters, and that they remained stable within time, shows that: (i) this is a spatial problem, and that (ii) it was probably a technological limitation in the method of delivering the electrical field, which was responsible for previous SCS device failure to recapture the new target. This spatial recapturing corresponds to a major added value of the technology connected to the adapter. Arising from this argumentation, we can conclude that LoE recovery, through LoC spatial recapturing, is clearly not to be attributed to any waveform change or temporal resolution adjustment of the electrical signal. We can thereby assume that “SCS resistance”, characterizing this first group of patients, is a topographic matter. This topographic matter has been addressed by spatial optimization of the signal in this study.
- − Examining the specificities of this new technology, the difference in terms of spatial targeting abilities, compared to other SCS systems, might come from anatomically guided neural targeting, using a three-dimensional finite element model of the spinal cord, derived from the Twente University computational bio-electrical model [45,46]. This model is applied to shape the electrical field, when delivering paresthesia-based SCS therapy, through multi-source generation of the electrical field, involving independent electrical sources at the level of the IPG. This signal processing, called “Multiple Independent Current Control (MICCTM)”, places a dynamic but constant electrical field along dorsal horn intra-spinal structures, in axial distribution, as well as a uniform electrical influence on longitudinal spinal cord tracts, thereby optimizing “sweet spot” targeting.
- − The second group includes patients facing LoE, without LoC, who have developed the notion of “SCS tolerance” (n = 16), leading to another type of FSCSS. The notion of electric tolerance has been introduced more recently and is still the subject of debate. Tolerance could correspond either to local neurochemical adaptation to the electrical field delivered by the SCS lead [47] or to neurochemical “desensitization” at the level of spinal pathways/supra-spinal projections, even at the cortical integration level [48,49] or local architectural plasticity, including fibrotic developments, or else neural plasticity observed at the level of the central nervous system pathways/cortex. It is interesting to note that SCS tolerance has been reported in both sub-categories of stimulation: tonic stimulation and novel sub-perception waveforms [50,51]. The reasons for this phenomenon are not completely understood, but neural plasticity and fibrosis around the electrodes have been postulated as contributing factors [13]. When exposed to the same stimulation input in the long term, changes in synaptic connections may indeed occur in the pain transmission neural network, which becomes less sensitive to SCS. This neural adaptation could explain why some patients lose the therapeutic effect over the long term despite a stable electrical field producing adequate pain coverage.
- − It appears here, in contrast to the LoC group, that the solution might not come from spatial retargeting, since the electric field generated by the new SCS device stimulates the same fibers as the initial device, while the susceptibility of these fibers has changed and could benefit from non-spatial modalities. This has been achieved in this study by temporal adjustments of the electrical signal, with a clinically significant outcome, for this specific population, using various waveforms and combinations (such as tonic in combination with burst or HF). As an echo to recent data regarding the interest of combination therapy and the ability to customize programs for patients so as to give them a certain level of autonomy to combine waveforms [16,52] by cycling them when necessary (e.g., patients switching to sub-perception mode when sleeping), our findings should help to personalize the therapy, encompassing patient variability in terms of electrical susceptibility, and even tolerance.
4.3. Proposal of a Salvage SCS Algorithm for Failed SCS Syndrome (FSCSS)
4.3.1. Proposal for LoC Group: SCS Resistance Characterized by LoE Associated with LoC
4.3.2. Proposal for SCStol Group: SCS Tolerance Characterized by LoE without LoC

4.4. Study Limitations
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, K.; Taylor, R.S.; Jacques, L.; Eldabe, S.; Meglio, M.; Molet, J.; Thomson, S.; O’Callaghan, J.; Eisenberg, E.; Milbouw, G.; et al. The Effects of Spinal Cord Stimulation in Neuropathic Pain Are Sustained: A 24-Month Follow-up of the Prospective Randomized Controlled Multicenter Trial of the Effectiveness of Spinal Cord Stimulation. Neurosurgery 2008, 63, 762–770, discussion 770. [Google Scholar] [CrossRef] [PubMed]
- Duarte, R.V.; McNicol, E.; Colloca, L.; Taylor, R.S.; North, R.B.; Eldabe, S. Randomized Placebo-/Sham-Controlled Trials of Spinal Cord Stimulation: A Systematic Review and Methodological Appraisal. Neuromodulation 2020, 23, 10–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duarte, R.V.; Nevitt, S.; McNicol, E.; Taylor, R.S.; Buchser, E.; North, R.B.; Eldabe, S. Systematic Review and Meta-Analysis of Placebo/Sham Controlled Randomised Trials of Spinal Cord Stimulation for Neuropathic Pain. Pain 2020, 161, 24–35. [Google Scholar] [CrossRef] [PubMed]
- Deer, T.R.; Grider, J.S.; Lamer, T.J.; Pope, J.E.; Falowski, S.; Hunter, C.W.; Provenzano, D.A.; Slavin, K.V.; Russo, M.; Carayannopoulos, A.; et al. A Systematic Literature Review of Spine Neurostimulation Therapies for the Treatment of Pain. Pain Med. 2020, 21, 1421–1432. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Thomson, S.; Raso, L.; Burton, A.; Deandres, J.; Buchser, E.; et al. The Appropriate Use of Neurostimulation: Avoidance and Treatment of Complications of Neurostimulation Therapies for the Treatment of Chronic Pain. Neuromodulation 2014, 17, 571–598. [Google Scholar] [CrossRef]
- Reddy, R.D.; Moheimani, R.; Yu, G.G.; Chakravarthy, K.V. A Review of Clinical Data on Salvage Therapy in Spinal Cord Stimulation. Neuromodulation 2020, 23, 562–571. [Google Scholar] [CrossRef]
- Hayek, S.M.; Veizi, E.; Hanes, M. Treatment-Limiting Complications of Percutaneous Spinal Cord Stimulator Implants: A Review of Eight Years of Experience from an Academic Center Database. Neuromodulation 2015, 18, 603–608. [Google Scholar] [CrossRef]
- Van Buyten, J.P.; Wille, F.; Smet, I.; Wensing, C.; Breel, J.; Karst, E.; Devos, M.; Pöggel-Krämer, K.; Vesper, J. Therapy-Related Explants After Spinal Cord Stimulation: Results of an International Retrospective Chart Review Study. Neuromodulation 2017, 20, 642–649. [Google Scholar] [CrossRef] [Green Version]
- Levy, R.M.; Mekhail, N.; Kramer, J.; Poree, L.; Amirdelfan, K.; Grigsby, E.; Staats, P.; Burton, A.W.; Burgher, A.H.; Scowcroft, J.; et al. Therapy Habituation at 12 Months: Spinal Cord Stimulation Versus Dorsal Root Ganglion Stimulation for Complex Regional Pain Syndrome Type I and II. J. Pain 2019, 21, 399–408. [Google Scholar] [CrossRef]
- Aiudi, C.M.; Dunn, R.Y.; Burns, S.M.; Roth, S.A.; Opalacz, A.; Zhang, Y.; Chen, L.; Mao, J.; Ahmed, S.U. Loss of Efficacy to Spinal Cord Stimulator Therapy: Clinical Evidence and Possible Causes. Pain Physician 2017, 20, E1073–E1080. [Google Scholar] [CrossRef]
- Ghosh, P.E.; Gill, J.S.; Simopoulos, T. The Evolving Role of High-Frequency Spinal Cord Stimulation as Salvage Therapy in Neurostimulation. Pain Pract. 2020, 20, 706–713. [Google Scholar] [CrossRef]
- Cameron, T. Safety and Efficacy of Spinal Cord Stimulation for the Treatment of Chronic Pain: A 20-Year Literature Review. J. Neurosurg. Spine 2004, 100, 254–267. [Google Scholar] [CrossRef] [Green Version]
- Kumar, K.; Hunter, G.; Demeria, D. Spinal Cord Stimulation in Treatment of Chronic Benign Pain: Challenges in Treatment Planning and Present Status, a 22-Year Experience. Neurosurgery 2006, 58, 481–494. [Google Scholar] [CrossRef]
- Deer, T.R.; Mekhail, N.; Provenzano, D.; Pope, J.; Krames, E.; Leong, M.; Levy, R.M.; Abejon, D.; Buchser, E.; Burton, A.; et al. The Appropriate Use of Neurostimulation of the Spinal Cord and Peripheral Nervous System for the Treatment of Chronic Pain and Ischemic Diseases: The Neuromodulation Appropriateness Consensus Committee. Neuromodulation 2014, 17, 515–550. [Google Scholar] [CrossRef]
- Kumar, K. Complications of Spinal Cord Stimulation, Suggestions to Improve Outcome, and Financial Impact. J. Neurosurg. Spine 2006, 5, 191–203. [Google Scholar] [CrossRef]
- Metzger, C.S.; Hammond, M.B.; Pyles, S.T.; Washabaugh, E.P., 3rd; Waghmarae, R.; Berg, A.P.; North, J.M.; Pei, Y.; Jain, R. Pain Relief Outcomes Using an SCS Device Capable of Delivering Combination Therapy with Advanced Waveforms and Field Shapes. Expert Rev. Med. Devices 2020, 17, 951–957. [Google Scholar] [CrossRef]
- Veizi, E.; Hayek, S.M.; North, J.; Brent Chafin, T.; Yearwood, T.L.; Raso, L.; Frey, R.; Cairns, K.; Berg, A.; Brendel, J.; et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. 2017, 18, 1534–1548. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.; Heiden, P. 1.2 KHz High-Frequency Stimulation as a Rescue Therapy in Patients With Chronic Pain Refractory to Conventional Spinal Cord Stimulation. Neuromodul. Technol. Neural Interface 2021, 24, 540–545. [Google Scholar] [CrossRef]
- Hunter, C.W.; Carlson, J.; Yang, A.; Patterson, D.; Lowry, B.; Mehta, P.; Rowe, J.; Deer, T. BURST(Able): A Retrospective, Multicenter Study Examining the Impact of Spinal Cord Stimulation with Burst on Pain and Opioid Consumption in the Setting of Salvage Treatment and “Upgrade”. Pain Physician 2020, 23, E643–E658. [Google Scholar]
- Kallewaard, J.W.; Paz-Solis, J.F.; De Negri, P.; Canós-Verdecho, M.A.; Belaid, H.; Thomson, S.J.; Abejón, D.; Vesper, J.; Mehta, V.; Rigoard, P.; et al. Real-World Outcomes Using a Spinal Cord Stimulation Device Capable of Combination Therapy for Chronic Pain: A European, Multicenter Experience. J. Clin. Med. 2021, 10, 4085. [Google Scholar] [CrossRef]
- Rigoard, P.; Nivole, K.; Blouin, P.; Monlezun, O.; Roulaud, M.; Lorgeoux, B.; Bataille, B.; Guetarni, F. A Novel, Objective, Quantitative Method of Evaluation of the Back Pain Component Using Comparative Computerized Multi-Parametric Tactile Mapping before/after Spinal Cord Stimulation and Database Analysis: The “Neuro-Pain’t” Software. Neurochirurgie 2015, 61, S99–S108. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Louis, P.-Y.; Slaoui, Y.; Roulaud, M.; Naiditch, N.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. J. Clin. Med. 2021, 10, 4910. [Google Scholar] [CrossRef]
- Rigoard, P.; Billot, M.; Ingrand, P.; Durand-Zaleski, I.; Roulaud, M.; Peruzzi, P.; Dam Hieu, P.; Voirin, J.; Raoul, S.; Page, P.; et al. How Should We Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation 2021, 24, 86–101. [Google Scholar] [CrossRef]
- Rigoard, P.; Ounajim, A.; Goudman, L.; Bouche, B.; Roulaud, M.; Page, P.; Lorgeoux, B.; Baron, S.; Nivole, K.; Many, M.; et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: A Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. J. Clin. Med. 2021, 10, 5094. [Google Scholar] [CrossRef]
- Maldonado, G.; Greenland, S. Simulation Study of Confounder-Selection Strategies. Am. J. Epidemiol. 1993, 138, 923–936. [Google Scholar] [CrossRef] [Green Version]
- Unnebrink, K.; Windeler, J. Sensitivity Analysis by Worst and Best Case Assessment: Is It Really Sensitive? Drug Inf. J. 1999, 33, 835–839. [Google Scholar] [CrossRef]
- Cook, T.; Zea, R. Missing Data and Sensitivity Analysis for Binary Data with Implications for Sample Size and Power of Randomized Clinical Trials. Stat. Med. 2020, 39, 192–204. [Google Scholar] [CrossRef]
- Rothman, K.J. No Adjustments Are Needed for Multiple Comparisons. Epidemiology 1990, 1, 43–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Althouse, A.D. Adjust for Multiple Comparisons? It’s Not That Simple. Ann. Thorac. Surg. 2016, 101, 1644–1645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, J.E.; Deer, T.R.; Falowski, S.; Provenzano, D.; Hanes, M.; Hayek, S.M.; Amrani, J.; Carlson, J.; Skaribas, I.; Parchuri, K.; et al. Multicenter Retrospective Study of Neurostimulation With Exit of Therapy by Explant. Neuromodulation 2017, 20, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Kemler, M.A.; de Vet, H.C.W.; Barendse, G.A.M.; van den Wildenberg, F.A.J.M.; van Kleef, M. Effect of Spinal Cord Stimulation for Chronic Complex Regional Pain Syndrome Type I: Five-Year Final Follow-up of Patients in a Randomized Controlled Trial. J. Neurosurg. 2008, 108, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Mann, S.A.; Sparkes, E.; Duarte, R.V.; Raphael, J.H. Attrition with Spinal Cord Stimulation. Br. J. Neurosurg. 2015, 29, 823–828. [Google Scholar] [CrossRef]
- Duse, G.; Reverberi, C.; Dario, A. Effects of Multiple Waveforms on Patient Preferences and Clinical Outcomes in Patients Treated With Spinal Cord Stimulation for Leg and/or Back Pain. Neuromodul. Technol. Neural Interface 2018, 22, 200–207. [Google Scholar] [CrossRef]
- Deer, T.; Slavin, K.V.; Amirdelfan, K.; North, R.B.; Burton, A.W.; Yearwood, T.L.; Tavel, E.; Staats, P.; Falowski, S.; Pope, J.; et al. Success Using Neuromodulation With BURST (SUNBURST) Study: Results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation 2018, 21, 56–66. [Google Scholar] [CrossRef]
- Thomson, S.J.; Tavakkolizadeh, M.; Love-Jones, S.; Patel, N.K.; Gu, J.W.; Bains, A.; Doan, Q.; Moffitt, M. Effects of Rate on Analgesia in Kilohertz Frequency Spinal Cord Stimulation: Results of the PROCO Randomized Controlled Trial. Neuromodulation 2018, 21, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Mekhail, N.; Levy, R.M.; Deer, T.R.; Kapural, L.; Li, S.; Amirdelfan, K.; Hunter, C.W.; Rosen, S.M.; Costandi, S.J.; Falowski, S.M.; et al. Long-Term Safety and Efficacy of Closed-Loop Spinal Cord Stimulation to Treat Chronic Back and Leg Pain (Evoke): A Double-Blind, Randomised, Controlled Trial. Lancet Neurol. 2020, 19, 123–134. [Google Scholar] [CrossRef]
- Billot, M.; Naiditch, N.; Brandet, C.; Lorgeoux, B.; Baron, S.; Ounajim, A.; Roulaud, M.; Roy-Moreau, A.; de Montgazon, G.; Charrier, E.; et al. Comparison of Conventional, Burst and High-Frequency Spinal Cord Stimulation on Pain Relief in Refractory Failed Back Surgery Syndrome Patients: Study Protocol for a Prospective Randomized Double-Blinded Cross-over Trial (MULTIWAVE Study). Trials 2020, 21, 696. [Google Scholar] [CrossRef]
- Ounajim, A.; Billot, M.; Louis, P.-Y.; Slaoui, Y.; Frasca, D.; Goudman, L.; Roulaud, M.; Naiditch, N.; Lorgeoux, B.; Baron, S.; et al. Finite Mixture Models Based on Pain Intensity, Functional Disability and Psychological Distress Composite Assessment Allow Identification of Two Distinct Classes of Persistent Spinal Pain Syndrome after Surgery Patients Related to Their Quality of Life. J. Clin. Med. 2021, 10, 4676. [Google Scholar] [CrossRef]
- Karri, J.; Orhurhu, V.; Wahezi, S.; Tang, T.; Deer, T.; Abd-Elsayed, A. Comparison of Spinal Cord Stimulation Waveforms for Treating Chronic Low Back Pain: Systematic Review and Meta-Analysis. Pain Physician 2020, 23, 451–460. [Google Scholar]
- Haider, N.; Ligham, D.; Quave, B.; Harum, K.E.; Garcia, E.A.; Gilmore, C.A.; Miller, N.; Moore, G.A.; Bains, A.; Lechleiter, K.; et al. Spinal Cord Stimulation ( SCS ) Trial Outcomes After Conversion to a Multiple Waveform SCS System. Neuromodul. Technol. Neural Interface 2018, 21, 504–507. [Google Scholar] [CrossRef]
- Berg, A.P.; Mekel-Bobrov, N.; Goldberg, E.; Huynh, D.; Jain, R. Utilization of Multiple Spinal Cord Stimulation (SCS) Waveforms in Chronic Pain Patients. Expert Rev. Med. Devices 2017, 14, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Goudman, L.; De Smedt, A.; Eldabe, S.; Rigoard, P.; Linderoth, B.; De Jaeger, M.; Moens, M. Discover Consortium High-Dose Spinal Cord Stimulation for Patients with Failed Back Surgery Syndrome: A Multicenter Effectiveness and Prediction Study. Pain 2021, 162, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Foss, J.M.; Apkarian, A.V.; Chialvo, D.R.; Jennifer, M.; Apkarian, A.V.; Dy-, D.R.C. Dynamics of Pain: Fractal Dimension of Temporal Variability of Spontaneous Pain Differentiates Between Pain States. J. Neurophysiol. 2006, 95, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Marchettini, P.; Lacerenza, M. Painful Peripheral Neuropathies. Curr. Neuropharmacol. 2006, 4, 175–181. [Google Scholar] [CrossRef] [Green Version]
- Holsheimer, J. Which Neuronal Elements Are Activated Directly by Spinal Cord Stimulation: Neuronal Elements Targeted by SCS. Neuromodul. Technol. Neural Interface 2002, 5, 25–31. [Google Scholar] [CrossRef]
- Manola, L.; Holsheimer, J.; Veltink, P.H.; Bradley, K.; Peterson, D. Theoretical Investigation Into Longitudinal Cathodal Field Steering in Spinal Cord Stimulation: Longitudinal Field Steering in SCS. Neuromodul. Technol. Neural Interface 2007, 10, 120–132. [Google Scholar] [CrossRef]
- Kriek, N.; Groeneweg, J.G.; Stronks, D.L.; de Ridder, D.; Huygen, F.J.P.M. Preferred Frequencies and Waveforms for Spinal Cord Stimulation in Patients with Complex Regional Pain Syndrome: A Multicentre, Double-Blind, Randomized and Placebo-Controlled Crossover Trial. Eur. J. Pain 2017, 21, 507–519. [Google Scholar] [CrossRef]
- Deer, T.R.; Patterson, D.G.; Baksh, J.; Pope, J.E.; Mehta, P.; Raza, A.; Agnesi, F.; Chakravarthy, K.V. Novel Intermittent Dosing Burst Paradigm in Spinal Cord Stimulation. Neuromodul. Technol. Neural Interface 2021, 24, 566–573. [Google Scholar] [CrossRef] [Green Version]
- de Leon-Casasola, O.; Yarussi, A. Pathophysiology of Opioid Tolerance and Clinical Approach to the Opioid-Tolerant Patient. Curr. Rev. Pain 2000, 4, 203–205. [Google Scholar] [CrossRef]
- Wang, V.C.; Bounkousohn, V.; Fields, K.; Bernstein, C.; Paicius, R.M.; Gilligan, C. Explantation Rates of High Frequency Spinal Cord Stimulation in Two Outpatient Clinics. Neuromodulation 2021, 24, 507–511. [Google Scholar] [CrossRef]
- Al-Kaisy, A.; Royds, J.; Al-Kaisy, O.; Palmisani, S.; Pang, D.; Smith, T.; Padfield, N.; Harris, S.; Wesley, S.; Yearwood, T.L.; et al. Explant Rates of Electrical Neuromodulation Devices in 1177 Patients in a Single Center over an 11-Year Period. Reg. Anesth. Pain Med. 2020, 45, 883–890. [Google Scholar] [CrossRef]
- Metzger, C.S.; Hammond, M.B.; Paz-Solis, J.F.; Newton, W.J.; Thomson, S.J.; Pei, Y.; Jain, R.; Moffitt, M.; Annecchino, L.; Doan, Q. A Novel Fast-Acting Sub-Perception Spinal Cord Stimulation Therapy Enables Rapid Onset of Analgesia in Patients with Chronic Pain. Expert Rev. Med. Devices 2021, 18, 299–306. [Google Scholar] [CrossRef]
- Rigoard, P.; Roulaud, M.; Goudman, L.; Adjali, N.; Ounajim, A.; Voirin, J.; Perruchoud, C.; Bouche, B.; Page, P.; Guillevin, R.; et al. Comparison of Spinal Cord Stimulation vs. Dorsal Root Ganglion Stimulation vs. Association of Both in Patients with Refractory Chronic Back and/or Lower Limb Neuropathic Pain: An International, Prospective, Randomized, Double-Blinded, Crossover Trial (BOOST-DRG Study). Medicina 2022, 58, 7. [Google Scholar] [CrossRef]


| Variable | (n = 27) |
|---|---|
| Age mean ± SD | 53.2 ± 11.6 |
| Sex: Female/Male (n, percentage) | 12 (44.4%)/15 (55.6%) |
| Lead type | |
| Octade | 45 (18.5%) |
| Quad | 4 (14.8%) |
| Octrode | 1 (3.7%) |
| Penta | 3 (11.1%) |
| Octade + quad | 1 (3.7%) |
| 5-6-5 | 3 (11.1%) |
| Octade + linear 3-6 | 2 (7.4%) |
| Vectris | 2 (7.4%) |
| 5-6-5 + linear 3-6 | 12 (37.84%) |
| 5-6-5 + octade | 1 (3.7%) |
| 5-6-5 + quad | 1 (3.7%) |
| vectris + octade | 1 (3.7%) |
| Infinion + octade | 1 (3.7%) |
| Octade | 45 (18.5%) |
| Quad | 4 (14.8%) |
| Implantation | |
| Percutaneous | 7 (26.9%) |
| Subcutaneous | 2 (7.4%) |
| Surgical | 9 (33.3%) |
| Percutaneous + subcutaneous | 5 (18.5%) |
| Surgical + subcutaneous | 4 (14.8%) |
| Pain localization | |
| Leg pain | 2 (7.4%) |
| Upper limbs pain | 3 (11.1%) |
| Cluster headache | 1 (3.7%) |
| Back and leg pain | 19 (70.4%) |
| Groin pain | 2 (7.4%) |
| Lead placement | |
| Thoracic | 13 (48.1%) |
| Cervical | 2 (7.4%) |
| Occipital | 1 (3.7%) |
| Thoracic + subcutaneous | 9 (33.3%) |
| Conus terminalis | 2 (7.4%) |
| Duration between previous SCS and adapter implantation (years) | 5.9 (5.2) |
| Follow-up duration | |
| Baseline | 27 |
| 1 month | 19/27 (70.4%) |
| 3 months | 16/27 (59.3%) |
| 6 months | 18/27 (66.7%) |
| 12 months | 16/27 (59.3%) |
| Baseline VAS (mean ± SD) | 75.1 (14.9) |
| Baseline ODI (mean ± SD) | 48.5 (15.9) |
| Baseline EQ5D (mean ± SD) | 0.25 (0.2) |
| Outcomes | Before Mean ± SD | After Mean ± SD | CI95% of the Difference | p-Value |
|---|---|---|---|---|
| 1-month follow-up (n = 19) | ||||
| Global VAS | 75.26 ± 16.45 | 45.56 ± 24.31 | [16.04; 43.96] | 0.001 |
| ODI score | 48.81 ± 12.55 | 31.69 ± 12.78 | [6.61; 23.21] | 0.001 |
| EQ-5D index | 0.25 ± 0.17 | 0.53 ± 0.23 | [−0.38; −0.13] | 0.004 |
| HADS depression | 5.82 ± 3.52 | 3.6 ± 2.56 | [0; 4] | 0.044 |
| HADS anxiety | 8.88 ± 4.55 | 6.53 ± 3.78 | [0; 4] | 0.049 |
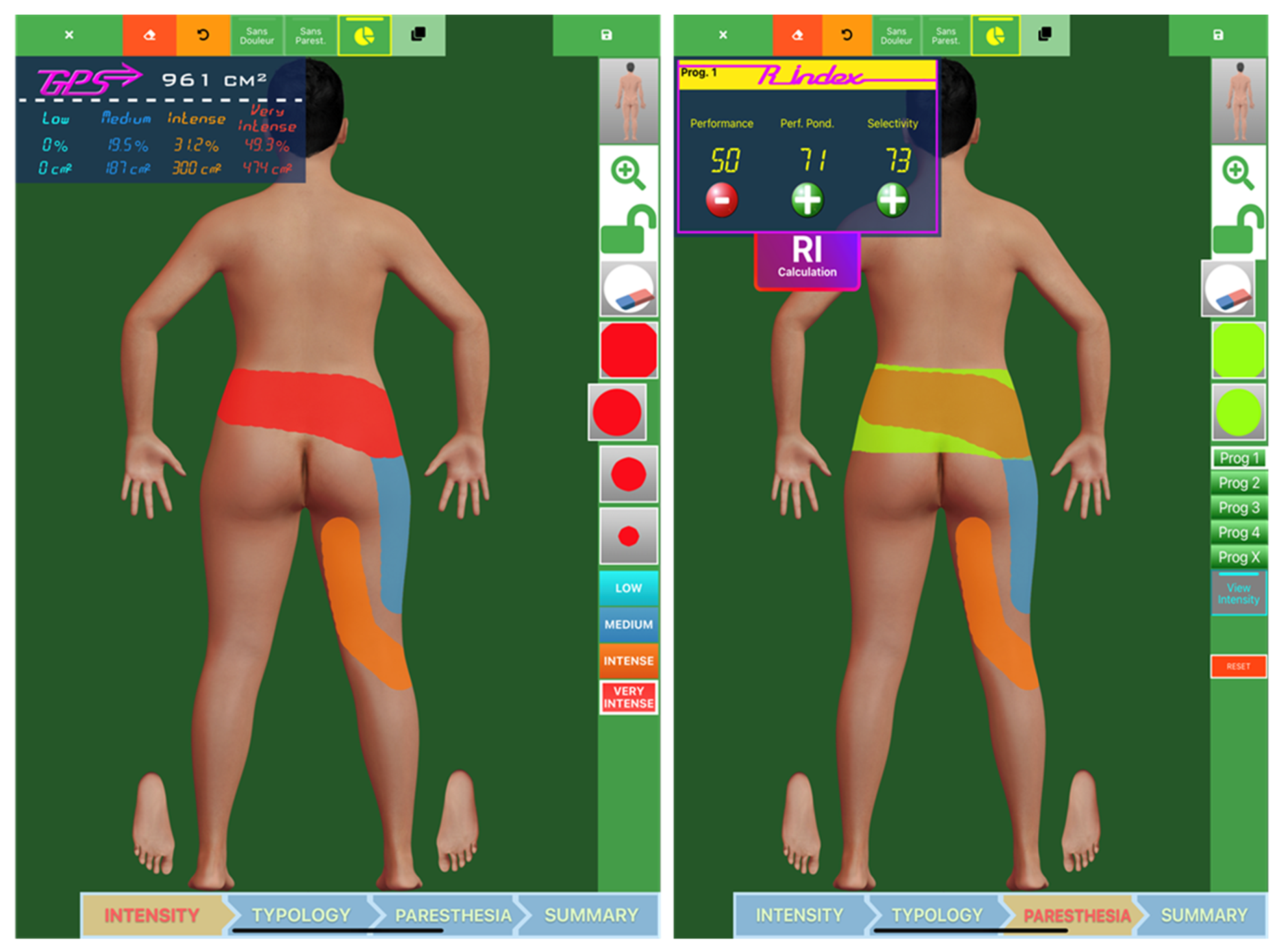
| Pain surface (cm²) | 938.26 ± 840.56 | 441.82 ± 597.72 | [182.96; 903.51] | 0.001 |
| Very intense pain surface (cm²) | 638.33 ± 862.57 | 28.24 ± 82.65 | [192; 1103.3] | 0.002 |
| Perceived pain relief | 68.3/100 ± 21.6 | |||
| PGIC | 6.1 ± 1.0 | |||
| 3-month follow-up (n = 16) | ||||
| Global VAS | 71.88 ± 16.82 | 46.25 ± 19.96 | [13.17; 38.08] | 0.005 |
| ODI score | 45.4 ± 16.97 | 28.12 ± 12.36 | [5.22; 36.23] | 0.020 |
| EQ-5D index | 0.29 ± 0.21 | 0.53 ± 0.19 | [−0.45; −0.07] | 0.021 |
| HADS depression | 6.08 ± 3.87 | 3.5 ± 3.03 | [0.26; 6.41] | 0.042 |
| HADS anxiety | 8.0 ± 4.84 | 6.7 ± 3.8 | [0.08; 3.25] | 0.056 |
| Pain surface (cm²) | 919.75 ± 926.34 | 569.79 ± 878.56 | [68.09; 403.77] | 0.014 |
| Very intense pain surface (cm²) | 493.12 ± 920.89 | 288.5 ± 913.49 | [−57.82; 507.82] | 0.021 |
| Perceived relief | 72.2 ± 14.8 | |||
| PGIC | 6.0 ± 0.7 | |||
| 6-month follow-up (n = 18) | ||||
| Global VAS | 78.33 ± 12 | 36.67 ± 23.26 | [28.76; 54.57] | <0.001 |
| ODI score | 43.11 ± 15.77 | 31.9 ± 13.85 | [−0.12; 25.52] | 0.042 |
| EQ-5D index | 0.32 ± 0.22 | 0.53 ± 0.26 | [−0.43; −0.08] | 0.022 |
| HADS depression | 5.07 ± 3.34 | 4.3 ± 3.89 | [0.61; 2.51] | 0.019 |
| HADS anxiety | 7.93 ± 4.62 | 6.6 ± 6.15 | [−0.1; 2.54] | 0.055 |
| Pain surface (cm²) | 848.22 ± 778.08 | 711.89 ± 969.23 | [−106.8; 379.47] | 0.088 |
| Very intense pain surface (cm²) | 562.53 ± 903.3 | 223.61 ± 809.48 | [45.48; 606.05] | 0.021 |
| Perceived relief | 72.8 ± 21.9 | |||
| PGIC | 5.8 ± 0.8 | |||
| 12-month follow-up (n = 16) | ||||
| Global VAS | 78.12 ± 11.09 | 33.75 ± 21.87 | [33.03; 55.72] | <0.001 |
| ODI score | 47.71 ± 14.62 | 34 ± 22.84 | [−6.66; 73.86] | 0.098 |
| EQ-5D index | 0.27 ± 0.19 | 0.48 ± 0.36 | [−0.88; 0.1] | 0.181 |
| HADS depression | 6.33 ± 3.85 | 5 ± 5.55 | [−2.13; 13.13] | 0.125 |
| HADS anxiety | 8.75 ± 5.19 | 8 ± 5.8 | [−1.5; 8] | 0.181 |
| Pain surface (cm²) | 887 ± 804.4 | 545.12 ± 859.58 | [60.19; 623.56] | 0.012 |
| Very intense pain surface (cm²) | 573.4 ± 950.46 | 240.12 ± 858.75 | [16.84; 617.7] | 0.019 |
| Perceived relief | 67.5 ± 28.9 | |||
| PGIC | 6.4 ± 0.5 | |||
| Outcomes | Before Mean ± SD | After Mean ± SD | CI95% of the Difference | p-Value |
|---|---|---|---|---|
| Global VAS | 75.06 ± 14.91 | 39.13 ± 27.90 | [23.7; 48.16] | <0.001 |
| ODI score | 48.48 ± 15.93 | 38.89 ± 20.45 | [2.05; 28.15] | 0.021 |
| EQ5D | 0.25 ± 0.20 | 0.39 ± 0.30 | [−0.34; −0.05] | 0.023 |
| HADS depression | 6.19 ± 3.71 | 5.08 ± 4.55 | [−0.45; 5.25] | 0.067 |
| HADS anxiety | 8.52 ± 4.32 | 7.50 ± 4.15 | [0.35; 3.65] | 0.036 |
| Pain surface | 1085.19 ± 1360.69 | 718.85 ± 1430.0 | [117.26; 509.82] | 0.001 |
| Variable | LoC Group (n = 11) | SCStol Group (n = 16) | p-Value |
|---|---|---|---|
| Age | 54.6 ± 11.6 | 52.2 ± 11.8 | 0.68 |
| Sex | 0.76 | ||
| Male | 7 (63.6%) | 8 (50.0%) | |
| Female | 4 (36.4%) | 8 (50.0%) | |
| Duration between SCS implantation and adapter rescue therapy (years) | 6.4 ± 5.1 | 5.6 ± 5.4 | 0.73 |
| 1-month follow-up | n = 10 | n = 9 | |
| Percentage of VAS decrease | 32.56 ± 41.81 | 26.13 ± 33.33 | 0.60 |
| Percentage of pain surface decrease | 64.3 ± 33.86 | 11.77 ± 65.81 | 0.034 |
| Paresthesia coverage | 73.88 ± 35.77 | 27.6 ± 32.99 | 0.048 |
| 3-month follow-up | n = 8 | n = 8 | |
| Percentage of VAS decrease | 36.89 ± 29.98 | 31.75 ± 39.61 | 0.75 |
| Percentage of pain surface decrease | 47.01 ± 38.69 | 20.13 ± 69.85 | 0.047 |
| Paresthesia coverage | 55.57 ± 46.23 | 30.5 ± 47.49 | 0.35 |
| 6-month follow-up | n = 8 | n = 10 | |
| Percentage of VAS decrease | 50.56 ± 31.83 | 36.36 ± 43.69 | 0.59 |
| Percentage of pain surface decrease | 34.47 ± 44.77 | 18.13 ± 64.32 | 0.60 |
| Paresthesia coverage | 51.25 ± 48.66 | 38.56 ± 52.7 | 0.92 |
| 12-month follow-up | n = 8 | n = 8 | |
| Percentage of VAS decrease | 58.68 ± 32.79 | 35.77 ± 44.09 | 0.19 |
| Percentage of pain surface decrease | 51.32 ± 42.31 | 26.61 ± 54.39 | 0.21 |
| Paresthesia coverage | 63.62 ± 46.26 | 21.5 ± 47.25 | 0.14 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigoard, P.; Ounajim, A.; Goudman, L.; Banor, T.; Héroux, F.; Roulaud, M.; Babin, E.; Bouche, B.; Page, P.; Lorgeoux, B.; et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study. J. Clin. Med. 2022, 11, 272. https://doi.org/10.3390/jcm11010272
Rigoard P, Ounajim A, Goudman L, Banor T, Héroux F, Roulaud M, Babin E, Bouche B, Page P, Lorgeoux B, et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study. Journal of Clinical Medicine. 2022; 11(1):272. https://doi.org/10.3390/jcm11010272
Chicago/Turabian StyleRigoard, Philippe, Amine Ounajim, Lisa Goudman, Tania Banor, France Héroux, Manuel Roulaud, Etienne Babin, Bénédicte Bouche, Philippe Page, Bertille Lorgeoux, and et al. 2022. "The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study" Journal of Clinical Medicine 11, no. 1: 272. https://doi.org/10.3390/jcm11010272
APA StyleRigoard, P., Ounajim, A., Goudman, L., Banor, T., Héroux, F., Roulaud, M., Babin, E., Bouche, B., Page, P., Lorgeoux, B., Baron, S., Adjali, N., Nivole, K., Many, M., Charrier, E., Rannou, D., Poupin, L., Wood, C., David, R., ... Billot, M. (2022). The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment: A Real Life Retrospective Study. Journal of Clinical Medicine, 11(1), 272. https://doi.org/10.3390/jcm11010272








