Urinary Tract Infections in Kidney Transplant Recipients—Is There a Need for Antibiotic Stewardship?
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Immunosuppressive Protocol
2.3. Perioperative Antibiotic Prophylaxis
2.4. Treatment Protocols for Urinary Tract Infections
2.5. Urinary Catheter and Double-J-Stent Management
2.6. Postoperative Follow-Up
2.7. Clinical Definitions
2.8. Microbiological Culture, Identification of Strains and Resistance Testing
2.9. Statistics
3. Results
3.1. Clinical Characteristics
3.2. Microbiological Results
3.3. Antibiotic Prescription
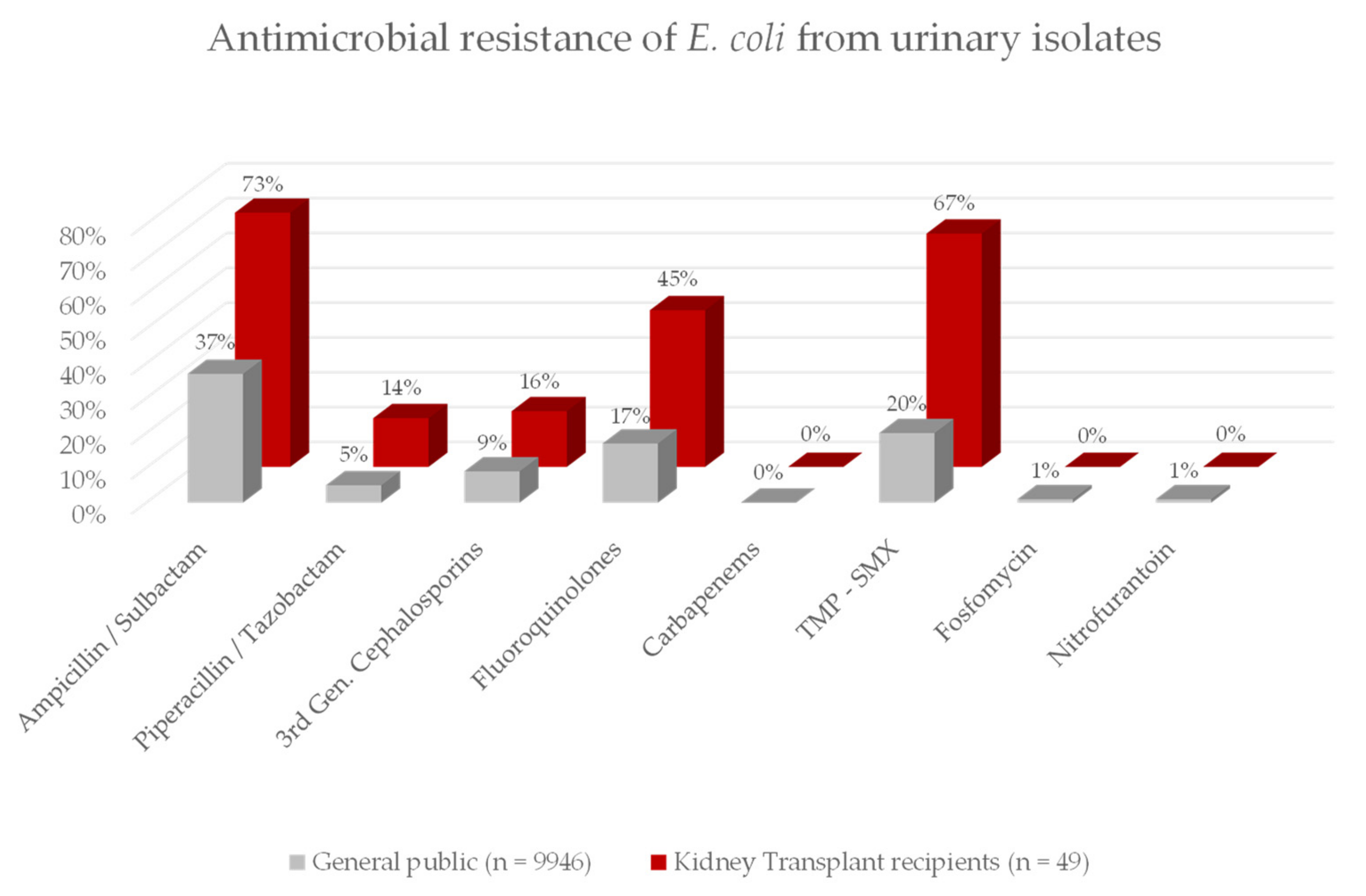
3.4. Antibiotic Resistance
3.5. Bacterial Resistance to Empiric Antibiotic Treatment Options
4. Discussion
Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASB | Asymptomatic Bacteriuria |
| BMI | Body Mass Index |
| CG | Control Group |
| CI | Confidence Interval |
| FET | Fisher’s Exact Test |
| GFR | Glomerular Filtration Rate |
| KT | Kidney Transplantation |
| MRGN | Multi-resistant Gram-Negative |
| MWU | Mann-Whitney U-Test |
| PAP | Perioperative Antibiotic Prophylaxis |
| POD | Postoperative Day |
| SD | Standard Deviation |
| SSI | Surgical Site Infection |
| TMP-SMX | Trimethoprim-Sulfamethoxazole |
| UTI | Urinary Tract Infection |
| Χ² | Chi-Square test |
References
- Cowan, J.; Bennett, A.; Fergusson, N.; McLean, C.; Mallick, R.; Cameron, D.W.; Knoll, G. Incidence Rate of Post-Kidney Transplant In-fection: A Retrospective Cohort Study Examining Infection Rates at a Large Canadian Multicenter Tertiary-Care Facility. Can. J. Kidney Health Dis. 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Coussement, J.; Abramowicz, D. Should we treat asymptomatic bacteriuria after renal transplantation? Nephrol. Dial. Transpl. 2014, 29, 260–262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hollyer, I.; Ison, M.G. The challenge of urinary tract infections in renal transplant recipients. Transpl. Infect. Dis. 2018, 20, e12828. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, K.; Kennedy, C.; Skally, M.; Foley, M.; Alex, S.; Magee, C.; Davis, N.F.; Humphreys, H.; Burns, K. Surveillance of common infections in the early period after renal transplantation in a national center: 2014–2017. Transpl. Infect. Dis. 2020, 22, e13261. [Google Scholar]
- Van Delden, C.; Stampf, S.; Hirsch, H.H.; Manuel, O.; Meylan, P.; Cusini, A.; Hirzel, C.; Khanna, N.; Weisser, M.; Garzoni, C.; et al. Burden and timeline of infectious diseases in the first year after solid organ transplantation in the Swiss transplant cohort study. Clin. Infect. Dis. 2020, 71, e159–e169. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Beam, E.; Lesnick, T.G.; Kremers, W.K.; Cosio, F.G.; Razonable, R.R. Urinary tract infections in kidney transplant recipients: Role of gender, urologic abnormalities, and antimicrobial prophylaxis. Ann. Transpl. 2013, 18, 195–204. [Google Scholar] [CrossRef]
- Wiley, Z.; Jacob, J.T.; Burd, E.M. Targeting Asymptomatic Bacteriuria in Antimicrobial Stewardship: The Role of the Microbiology Laboratory. J. Clin. Microbiol. 2020, 58, e00518–18. [Google Scholar] [CrossRef]
- Goh, Y.S.B.; Deng, Z.; Cheong, P.S.C.; Raman, L.; Goh, T.H.A.; Vathsala, A.; Tiong, H.Y. Screening for asymptomatic bacteruria at one month after adult kidney transplantation: Clinical factors and implications. Clin. Transpl. 2017, 31, e12954. [Google Scholar] [CrossRef]
- Nicolle, L.E.; Gupta, K.; Bradley, S.F.; Colgan, R.; DeMuri, G.P.; Drekonja, D.; Eckert, L.O.; Geerlings, S.E.; Köves, B.; Hooton, T.M.; et al. Clinical Practice Guideline for the Management of Asymptomatic Bacteriuria: 2019 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2019, 68, e83–e110. [Google Scholar] [CrossRef]
- Coussement, J.; Scemla, A.; Abramowicz, D.; Nagler, E.V.; Webster, A.C. Antibiotics for asymptomatic bacteriuria in kidney trans-plant recipients. Cochrane Database Syst. Rev. 2018, 2, Cd011357. [Google Scholar]
- Gomez-Ochoa, S.A.; Vega-Vera, A. Systematic review and meta-analysis of asymptomatic bacteriuria after renal transplantation: Incidence, risk of complications, and treatment outcomes. Transpl. Infect. Dis. 2020, 22, e13221. [Google Scholar] [CrossRef]
- Bohn, B.C.; Athans, V.; Kovacs, C.S.; Stephany, B.R.; Spinner, M.L. Impact of asymptomatic bacteriuria incidence and management post–kidney transplantation. Clin. Transpl. 2019, 33, e13583. [Google Scholar] [CrossRef]
- Ariza-Heredia, E.J.; Beam, E.; Lesnick, T.G.; Cosio, F.G.; Kremers, W.K.; Razonable, R.R. Impact of urinary tract infection on allograft function after kidney transplantation. Clin. Transpl. 2014, 28, 683–690. [Google Scholar] [CrossRef]
- Tekkarışmaz, N.; Özelsancak, R.; Micozkadıoğlu, H.; Çalışkan, K.; Demiroğlu, Y.Z.; Arslan, A.H.; Haberal, M. Risk Factors for Urinary Tract Infection After Kidney Transplant: A Retrospective Analysis. Exp. Clin. Transpl. 2020, 18, 306–312. [Google Scholar] [CrossRef]
- Sánchez, M.P.R.; Rubio, D.C.A.; Luna, I.M.; Padilla, P.K.G.; Villamizar, K.M.C.; González, C.A.G.; Trejos, J.A.P. Impact of Complicated Urinary Tract Infection on Renal Graft Function. Transpl. Proc. 2020, 52, 1173–1177. [Google Scholar] [CrossRef]
- Wu, X.; Dong, Y.; Liu, Y.; Li, Y.; Sun, Y.; Wang, J.; Wang, S. The prevalence and predictive factors of urinary tract infection in patients undergoing renal transplantation: A meta-analysis. Am. J. Infect. Control. 2016, 44, 1261–1268. [Google Scholar] [CrossRef]
- Bachmann, F.; Adam, T.; Friedersdorff, F.; Liefeldt, L.; Slowinski, T.; Budde, K.; Waiser, J. Perioperative antibiotic prophylaxis in renal transplantation: A single-center comparison between two regimens and a brief survey among the Eurotransplant renal trans-plantation centers. World J. Urol. 2019, 37, 957–967. [Google Scholar] [CrossRef]
- Faba, O.R.; Boissier, R.; Budde, K.; Figueiredo, A.; Taylor, C.F.; Hevia, V.; García, E.L.; Regele, H.; Zakri, R.H.; Olsburgh, J.; et al. European Association of Urology Guidelines on Renal Transplantation: Update 2018. Eur. Urol. Focus. 2018, 4, 208–215. [Google Scholar] [CrossRef]
- Orlando, G.; Manzia, T.M.; Sorge, R.; Iaria, G.; Angelico, R.; Sforza, D.; Toti, L.; Peloso, A.; Patel, T.; Katari, R.; et al. One-shot versus multidose perioperative antibiotic prophylaxis after kidney transplantation: A randomized, controlled clinical trial. Surgery 2015, 157, 104–110. [Google Scholar] [CrossRef]
- Graninger, W.; Wenisch, C.; Presterl, E. Quinolones in the treatment of complicated urinary tract infection. Int. J. Antimicrob. Agents 1994, 4, S29–S37. [Google Scholar] [CrossRef]
- Vidal, E.; Torre-Cisneros, J.; Blanes, M.; Montejo, M.; Cervera, C.; Aguado, J.; Len, O.; Carratalá, J.; Cordero, E.; Bou, G.; et al. Bacterial urinary tract infection after solid organ transplantation in the RESITRA cohort. Transpl. Infect. Dis. 2012, 14, 595–603. [Google Scholar] [CrossRef]
- Lebel, M. Ciprofloxacin: Chemistry, Mechanism of Action, Resistance, Antimicrobial Spectrum, Pharmacokinetics, Clinical Trials, and Adverse Reactions. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1988, 8, 3–30. [Google Scholar] [CrossRef]
- Andriole, V.T. Use of quinolones in treatment of prostatitis and lower urinary tract infections. Eur. J. Clin. Microbiol. Infect. Dis. 1991, 10, 342–350. [Google Scholar] [CrossRef]
- Administration UFaD. FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. 2018. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-updates-warnings-oral-and-injectable-fluoroquinolone-antibiotics (accessed on 30 December 2021).
- Warzyszyńska, K.; Zawistowski, M.; Karpeta, E.; Dziewa, N.; Kosieradzki, M. How Early Postoperative Urinary Tract Infections Affect Renal Graft Function at 1-Year Follow-up. Transpl. Proc. 2020, 52, 2403–2408. [Google Scholar] [CrossRef]
- Bodro, M.; Sanclemente, G.; Lipperheide, I.; Allali, M.; Marco, F.; Bosch, J.; Cofan, F.; Ricart, M.; Esforzado, N.; Oppenheimer, F.; et al. Impact of urinary tract infections on short-term kidney graft outcome. Clin. Microbiol. Infect. 2015, 21, 1104.e1–1104.e8. [Google Scholar] [CrossRef][Green Version]
- Pesce, F.; Martino, M.; Fiorentino, M.; Rollo, T.; Simone, S.; Gallo, P.; Stallone, G.; Grandaliano, G.; Schena, A.; Margiotta, M.; et al. Recurrent urinary tract infections in kidney transplant recipients during the first-year influence long-term graft function: A single-center retrospective cohort study. J. Nephrol. 2019, 32, 661–668. [Google Scholar] [CrossRef]
- Al Midani, A.; Elands, S.; Collier, S.; Harber, M.; Shendi, A.M. Impact of Urinary Tract Infections in Kidney Transplant Recipients: A 4-Year Single-Center Experience. Transpl. Proc. 2018, 50, 3351–3355. [Google Scholar] [CrossRef]
- Morales-Alvarez, M.C. Nephrotoxicity of Antimicrobials and Antibiotics. Adv. Chronic Kidney Dis. 2020, 27, 31–37. [Google Scholar] [CrossRef]
- Cox, L.; He, C.; Bevins, J.; Clemens, J.Q.; Stoffel, J.; Cameron, A.P. Gentamicin bladder instillations decrease symptomatic urinary tract infections in neurogenic bladder patients on intermittent catheterization. Can. Urol. Assoc. J. 2017, 11, E350–E354. [Google Scholar] [CrossRef]
- Liu, S.; Luo, G.; Sun, B.; Lu, J.; Zu, Q.; Yang, S.; Zhang, X.; Dong, J. Early Removal of Double-J Stents Decreases Urinary Tract Infections in Living Donor Renal Transplantation: A Prospective, Randomized Clinical Trial. Transpl. Proc. 2017, 49, 297–302. [Google Scholar] [CrossRef]
- Visser, I.J.; van der Staaij, J.P.T.; Muthusamy, A.; Willicombe, M.; Lafranca, J.A.; Dor, F. Timing of Ureteric Stent Removal and Oc-currence of Urological Complications after Kidney Transplantation: A Systematic Review and Meta-Analysis. J. Clin. Med. 2019, 8, 689. [Google Scholar] [CrossRef] [PubMed]
- Moysés Neto, M.; Costa, R.S.; Reis, M.A.; Ferraz, A.S.; Saber, L.T.; Batista, M.E.; Muglia, V.; Garcia, T.M.; Figueiredo, J.F. Use of ciprofloxacin as a prophylactic agent in urinary tract infections in renal transplant recipients. Clin. Transpl. 1997, 11 Pt 1, 446–452. [Google Scholar]
- Rosado-Canto, R.; Parra-Avila, I.; Tejeda-Maldonado, J.; Kauffman-Ortega, C.; Rodriguez-Covarrubias, F.T.; Trujeque-Matos, M.; Cruz-Martínez, R.; Maravilla-Franco, E.; Criollo-Mora, E.; Arreola-Guerra, J.M.; et al. Perioperative fosfomycin disodium prophylaxis against urinary tract infection in renal transplant recipients: A randomized clinical trial. Nephrol. Dial. Transpl. 2020, 35, 1996–2003. [Google Scholar] [CrossRef] [PubMed]
- Horwedel, T.; Bowman, L.; Saab, G.; Brennan, D. Benefits of sulfamethoxazole-trimethoprim prophylaxis on rates of sepsis after kidney transplant. Transpl. Infect. Dis. 2014, 16, 261–269. [Google Scholar] [CrossRef]
- Singh, R.; Bemelman, F.J.; Hodiamont, C.J.; Idu, M.M.; Berge, I.J.M.T.; Geerlings, S.E. The impact of trimethoprim-sulfamethoxazole as Pneumocystis jiroveci pneumonia prophylaxis on the occurrence of asymptomatic bacteriuria and urinary tract infections among renal allograft recipients: A retrospective before-after study. BMC Infect. Dis. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Wolters, H.; Palmes, D.; Lordugin, E.; Bahde, R.; Senninger, N.; Hölzen, J.-P.; Kebschull, L. Antibiotic Prophylaxis at Urinary Catheter Removal Prevents Urinary Tract Infection After Kidney Transplantation. Transpl. Proc. 2014, 46, 3463–3465. [Google Scholar] [CrossRef]
- Lee, J.H.; Muthukumar, T.; Kim, J.; Aull, M.J.; Watkins, A.; Kapur, S.; Hartono, C. Antibiotic prophylaxis for ureteral stent removal after kidney transplantation. Clin. Transpl. 2019, 33, e13491. [Google Scholar] [CrossRef]
- Coussement, J.; Maggiore, U.; Manuel, O.; Scemla, A.; López-Medrano, F.; Nagler, E.V.; Aguado, J.M.; Abramowicz, D. Diagnosis and management of asymp-tomatic bacteriuria in kidney transplant recipients: A survey of current practice in Europe. Nephrol. Dial. Transpl. 2018, 33, 1661–1668. [Google Scholar] [CrossRef]
- Kotagiri, P.; Chembolli, D.; Ryan, J.; Hughes, P.D.; Toussaint, N.D. Urinary Tract Infections in the First Year Post-Kidney Trans-plantation: Potential Benefits of Treating Asymptomatic Bacteriuria. Transpl. Proc. 2017, 49, 2070–2075. [Google Scholar] [CrossRef]
- Lee, J.R.; Bang, H.; Dadhania, D.; Hartono, C.; Aull, M.J.; Satlin, M.; August, P.; Suthanthiran, M.; Muthukumar, T. Independent risk factors for urinary tract infection and for subsequent bacteremia or acute cellular rejection: A single-center report of 1166 kidney allograft recipients. Transplantation 2013, 96, 732–738. [Google Scholar] [CrossRef]
- Origüen, J.; López-Medrano, F.; Fernández-Ruiz, M.; Polanco, N.; Gutiérrez, E.; González, E.; Mérida, E.; Ruiz-Merlo, T.; Morales-Cartagena, A.; Pérez-Jacoiste Asín, M.A.; et al. Should Asymptomatic Bacteriuria Be Systematically Treated in Kidney Transplant Recipients? Results from a Randomized Controlled Trial. Am. J. Transplant. 2016, 16, 2943–2953. [Google Scholar] [CrossRef]
- Coussement, J.; Kamar, N.; Matignon, M.; Weekers, L.; Scemla, A.; Giral, M.; Racapé, J.; Alamartine, É.; Mesnard, L.; Kianda, M.; et al. Antibiotics versus no therapy in kidney transplant recipients with asymptomatic bacteriuria (BiRT): A pragmatic, multicentre, randomized, controlled trial. Clin. Microbiol. Infect. 2020, 27, 398–405. [Google Scholar] [CrossRef]
- Coussement, J.; Scemla, A.; Hougardy, J.M.; Sberro-Soussan, R.; Amrouche, L.; Catalano, C.; Johnson, J.R.; Abramowicz, D. Prevalence of asymptomatic bac-teriuria among kidney transplant recipients beyond two months post-transplant: A multicenter, prospective, cross-sectional study. PLoS ONE 2019, 14, e0221820. [Google Scholar] [CrossRef]
- Richards, K.A.; Cesario, S.; Best, S.L.; Deeren, S.M.; Bushman, W.; Safdar, N. Reflex urine culture testing in an ambulatory urology clinic: Implications for antibiotic stewardship in urology. Int. J. Urol. 2018, 26, 69–74. [Google Scholar] [CrossRef]
- López-Medrano, F.; Silva, J.T.; Fernández-Ruiz, M.; Vidal, E.; Origüen, J.; Calvo-Cano, A.; Luna-Huerta, E.; Merino, E.; Hernández, D.; Jironda-Gallegos, C.; et al. Oral fosfomycin for the treatment of lower urinary tract infections among kidney transplant recipients—Results of a Spanish multicenter cohort. Arab. Archaeol. Epigr. 2019, 20, 451–462. [Google Scholar] [CrossRef]
- Ten Doesschate, T.; van Werkhoven, H.; Meijvis, S.; Stalenhoef, J.; van Zuilen, A.; de Vries, A.; Bonten, M. Fosfomycin-trometamol for urinary tract infections in kidney transplant recipients. Transplantation 2019, 103, 1272–1276. [Google Scholar] [CrossRef]
- Mercuro, N.J.; Davis, S.L.; Zervos, M.J.; Herc, E.S. Combatting resistant enterococcal infections: A pharmacotherapy review. Expert Opin. Pharmacother. 2018, 19, 979–992. [Google Scholar] [CrossRef]
- Schneidewind, L.; Kranz, J.; Tandogdu, Z. Rising significance of antibiotic stewardship in urology and urinary tract infections—A rapid review. Curr. Opin. Urol. 2021, 31, 285–290. [Google Scholar] [CrossRef]
- Frenette, C.; Sperlea, D.; Leharova, Y.; Thirion, D.J.G. Impact of an Infection Control and Antimicrobial Stewardship Program on Solid Organ Transplantation and Hepatobiliary Surgical Site Infections. Infect. Control. Hosp. Epidemiol. 2016, 37, 1468–1474. [Google Scholar] [CrossRef]
- Brakemeier, S.; Taxeidi, S.I.; Zukunft, B.; Schmidt, D.; Gaedeke, J.; Dürr, M.; Hansen, S.; Budde, K. Extended-Spectrum Beta-Lactamase-Producing Enterobacteriaceae-Related Urinary Tract Infection in Kidney Transplant Recipients: Risk Factors, Treatment, and Long-Term Outcome. Transpl Proc. 2017, 49, 1757–1765. [Google Scholar] [CrossRef]
- Alevizakos, M.; Nasioudis, D.; Mylonakis, E. Urinary tract infections caused by ESBL-producing Enterobacteriaceae in renal transplant recipients: A systematic review and meta-analysis. Transpl. Infect. Dis. 2017, 19, e12759. [Google Scholar] [CrossRef]

| No UTI (n = 139) | UTI (n = 68) | Total | p | |
|---|---|---|---|---|
| Gender m (Percentage) | 86 (62%) | 33 (49%) | 119 (57%) | 0.068 |
| Living Donor Kidney Transplantation (Percentage) | 54 (38%) | 19 (30%) | 73 (35%) | 0.123 |
| Median age in years (Range) | 54 (19–82) | 60 (18–77) | 55 (18–82) | 0.034 |
| Mean Body mass index (BMI) in kg/m² (±Standard Deviation (SD)) | 24.8 (±3.9) | 25.6 (±4.1) | 25.3 (±4.0) | 0.035 |
| Diagnosis Glomerulopathy Polycystic Kidney Disease Hypertension Diabetes Ureteral Disease and Reflux Graft Loss Other | 45 (32%) 25 (18%) 9 (6%) 8 (6%) 2 (1%) 12 (9%) 38 (27%) | 26 (38%) 14 (21%) 5 (7%) 5 (7%) 3 (4%) 5 (7%) 10 (15%) | 71 39 14 13 5 17 48 | 0.450 |
| Induction Immunosuppression Basiliximab Thymoglobulin Alemtuzumab | 88 (63%) 32 (23%) 19 (14%) | 51 (75%) 8 (12%) 9 (13%) | 139 40 28 | 0.140 |
| Mean Kidney function in Glomerular Filtration Rate in ml/min (±SD) Discharge 3 Months Follow-Up 12 Months Follow-Up | 46.5 (±20) 49.1 (±9) 51.1 (±18) | 38.9 (±17) 41.2 (±15) 41.1 (±15) | 44.8 (±19) 46.8 (±18) 48.0 (±18) | 0.013 0.005 <0.001 |
| Trimetroprime-Sulfamethoxazole Prophylaxis (Percentage) | 86 (62%) | 31 (46%) | 117 (57%) | 0.036 |
| UTI | ASB | n | |
|---|---|---|---|
| E. coli Klebsiella pneumoniae Klebsiella oxytoca Klebsiella variicola Pseudomonas aeruginosa Enterobacter cloacae Serratia marcescens Citrobacter species Proteus mirabilis Raoultella planticola Ureaplasma urealyticum Acinetobacter baumanii E. faecalis E. faecium | 31 13 3 1 2 1 2 2 1 1 6 10 | 18 1 3 4 2 1 1 1 1 17 13 | 49 14 6 1 6 3 3 3 1 1 1 1 23 23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strohaeker, J.; Aschke, V.; Koenigsrainer, A.; Nadalin, S.; Bachmann, R. Urinary Tract Infections in Kidney Transplant Recipients—Is There a Need for Antibiotic Stewardship? J. Clin. Med. 2022, 11, 226. https://doi.org/10.3390/jcm11010226
Strohaeker J, Aschke V, Koenigsrainer A, Nadalin S, Bachmann R. Urinary Tract Infections in Kidney Transplant Recipients—Is There a Need for Antibiotic Stewardship? Journal of Clinical Medicine. 2022; 11(1):226. https://doi.org/10.3390/jcm11010226
Chicago/Turabian StyleStrohaeker, Jens, Victoria Aschke, Alfred Koenigsrainer, Silvio Nadalin, and Robert Bachmann. 2022. "Urinary Tract Infections in Kidney Transplant Recipients—Is There a Need for Antibiotic Stewardship?" Journal of Clinical Medicine 11, no. 1: 226. https://doi.org/10.3390/jcm11010226
APA StyleStrohaeker, J., Aschke, V., Koenigsrainer, A., Nadalin, S., & Bachmann, R. (2022). Urinary Tract Infections in Kidney Transplant Recipients—Is There a Need for Antibiotic Stewardship? Journal of Clinical Medicine, 11(1), 226. https://doi.org/10.3390/jcm11010226






