Perioperative Management of Polytrauma Patients with Severe Traumatic Brain Injury Undergoing Emergency Extracranial Surgery: A Narrative Review
Abstract
:1. Introduction
2. Perioperative Cardiorespiratory Optimization
2.1. Hemodynamic Management
2.2. Respiratory Management
2.2.1. Hypoxemia
2.2.2. Hyperoxemia
2.2.3. Hypercapnia/Hypocapnia
3. Coagulation (Including Transfusion) Management
3.1. Hemoglobin-Based Transfusion Thresholds
3.2. Coagulation Management
3.3. Transfusion Ratios
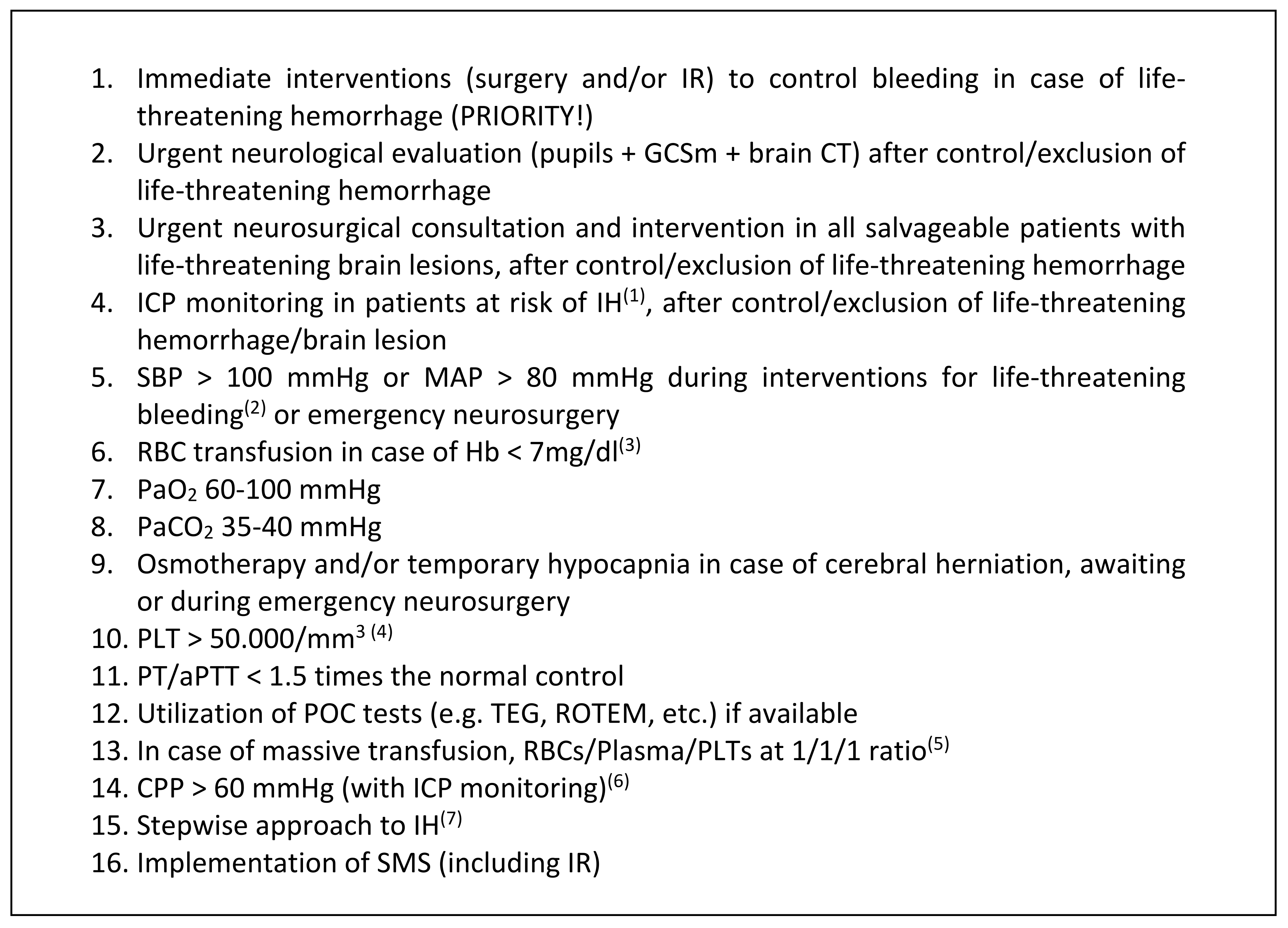
4. Perioperative Brain Protection
4.1. Intracranial Pressure (ICP)/Cerebral Prefusion Pressure (CPP) Monitoring in Polytrauma Patients with TBI
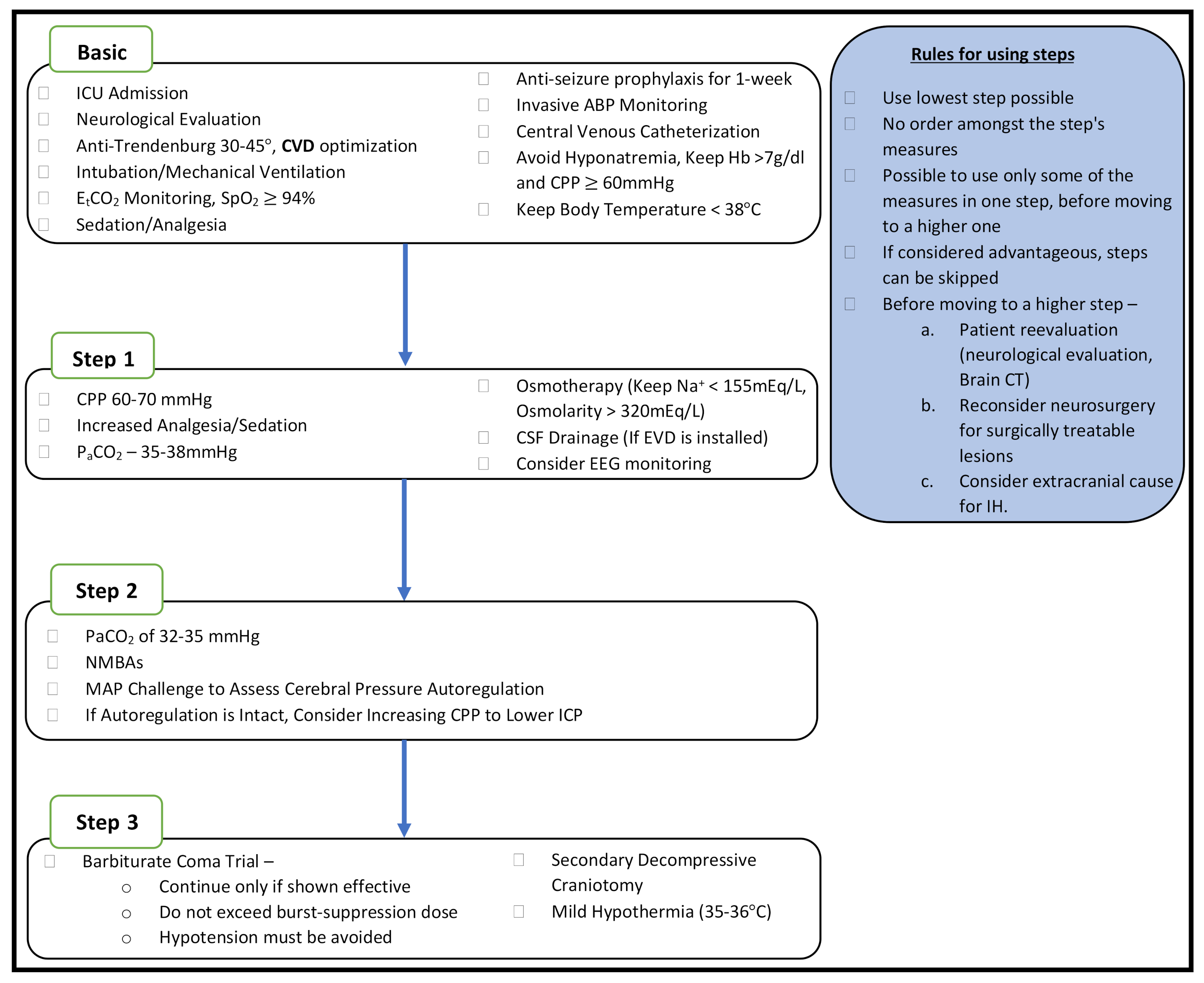
4.2. Perioperative ICP and CPP Management
4.3. Brain Trauma Foundation (BTF) Guidelines for the Management of Severe TBI
- -
- The use of information from ICP monitoring is recommended to reduce in-hospital and two-week post-injury mortality (LEVEL II B).
- -
- Treat patients with ICP > 22 mmHg is recommended because values above this level are associated with increased mortality (LEVEL II B).
- -
- CPP monitoring is recommended to decrease two-week mortality (LEVEL II B).
- -
- CPP value for survival and favorable outcomes is 60–70 mmHg depending upon the autoregulatory status of the patient (LEVEL II B).
- -
- Avoid aggressive attempts to maintain CPP > 70 mmHg with fluids and pressors should be considered because of the risk of adult respiratory failure (LEVEL III).
- -
- Jugular bulb monitoring of arteriovenous oxygen content difference (AVDO2), as a source of information for management decisions, may be considered to reduce mortality and improve outcomes at 3 and 6 months post-injury (LEVEL III).
- -
- Avoid jugular venous saturation <50% to reduce mortality and improve outcomes (LEVEL III).
- -
- SBP ≥ 100 mmHg for patients 50 to 69 years old or≥ 110 mmHg or above for patients 15 to 49 or >70 years old may be considered to decrease mortality and improve outcomes (LEVEL III).
- -
- A combination of ICP values and clinical and brain CT findings may be used to make management decisions (LEVEL III).
- -
- Prophylactic hypothermia is not recommended to improve outcomes in patients with diffuse injury (LEVEL II B).
- -
- Secondary decompressive craniectomy (DC) performed for late refractory ICP elevation is recommended to improve mortality and favorable outcomes (LEVEL II A).
- -
- Secondary DC performed for early refractory ICP elevation is not recommended to improve mortality and favorable outcomes (LEVEL II A).
- -
- A large frontotemporoparietal DC (not less than 12 × 15 cm or 15 cm in diameter) is recommended to reduce mortality and to improve neurological outcomes in patients with severe TBI (LEVEL II A).
- -
- Secondary DC, for early or late refractory ICP elevation, is suggested to reduce ICP and the duration of intensive care, although the relationship between these effects and a favorable outcome is uncertain (LEVEL II A).
- -
- An external ventricular drain (EVD) system zeroed at the midbrain with continuous drainage of cerebrospinal fluid (CSF) may be considered to lower ICP burden more effectively than intermittent use (LEVEL III).
- -
- The use of CSF drainage to lower ICP in patients with an initial GCS < 6 during the first 12 h after injury may be considered (LEVEL III).
- -
- Antimicrobial-impregnated catheters may be considered to prevent catheter-related infections during EVD (LEVEL III).
- -
- Prolonged prophylactic hyperventilation with PaCO2 ≤ 25 mmHg is not recommended (LEVEL II B).
- -
- Administration of barbiturates to induce burst suppression as prophylaxis against the development of intracranial hypertension is not recommended (LEVEL II B).
- -
- High-dose barbiturate administration is recommended to control elevated ICP refractory to maximum standard medical and surgical treatment. Hemodynamic stability is essential before and during barbiturate therapy (LEVEL II B).
- -
- Although propofol is recommended for the control of ICP, it is not recommended for improvement in mortality or six-month outcomes (LEVEL II B).
- -
- The use of steroids is not recommended for improving outcomes or reducing ICP (LEVEL I).
- -
- Feeding patients to attain basal caloric replacement at least by the fifth day and, at most, by the seventh day post-injury is recommended to decrease mortality (LEVEL II A).
- -
- Transgastric jejunal feeding is recommended to reduce the incidence of ventilator-associated pneumonia (VAP) (LEVEL II B).
- -
- Early tracheostomy is recommended to reduce mechanical ventilation days when the overall benefit is thought to outweigh the complications associated with such a procedure. However, there is no evidence that early tracheostomy reduces mortality or the rate of nosocomial pneumonia (LEVEL II A).
- -
- The use of povidone-iodine oral care is not recommended to reduce VAP and may cause an increased risk of acute respiratory distress syndrome (ARDS) (LEVEL II A).
- -
- Low-molecular-weight heparin (LMWH) or low-dose unfractionated heparin may be used in combination with mechanical prophylaxis; however, there is an increased risk for expansion of intracranial hemorrhage (LEVEL III).
- -
- In addition to compression stockings, pharmacologic prophylaxis may be considered if the brain injury is stable and when the benefit is considered to outweigh the risk of increased intracranial hemorrhage (LEVEL III).
- -
- There is insufficient evidence to support recommendations regarding the preferred agent, dose, or timing of pharmacologic prophylaxis for deep vein thrombosis (LEVEL III).
- -
- Prophylactic phenytoin or valproate are not recommended for preventing late post-traumatic seizures (PTS) (LEVEL II A).
- -
- Phenytoin is recommended to decrease the incidence of early PTS (within 7 days of injury), when the overall benefit is thought to outweigh the complications associated with such treatment. However, early PTS have not been associated with worse outcomes (LEVEL II A).
- -
- At the present time, there is insufficient evidence to recommend levetiracetam as compared with phenytoin regarding efficacy in preventing early post-traumatic seizures and toxicity (LEVEL II A).
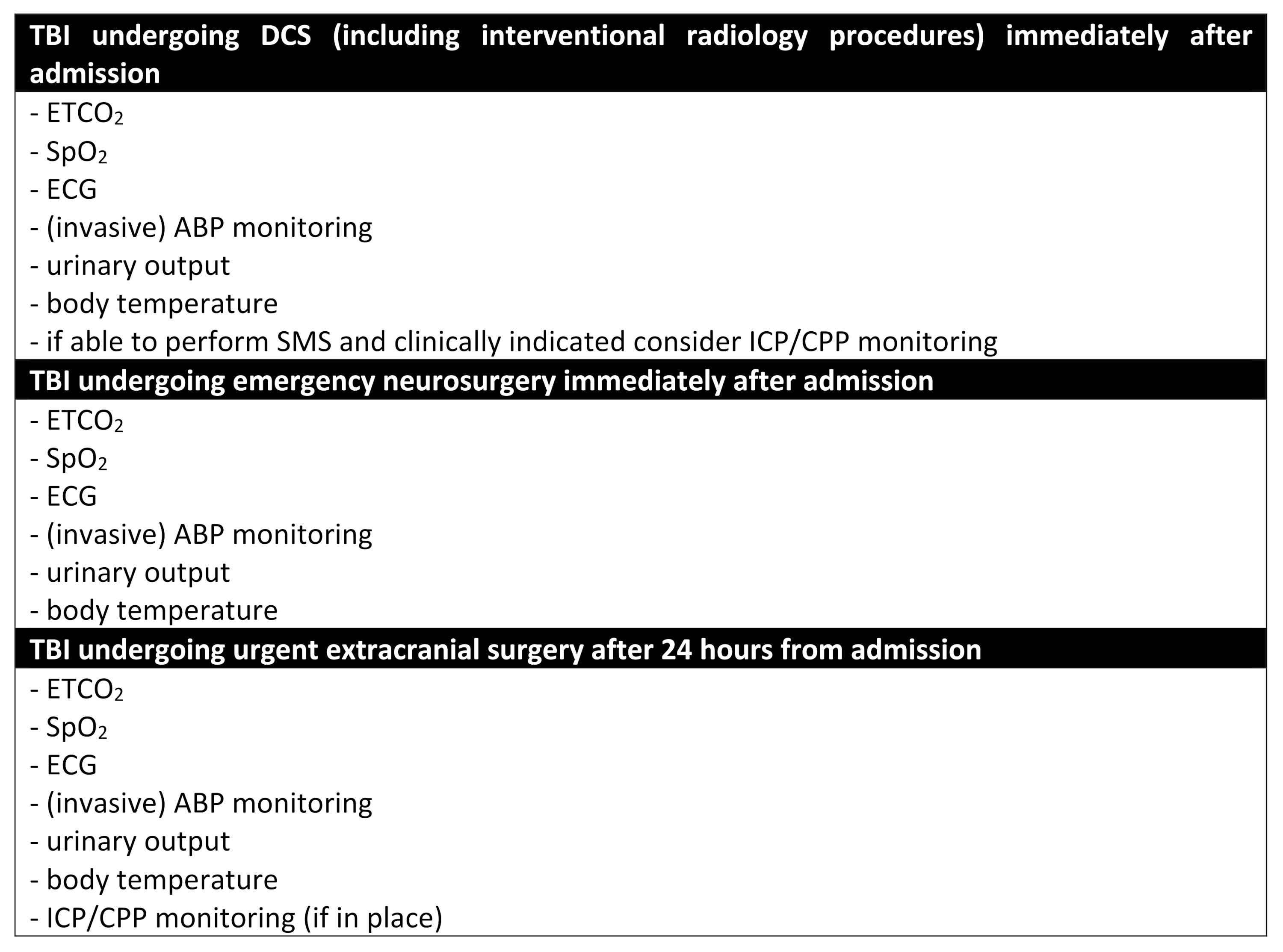
5. Simultaneous Multisystem Surgery (SMS)
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IR | Interventional Radiology |
| GCSm | Glasgow Coma Scale motor score |
| CT | Computed Tomography |
| ICP | Intracranial Pressure |
| IH | Intracranial Hypertension |
| SBP | Systolic Blood Pressure |
| MAP | Mean Arterial Pressure |
| RBC | Red Blood Cell |
| Hb | Hemoglobin |
| PaO2 | Arterial Partial Pressure of Oxygen |
| PaCO2 | Arterial Partial Pressure of Carbon Dioxide |
| PLT | Platelet |
| PT | Prothrombin Time |
| aPTT | Activated Partial Thromboplastin Time |
| POC | Point-Of-Care |
| TEG | Thromboelastography |
| ROTEM | Rotational Thromboelastometry |
| CPP | Cerebral Perfusion Pressure |
| SMS | Simultaneous Multisystem Surgery |
References
- Maas, A.I.R.; Menon, D.K.; Adelson, P.D.; Andelic, N.; Bell, M.J.; Belli, A.; Bragge, P.; Brazinova, A.; Büki, A.; Chesnut, R.M.; et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017, 16, 987–1048. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.C.; Rattani, A.; Gupta, S.; Baticulon, R.E.; Hung, Y.C.; Punchak, M.; Agrawal, A.; Adeleye, A.O.; Shrime, M.G.; Rubiano, A.M.; et al. Estimating the global incidence of traumatic brain injury. J. Neurosurg. 2018, 30, 1080–1097. [Google Scholar] [CrossRef] [Green Version]
- Pfeifer, R.; Tarkin, I.S.; Rocos, B.; Pape, H.C. Patterns of mortality and causes of death in polytrauma patients—Has anything changed? Injury 2009, 40, 907–911. [Google Scholar] [CrossRef]
- Pape, H.C.; Lefering, R.; Butcher, N.; Peitzman, A.; Leenen, L.; Marzi, I.; Lichte, P.; Josten, C.; Bouillon, B.; Schmucker, U.; et al. The definition of polytrauma revisited: An international consensus process and proposal of the new ‘Berlin definition’. J. Trauma Acute Care Surg. 2014, 77, 780–786. [Google Scholar] [CrossRef] [PubMed]
- Tisherman, S.A.; Schmicker, R.H.; Brasel, K.J.; Bulger, E.M.; Kerby, J.D.; Minei, J.P.; Powell, J.L.; Reiff, D.A.; Rizoli, S.B.; Schreiber, M.A. Detailed description of all deaths in both the shock and traumatic brain injury hypertonic saline trials of the Resuscitation Outcomes Consortium. Ann. Surg. 2015, 261, 586–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badjatia, N.; Carney, N.; Crocco, T.J.; Fallat, M.E.; Hennes, H.M.; Jagoda, A.S.; Jernigan, S.; Letarte, P.B.; Lerner, E.B.; Moriarty, T.M.; et al. Guidelines for prehospital management of traumatic brain injury 2nd edition. Prehosp. Emerg. Care 2008, 12, S1–S52. [Google Scholar] [CrossRef] [PubMed]
- Callcut, R.A.; Kornblith, L.Z.; Conroy, A.S.; Robles, A.J.; Meizoso, J.P.; Namias, N.; Meyer, D.E.; Haymaker, A.; Truitt, M.S.; Agrawal, V.; et al. The why and how our trauma patients die: A prospective Multicenter Western Trauma Association study. J. Trauma Acute Care Surg. 2019, 86, 864–870. [Google Scholar] [PubMed]
- Duchesne, J.C.; McSwain, N.E., Jr.; Cotton, B.A.; Hunt, J.P.; Dellavolpe, J.; Lafaro, K.; Marr, A.B.; Gonzalez, E.A.; Phelan, H.A.; Bilski, T.; et al. Damage control resuscitation: The new face of damage control. J. Trauma 2010, 69, 976–990. [Google Scholar] [CrossRef]
- Chovanes, J.; Cannon, J.W.; Nunez, T.C. The evolution of damage control surgery. Surg. Clin. 2012, 92, 859–875. [Google Scholar] [CrossRef] [PubMed]
- Cannon, J.W.; Khan, M.A.; Raja, A.S.; Cohen, M.J.; Como, J.J.; Cotton, B.A.; Dubose, J.J.; Fox, E.E.; Inaba, K.; Rodriguez, C.J.; et al. Damage control resuscitation in patients with severe traumatic hemorrhage: A practice management guideline from the Eastern Association for the Surgery of Trauma. J. Trauma Acute Care Surg. 2017, 82, 605–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 1852–1853. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.M.; Thomas, P.A.; Gruen, R.L.; Chan, P.; Rosenfeld, J.V. Simultaneous multisystem surgery: An important capability for the civilian trauma hospital. Clin. Neurol. Neurosurg. 2016, 148, 13–16. [Google Scholar] [CrossRef]
- Kinoshita, T.; Hayashi, M.; Yamakawa, K.; Watanabe, A.; Yoshimura, J.; Hamasaki, T.; Fujimi, S. Effect of the Hybrid Emergency Room System on Functional Outcome in Patients with Severe Traumatic Brain Injury. World Neurosurg. 2018, 118, e792–e799. [Google Scholar] [CrossRef] [PubMed]
- Carver, D.; Kirkpatrick, A.W.; D’Amours, S.; Hameed, S.M.; Beveridge, J.; Ball, C.G. A Prospective Evaluation of the Utility of a Hybrid Operating Suite for Severely Injured Patients: Overstated or Underutilized? Ann. Surg. 2020, 271, 958–961. [Google Scholar] [CrossRef]
- Vella, M.A.; Crandall, M.L.; Patel, M.B. Acute Management of Traumatic Brain Injury. Surg. Clin. 2017, 97, 1015–1030. [Google Scholar] [CrossRef]
- Hardcastle, T.C.; Muckart, D.J.J.; Maier, R.V. Ventilation in Trauma Patients: The First 24 h is Different! World J. Surg. 2017, 41, 1153–1158. [Google Scholar] [CrossRef]
- Cnossen, M.C.; Huijben, J.A.; van der Jagt, M.; Volovici, V.; van Essen, T.; Polinder, S.; Nelson, D.; Ercole, A.; Stocchetti, N.; Citerio, G.; et al. Variation in monitoring and treatment policies for intracranial hypertension in traumatic brain injury: A survey in 66 neurotrauma centers participating in the CENTER-TBI study. Crit. Care 2017, 21, 233. [Google Scholar] [CrossRef] [PubMed]
- Picetti, E.; Maier, R.V.; Rossi, S.; Kirkpatrick, A.W.; Biffl, W.L.; Stahel, P.F.; Moore, E.E.; Kluger, Y.; Baiocchi, G.L.; Ansaloni, L.; et al. Preserve encephalus in surgery of trauma: Online survey. (P.E.S.T.O). World J. Emerg. Surg. 2019, 14, 9. [Google Scholar] [CrossRef]
- Volovici, V.; Ercole, A.; Citerio, G.; Stocchetti, N.; Haitsma, I.K.; Huijben, J.A.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Nelson, D.; et al. Variation in Guideline Implementation and Adherence Regarding Severe Traumatic Brain Injury Treatment: A CENTER-TBI Survey Study in Europe. World Neurosurg. 2019, 125, e515–e520. [Google Scholar] [CrossRef] [PubMed]
- Tropeano, M.P.; Spaggiari, R.; Ileyassoff, H.; Park, K.B.; Kolias, A.G.; Hutchinson, P.J.; Servadei, F. A comparison of publication to TBI burden ratio of low- and middle-income countries versus high-income countries: How can we improve worldwide care of TBI? Neurosurg. Focus 2019, 47, E5. [Google Scholar] [CrossRef] [Green Version]
- Kuza, C.M.; Hatzakis, G.; Nahmias, J.T. The Assignment of American Society of Anesthesiologists Physical Status Classification for Adult Polytrauma Patients: Results from a Survey and Future Considerations. Anesth. Analg. 2017, 125, 1960–1966. [Google Scholar] [CrossRef]
- Gao, G.; Wu, X.; Feng, J.; Hui, J.; Mao, Q.; Lecky, F.; Lingsma, H.; Maas, A.I.R.; Jiang, J.; China CENTER-TBI Registry Participants. Clinical characteristics and outcomes in patients with traumatic brain injury in China: A prospective, multicentre, longitudinal, observational study. Lancet Neurol. 2020, 19, 670–677. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2017, 80, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Spahn, D.R.; Bouillon, B.; Cerny, V.; Duranteau, J.; Filipescu, D.; Hunt, B.J.; Komadina, R.; Maegele, M.; Nardi, G.; Riddez, L.; et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care 2019, 23, 98. [Google Scholar] [CrossRef] [Green Version]
- Marmarou, A.; Anderson, R.; Ward, J.; Choi, S.; Young, H. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg. 1991, 75, S59–S66. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef]
- Butcher, I.; Maas, A.I.; Lu, J.; Marmarou, A.; Murray, G.D.; Mushkudiani, N.A.; McHugh, G.S.; Steyerberg, E.W. Prognostic value of admission blood pressure in traumatic brain injury: Results from the IMPACT study. J. Neurotrauma 2007, 24, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Brenner, M.; Stein, D.M.; Hu, P.F.; Aarabi, B.; Sheth, K.; Scalea, T.M. Traditional systolic blood pressure targets underestimate hypotension-induced secondary brain injury. J. Trauma Acute Care Surg. 2012, 72, 1135–1139. [Google Scholar] [CrossRef]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Sherrill, D.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Viscusi, C.; Mullins, T.; et al. Mortality and Prehospital Blood Pressure in Patients with Major Traumatic Brain Injury: Implications for the Hypotension Threshold. JAMA Surg. 2017, 152, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Geeraerts, T.; Velly, L.; Abdennour, L.; Asehnoune, K.; Audibert, G.; Bouzat, P.; Bruder, N.; Carrillon, R.; Cottenceau, V.; Cotton, F.; et al. Management of severe traumatic brain injury (first 24 hours). Anaesth. Crit. Care Pain Med. 2018, 37, 171–186. [Google Scholar] [CrossRef]
- Manley, G.; Knudson, M.M.; Morabito, D.; Damron, S.; Erickson, V.; Pitts, L. Hypotension, hypoxia, and head injury: Frequency, duration, and consequences. Arch. Surg. 2001, 136, 1118–1123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picetti, E.; Rossi, S.; Abu-Zidan, F.M.; Ansaloni, L.; Armonda, R.; Baiocchi, G.L.; Bala, M.; Balogh, Z.J.; Berardino, M.; Biffl, W.L.; et al. WSES consensus conference guidelines: Monitoring and management of severe adult traumatic brain injury patients with polytrauma in the first 24 hours. World J. Emerg. Surg. 2019, 14, 53. [Google Scholar] [CrossRef]
- Stahel, P.; Heyde, C.; Ertel, W. Current concepts of polytrauma management. Eur. J. Trauma 2005, 31, 200–211. [Google Scholar] [CrossRef]
- Branson, R.D.; Johannigman, J.A. Pre-hospital oxygen therapy. Respir. Care 2013, 58, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockinger, Z.T.; Mcswain, N.E., Jr. Prehospital supplemental oxygen in trauma patients: Its efficacy and implications for military medical care. Mil. Med. 2004, 169, 609–612. [Google Scholar] [CrossRef] [Green Version]
- Ó Briain, D.; Nickson, C.; Pilcher, D.V.; Udy, A.A. Early Hyperoxia in Patients with Traumatic Brain Injury Admitted to Intensive Care in Australia and New Zealand: A Retrospective Multicenter Cohort Study. Neurocrit. Care 2018, 29, 443–445. [Google Scholar] [CrossRef]
- Kim, M.W.; Shin, S.D.; Song, K.J.; Ro, Y.S.; Kim, Y.J.; Hong, K.J.; Jeong, J.; Kim, T.H.; Park, J.H.; Kong, S.Y. Interactive Effect between On-Scene Hypoxia and Hypotension on Hospital Mortality and Disability in Severe Trauma. Prehosp. Emerg. Care 2018, 22, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Stocchetti, N.; Furlan, A.; Volta, F. Hypoxemia and arterial hypotension at the accident scene in head injury. J. Trauma 1996, 40, 764–767. [Google Scholar] [CrossRef] [PubMed]
- Chi, J.H.; Knudson, M.M.; Vassar, M.J.; McCarthy, M.C.; Shapiro, M.B.; Mallet, S.; Holcroft, J.J.; Moncrief, H.; Noble, J.; Wisner, D.; et al. Prehospital hypoxia affects outcome in patients with traumatic brain injury: A prospective multicenter study. J. Trauma 2006, 61, 1134–1141. [Google Scholar] [CrossRef]
- Spaite, D.W.; Hu, C.; Bobrow, B.J.; Chikani, V.; Barnhart, B.; Gaither, J.B.; Denninghoff, K.R.; Adelson, P.D.; Keim, S.M.; Viscusi, C.; et al. The Effect of Combined Out-of-Hospital Hypotension and Hypoxia on Mortality in Major Traumatic Brain Injury. Ann. Emerg. Med. 2017, 69, 62–72. [Google Scholar] [CrossRef] [Green Version]
- Chu, D.K.; Kim, L.H.; Young, P.J.; Zamiri, N.; Almenawer, S.A.; Jaeschke, R.; Szczeklik, W.; Schünemann, H.J.; Neary, J.D.; Alhazzani, W. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): A systematic review and meta-analysis. Lancet 2018, 391, 1693–1705. [Google Scholar] [CrossRef]
- Brenner, M.; Stein, D.; Hu, P.; Kufera, J.; Wooford, M.; Scalea, T. Association between early hyperoxia and worse outcomes after traumatic brain injury. Arch. Surg. 2012, 147, 1042–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, D.P.; Meade, W.; Sise, M.J.; Kennedy, F.; Simon, F.; Tominaga, G.; Steele, J.; Coimbra, R. Both hypoxemia and extreme hyperoxemia may be detrimental in patients with severe traumatic brain injury. J. Neurotrauma 2009, 26, 2217–2223. [Google Scholar] [CrossRef]
- Baekgaard, J.S.; Abback, P.S.; Boubaya, M.; Moyer, J.D.; Garrigue, D.; Raux, M.; Champigneulle, B.; Dubreuil, G.; Pottecher, J.; Laitselart, P.; et al. Early hyperoxemia is associated with lower adjusted mortality after severe trauma: Results from a French registry. Crit. Care 2020, 24, 604. [Google Scholar] [CrossRef]
- Marhong, J.; Fan, E. Carbon dioxide in the critically ill: Too much or too little of a good thing? Respir. Care 2014, 59, 1597–1605. [Google Scholar] [CrossRef] [Green Version]
- Richter, T.; Ragaller, M. Ventilation in chest trauma. J. Emerg. Trauma Shock 2011, 4, 251–259. [Google Scholar]
- Muizelaar, J.P.; Marmarou, A.; Ward, J.D.; Kontos, H.A.; Choi, S.C.; Becker, D.P.; Gruemer, H.; Young, H.F. Adverse effects of prolonged hyperventilation in patients with severe head injury: A randomized clinical trial. J. Neurosurg. 1991, 75, 731–739. [Google Scholar] [CrossRef]
- Davis, D.P. Early ventilation in traumatic brain injury. Resuscitation 2008, 76, 333–340. [Google Scholar] [CrossRef]
- Stocchetti, N.; Maas, A.I.; Chieregato, A.; van der Plas, A.A. Hyperventilation in head injury: A review. Chest 2005, 127, 1812–1827. [Google Scholar] [CrossRef]
- Association of Hypercapnia and Hypercapnic Acidosis with Clinical Outcomes in Mechanically Ventilated Patients With Cerebral Injury. JAMA Neurol. 2018, 75, 818–826. [CrossRef] [PubMed]
- Chesnut, R.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for adult patients with both brain oxygen and intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2020, 46, 919–929. [Google Scholar] [CrossRef] [Green Version]
- Okonkwo, D.O.; Shutter, L.A.; Moore, C.; Temkin, N.R.; Puccio, A.M.; Madden, C.J.; Andaluz, N.; Chesnut, R.M.; Bullock, M.R.; Grant, G.A.; et al. Brain Oxygen Optimization in Severe Traumatic Brain Injury Phase-II: A Phase II Randomized Trial. Crit. Care Med. 2017, 45, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Badenes, R.; Oddo, M.; Suarez, J.I.; Antonelli, M.; Lipman, J.; Citerio, G.; Taccone, F.S. Hemoglobin concentrations and RBC transfusion thresholds in patients with acute brain injury: An international survey. Crit. Care 2017, 21, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hébert, P.C.; Wells, G.; Blajchman, M.A.; Marshall, J.; Martin, C.; Pagliarello, G.; Tweeddale, M.; Schweitzer, I.; Yetisir, E. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N. Engl. J. Med. 1999, 340, 409–417. [Google Scholar] [CrossRef]
- McIntyre, L.A.; Fergusson, D.A.; Hutchison, J.S.; Pagliarello, G.; Marshall, J.C.; Yetisir, E.; Hare, G.M.; Hébert, P.C. Effect of a liberal versus restrictive transfusion strategy on mortality in patients with moderate to severe head injury. Neurocrit. Care 2006, 5, 4–9. [Google Scholar] [CrossRef]
- French, C.J.; Glassford, N.J.; Gantner, D.; Higgins, A.M.; Cooper, D.J.; Nichol, A.; Skrifvars, M.B.; Imberger, G.; Presneill, J.; Bailey, M.; et al. Erythropoiesis-stimulating Agents in Critically Ill Trauma Patients: A Systematic Review and Meta-analysis. Ann. Surg. 2017, 265, 54–62. [Google Scholar] [CrossRef] [PubMed]
- Hawryluk, G.W.J.; Aguilera, S.; Buki, A.; Bulger, E.; Citerio, G.; Cooper, D.J.; Arrastia, R.D.; Diringer, M.; Figaji, A.; Gao, G.; et al. A management algorithm for patients with intracranial pressure monitoring: The Seattle International Severe Traumatic Brain Injury Consensus Conference (SIBICC). Intensive Care Med. 2019, 45, 1783–1794. [Google Scholar] [CrossRef] [Green Version]
- Carson, J.L.; Guyatt, G.; Heddle, N.M.; Grossman, B.J.; Cohn, C.S.; Fung, M.K.; Gernsheimer, T.; Holcomb, J.B.; Kaplan, L.J.; Katz, L.M.; et al. Clinical Practice Guidelines From the AABB: Red Blood Cell Transfusion Thresholds and Storage. JAMA 2016, 316, 2025–2035. [Google Scholar] [CrossRef]
- MacLeod, J.B.; Lynn, M.; McKenney, M.G.; Cohn, S.M.; Murtha, M. Early coagulopathy predicts mortality in trauma. J. Trauma 2003, 55, 39–44. [Google Scholar] [CrossRef]
- Allard, C.B.; Scarpelini, S.; Rhind, S.G.; Baker, A.J.; Shek, P.N.; Tien, H.; Fernando, M.; Tremblay, L.; Morrison, L.J.; Pinto, R.; et al. Abnormal coagulation tests are associated with progression of traumatic intracranial hemorrhage. J. Trauma 2009, 67, 959–967. [Google Scholar] [CrossRef]
- Yuan, Q.; Sun, Y.R.; Wu, X.; Yu, J.; Li, Z.Q.; Du, Z.Y.; Wu, X.H.; Zhou, L.F.; Hu, J. Coagulopathy in Traumatic Brain Injury and Its Correlation with Progressive Hemorrhagic Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2016, 33, 1279–1291. [Google Scholar] [CrossRef]
- Moore, E.E.; Moore, H.B.; Chapman, M.P.; Gonzalez, E.; Sauaia, A. Goal-directed hemostatic resuscitation for trauma induced coagulopathy: Maintaining homeostasis. J. Trauma Acute Care Surg. 2018, 84, S35–S40. [Google Scholar] [CrossRef] [PubMed]
- Kvint, S.; Schuster, J.; Kumar, M.A. Neurosurgical applications of viscoelastic hemostatic assays. Neurosurg. Focus 2017, 43, E9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baksaas-Aasen, K.; Gall, L.S.; Stensballe, J.; Juffermans, N.P.; Curry, N.; Maegele, M.; Brooks, A.; Rourke, C.; Gillespie, S.; Murphy, J.; et al. Viscoelastic haemostatic assay augmented protocols for major trauma haemorrhage (ITACTIC): A randomized, controlled trial. Intensive Care Med. 2021, 47, 49–59. [Google Scholar] [CrossRef]
- Taccone, F.S.; Citerio, G.; Stocchetti, N. Is tranexamic acid going to CRASH the management of traumatic brain injury? Intensive Care Med. 2020, 46, 1261–1263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CRASH-3 Trial Collaborators. Effects of tranexamic acid on death, disability, vascular occlusive events and other morbidities in patients with acute traumatic brain injury (CRASH-3): A randomised, placebo-controlled trial. Lancet 2019, 394, 1713–1723. [Google Scholar] [CrossRef] [Green Version]
- Rowell, S.E.; Meier, E.N.; McKnight, B.; Kannas, D.; May, S.; Sheehan, K.; Bulger, E.M.; Idris, A.H.; Christenson, J.; Morrison, L.J.; et al. Effect of Out-of-Hospital Tranexamic Acid vs. Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA 2020, 324, 961–974. [Google Scholar] [CrossRef]
- Bossers, S.M.; Loer, S.A.; Bloemers, F.W.; Den Hartog, D.; Van Lieshout, E.M.M.; Hoogerwerf, N.; van der Naalt, J.; Absalom, A.R.; Peerdeman, S.M.; Schwarte, L.A.; et al. Association between Prehospital Tranexamic Acid Administration and Outcomes of Severe Traumatic Brain Injury. JAMA Neurol. 2021, 78, 338–345. [Google Scholar] [CrossRef]
- Abuzeid, A.M.; O’Keeffe, T. Review of massive transfusion protocols in the injured, bleeding patient. Curr. Opin. Crit. Care 2019, 25, 661–667. [Google Scholar] [CrossRef]
- Holcomb, J.B.; Tilley, B.C.; Baraniuk, S.; Fox, E.E.; Wade, C.E.; Podbielski, J.M.; del Junco, D.J.; Brasel, K.J.; Bulger, E.M.; Callcut, R.A.; et al. Transfusion of plasma, platelets, and red blood cells in a 1:1:1 vs. a 1:1:2 ratio and mortality in patients with severe trauma: The PROPPR randomized clinical trial. JAMA 2015, 313, 471–482. [Google Scholar] [CrossRef]
- Vik, A.; Nag, T.; Fredriksli, O.A.; Skandsen, T.; Moen, K.G.; Schirmer-Mikalsen, K.; Manley, G.T. Relationship of “dose” of intracranial hypertension to outcome in severe traumatic brain injury. J. Neurosurg. 2008, 109, 678–684. [Google Scholar] [CrossRef] [PubMed]
- Güiza, F.; Depreitere, B.; Piper, I.; Citerio, G.; Chambers, I.; Jones, P.A.; Lo, T.Y.; Enblad, P.; Nillson, P.; Feyen, B.; et al. Visualizing the pressure and time burden of intracranial hypertension in adult and paediatric traumatic brain injury. Intensive Care Med. 2015, 41, 1067–1076. [Google Scholar] [CrossRef]
- Güiza, F.; Meyfroidt, G.; Piper, I.; Citerio, G.; Chambers, I.; Enblad, P.; Nillson, P.; Feyen, B.; Jorens, P.; Maas, A.; et al. Cerebral Perfusion Pressure Insults and Associations with Outcome in Adult Traumatic Brain Injury. J. Neurotrauma 2017, 34, 2425–2431. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Algarra, N.N.; Vavilala, M.S.; Prathep, S.; Prapruettham, S.; Sharma, D. Intraoperative secondary insults during extracranial surgery in children with traumatic brain injury. Childs Nerv. Syst. 2014, 30, 1201–1208. [Google Scholar] [CrossRef]
- Algarra, N.N.; Lele, A.V.; Prathep, S.; Souter, M.J.; Vavilala, M.S.; Qiu, Q.; Sharma, D. Intraoperative Secondary Insults During Orthopedic Surgery in Traumatic Brain Injury. J. Neurosurg. Anesthesiol. 2017, 29, 228–235. [Google Scholar] [CrossRef]
- Smith, M. Monitoring intracranial pressure in traumatic brain injury. Anesth. Analg. 2008, 106, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Servadei, F.; Picetti, E. Intracranial pressure monitoring and outcome in traumatic brain injury: The probe does matter? World Neurosurg. 2015, 83, 732–733. [Google Scholar] [CrossRef] [PubMed]
- Smith, M. Cerebral perfusion pressure. Br. J. Anaesth. 2015, 115, 488–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robba, C.; Graziano, F.; Rebora, P.; Elli, F.; Giussani, C.; Oddo, M.; Meyfroidt, G.; Helbok, R.; Taccone, F.S.; Prisco, L.; et al. Intracranial pressure monitoring in patients with acute brain injury in the intensive care unit (SYNAPSE-ICU): An international, prospective observational cohort study. Lancet Neurol. 2021, 20, 548–558. [Google Scholar] [CrossRef]
- Stocchetti, N.; Picetti, E.; Berardino, M.; Buki, A.; Chesnut, R.M.; Fountas, K.N.; Horn, P.; Hutchinson, P.J.; Iaccarino, C.; Kolias, A.G.; et al. Clinical applications of intracranial pressure monitoring in traumatic brain injury: Report of the Milan consensus conference. Acta Neurochir. 2014, 156, 1615–1622. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, R.; Videtta, W.; Vespa, P.; Le Roux, P.; Participants in the International Multidisciplinary Consensus Conference on Multimodality Monitoring. Intracranial pressure monitoring: Fundamental considerations and rationale for monitoring. Neurocrit. Care 2014, 21, S64–S84. [Google Scholar] [CrossRef]
- Picetti, E.; Iaccarino, C.; Servadei, F. Letter: Guidelines for the Management of Severe Traumatic Brain Injury Fourth Edition. Neurosurgery 2017, 81, E2. [Google Scholar] [CrossRef]
- Citerio, G.; Signorini, L.; Bronco, A.; Vargiolu, A.; Rota, M.; Latronico, N.; Infezioni LIquorali Catetere Correlate Study Investigators. External Ventricular and Lumbar Drain Device Infections in ICU Patients: A Prospective Multicenter Italian Study. Crit. Care Med. 2015, 43, 1630–1637. [Google Scholar] [CrossRef]
- Volovici, V.; Huijben, J.A.; Ercole, A.; Stocchetti, N.; Dirven, C.M.F.; van der Jagt, M.; Steyerberg, E.W.; Lingsma, H.F.; Menon, D.K.; Maas, A.I.R.; et al. Ventricular Drainage Catheters versus Intracranial Parenchymal Catheters for Intracranial Pressure Monitoring-Based Management of Traumatic Brain Injury: A Systematic Review and Meta-Analysis. J. Neurotrauma 2019, 36, 988–995. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamakawa, K.; Yoshimura, J.; Watanabe, A.; Matsumura, Y.; Ito, K.; Ohbe, H.; Hayashida, K.; Kushimoto, S.; Matsumoto, J.; et al. First clinical experiences of concurrent bleeding control and intracranial pressure monitoring using a hybrid emergency room system in patients with multiple injuries. World J. Emerg. Surg. 2018, 13, 56. [Google Scholar] [CrossRef] [Green Version]
- Stocchetti, N.; Maas, A.I. Traumatic intracranial hypertension. N. Engl. J. Med. 2014, 370, 2121–2130. [Google Scholar] [CrossRef] [Green Version]
- Smith, M. Multimodality Neuromonitoring in Adult Traumatic Brain Injury: A Narrative Review. Anesthesiology 2018, 128, 401–415. [Google Scholar] [CrossRef]
- Feyen, B.F.; Sener, S.; Jorens, P.G.; Menovsky, T.; Maas, A.I. Neuromonitoring in traumatic brain injury. Minerva Anestesiol. 2012, 78, 949–958. [Google Scholar]
- Hawryluk, G.W.J.; Rubiano, A.M.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; Shutter, L.; et al. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery 2020, 87, 427–434. [Google Scholar] [CrossRef]
- Hernandez, A.M.; Roguski, M.; Qiu, R.S.; Shepard, M.J.; Riesenburger, R.I. Surgeons’ perspectives on optimal patient positioning during simultaneous cranial procedures and exploratory laparotomy. South. Med. J. 2013, 106, 679–683. [Google Scholar] [CrossRef]
- Kinoshita, T.; Yamakawa, K.; Matsuda, H.; Yoshikawa, Y.; Wada, D.; Hamasaki, T.; Ono, K.; Nakamori, Y.; Fujimi, S. The Survival Benefit of a Novel Trauma Workflow that Includes Immediate Whole-body Computed Tomography, Surgery, and Interventional Radiology, All in One Trauma Resuscitation Room: A Retrospective Historical Control Study. Ann. Surg. 2019, 269, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Umemura, Y.; Watanabe, A.; Kinoshita, T.; Morita, N.; Yamakawa, K.; Fujimi, S. Hybrid emergency room shows maximum effect on trauma resuscitation when used in patients with higher severity. J. Trauma Acute Care Surg. 2021, 90, 232–239. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Moriwaki, K.; Hanaki, N.; Kitamura, T.; Yamakawa, K.; Fukuda, T.; Hunink, M.G.M.; Fujimi, S. Cost-effectiveness of a hybrid emergency room system for severe trauma: A health technology assessment from the perspective of the third-party payer in Japan. World J. Emerg. Surg. 2021, 16, 2. [Google Scholar] [CrossRef] [PubMed]
- Ito, K.; Nagao, T.; Nakazawa, K.; Kato, A.; Chiba, H.; Kondo, H.; Miyake, Y.; Sakamoto, T.; Fujita, T. Simultaneous damage control surgery and endovascular procedures for patients with blunt trauma in the hybrid emergency room system: New multidisciplinary trauma team building. J. Trauma Acute Care Surg. 2019, 86, 160–162. [Google Scholar] [PubMed]
- Kirkpatrick, A.W.; Vis, C.; Dubé, M.; Biesbroek, S.; Ball, C.G.; Laberge, J.; Shultz, J.; Rea, K.; Sadler, D.; Holcomb, J.B.; et al. The evolution of a purpose designed hybrid trauma operating room from the trauma service perspective: The RAPTOR (Resuscitation with Angiography Percutaneous Treatments and Operative Resuscitations). Injury 2014, 45, 1413–1421. [Google Scholar] [PubMed]
- Founding Members of the Japanese Association for Hybrid Emergency Room System (JA-HERS). The hybrid emergency room system: A novel trauma evaluation and care system created in Japan. Acute Med. Surg. 2019, 6, 247–251. [Google Scholar]
- Loftus, T.J.; Croft, C.A.; Rosenthal, M.D.; Mohr, A.M.; Efron, P.A.; Moore, F.A.; Upchurch, G.R., Jr.; Smith, R.S. Clinical Impact of a Dedicated Trauma Hybrid Operating Room. J. Am. Coll. Surg. 2021, 232, 560–570. [Google Scholar] [CrossRef]
- Wiegers, E.J.A.; Lingsma, H.F.; Huijben, J.A.; Cooper, D.J.; Citerio, G.; Frisvold, S.; Helbok, R.; Maas, A.I.R.; Menon, D.K.; Moore, E.M.; et al. Fluid balance and outcome in critically ill patients with traumatic brain injury (CENTER-TBI and OzENTER-TBI): A prospective, multicentre, comparative effectiveness study. Lancet Neurol. 2021, 20, 627–638. [Google Scholar] [CrossRef]
- Watson, X.; Cecconi, M. Haemodynamic monitoring in the peri-operative period: The past, the present and the future. Anaesthesia 2017, 72, 7–15. [Google Scholar] [CrossRef] [Green Version]
- Badenes, R.; Bilotta, F. Neurocritical care for intracranial haemorrhage: A systematic review of recent studies. Br. J. Anaesth. 2015, 115, ii68–ii74. [Google Scholar] [CrossRef] [Green Version]
- Chesnut, R.M.; Temkin, N.; Videtta, W.; Petroni, G.; Lujan, S.; Pridgeon, J.; Dikmen, S.; Chaddock, K.; Barber, J.; Machamer, J.; et al. Consensus-Based Management Protocol (CREVICE Protocol) for the Treatment of Severe Traumatic Brain Injury Based on Imaging and Clinical Examination for Use When Intracranial Pressure Monitoring Is Not Employed. J. Neurotrauma 2020, 37, 1291–1299. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Sharma, D.; Kannan, N.; Prapruettham, S.; Mock, C.; Wang, J.; Qiu, Q.; Pandey, R.M.; Mahapatra, A.; Dash, H.H.; et al. Guideline Adherence and Outcomes in Severe Adult Traumatic Brain Injury for the CHIRAG (Collaborative Head Injury and Guidelines) Study. World Neurosurg. 2016, 89, 169–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Reference Number | Title | Journal | Year |
|---|---|---|---|
| [74] | Intraoperative Secondary Insults during Extracranial Surgery in children with Traumatic Brain Injury | Child’s Nervous System | 2014 |
| [95] | The Evolution of a Purpose Designed Hybrid Trauma Operating Room from the Trauma Service Perspective: The RAPTOR (Resuscitation with Angiography Percutaneous Treatments and Operative Resuscitations) | Injury | 2014 |
| [12] | Simultaneous Multisystem Surgery: An Important Capability for the Civilian Trauma Hospital | Clinical Neurology and Neurosurgery | 2016 |
| [75] | Intraoperative Secondary Insults during Orthopedic Surgery in Traumatic Brain Injury | Journal of Neurosurgical Anesthesiology | 2017 |
| [13] | Effect of the Hybrid Emergency Room System on Functional Outcome in Patients with Severe Traumatic Brain Injury | World Neurosurgery | 2018 |
| [85] | First Clinical Experiences of Concurrent Bleeding Control and Intracranial Pressure Monitoring Using a Hybrid Emergency Room System in Patients with Multiple Injuries | World Journal of Emergency Surgery | 2018 |
| [96] | The Hybrid Emergency Room System: A Novel Trauma Evaluation and Care System Created in Japan | Acute Medicine & Surgery | 2019 |
| [94] | Simultaneous Damage Control Surgery and Endovascular Procedures for Patients with Blunt Trauma in the Hybrid Emergency Room SYSTEM: New Multidisciplinary Trauma Team Building | Journal of Trauma and Acute Care Surgery | 2019 |
| [91] | The Survival Benefit of a Novel Trauma Workflow that Includes Immediate Whole-body Computed Tomography, Surgery, and Interventional Radiology, All in One Trauma Resuscitation Room: A Retrospective Historical Control Study | Annals of Surgery | 2019 |
| [18] | Preserve Encephalus in Surgery of Trauma: Online Survey (P.E.S.T.O) | World Journal of Emergency Surgery | 2019 |
| [32] | WSES Consensus Conference Guidelines: Monitoring and Management of Severe Adult Traumatic Brain Injury Patients with Polytrauma in the First 24 Hours | World Journal of Emergency Surgery | 2019 |
| [14] | A Prospective Evaluation of the Utility of a Hybrid Operating Suite for Severely Injured Patients: Overstated or Underutilized? | Annals of Surgery | 2020 |
| [93] | Cost-Effectiveness of a Hybrid Emergency Room System for Severe Trauma: A Health Technology Assessment from the Perspective of the Third-Party Payer in Japan | World Journal of Emergency Surgery | 2021 |
| [97] | Clinical Impact of a Dedicated Trauma Hybrid Operating Room | Journal of the American College of Surgeons | 2021 |
| [92] | Hybrid Emergency Room Shows Maximum Effect on Trauma Resuscitation When Used in Patients with Higher Severity | Journal of Trauma and Acute Care Surgery | 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Picetti, E.; Rosenstein, I.; Balogh, Z.J.; Catena, F.; Taccone, F.S.; Fornaciari, A.; Votta, D.; Badenes, R.; Bilotta, F. Perioperative Management of Polytrauma Patients with Severe Traumatic Brain Injury Undergoing Emergency Extracranial Surgery: A Narrative Review. J. Clin. Med. 2022, 11, 18. https://doi.org/10.3390/jcm11010018
Picetti E, Rosenstein I, Balogh ZJ, Catena F, Taccone FS, Fornaciari A, Votta D, Badenes R, Bilotta F. Perioperative Management of Polytrauma Patients with Severe Traumatic Brain Injury Undergoing Emergency Extracranial Surgery: A Narrative Review. Journal of Clinical Medicine. 2022; 11(1):18. https://doi.org/10.3390/jcm11010018
Chicago/Turabian StylePicetti, Edoardo, Israel Rosenstein, Zsolt J. Balogh, Fausto Catena, Fabio S. Taccone, Anna Fornaciari, Danilo Votta, Rafael Badenes, and Federico Bilotta. 2022. "Perioperative Management of Polytrauma Patients with Severe Traumatic Brain Injury Undergoing Emergency Extracranial Surgery: A Narrative Review" Journal of Clinical Medicine 11, no. 1: 18. https://doi.org/10.3390/jcm11010018
APA StylePicetti, E., Rosenstein, I., Balogh, Z. J., Catena, F., Taccone, F. S., Fornaciari, A., Votta, D., Badenes, R., & Bilotta, F. (2022). Perioperative Management of Polytrauma Patients with Severe Traumatic Brain Injury Undergoing Emergency Extracranial Surgery: A Narrative Review. Journal of Clinical Medicine, 11(1), 18. https://doi.org/10.3390/jcm11010018










