Homocysteine Plasmatic Concentration in Brain-Injured Neurocritical Care Patients: Systematic Review of Clinical Evidence
Abstract
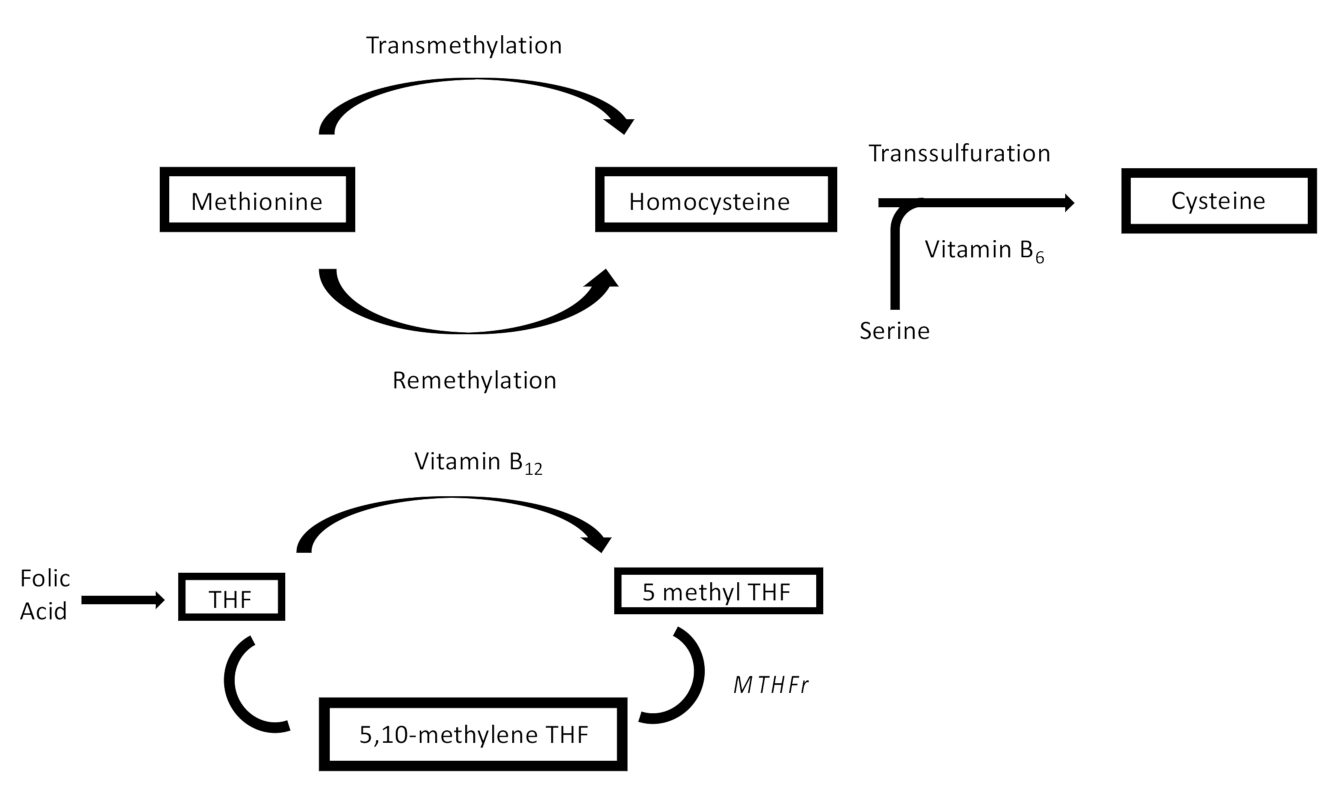
1. Introduction
2. Materials and Methods
3. Results
3.1. Hcy in AIS
3.2. Hcy in TBI
3.3. Hcy in ICH and SAH
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Search | (homocysteine) AND (cerebrovascular disease) |
| Filters | in the last 10 years, Humans, English, Adult: 19+ years [All Fields] OR [MeSH Subheading] OR [MeSH Terms] FOR: ((“homocystein” “homocysteine” “homocysteine” “homocysteine s” “homocysteines”) AND (“cerebrovascular disorders” (“cerebrovascular” AND “disorders”) “cerebrovascular disorders” (“cerebrovascular” AND “disease”) “cerebrovascular disease”)) AND ((y_10[Filter]) AND (humans[Filter]) AND (english[Filter]) AND (alladult[Filter])) |
| Translations | homocysteine: “homocystein” “homocysteine” “homocysteine” “homocysteine’s” “homocysteines” cerebrovascular disease: “cerebrovascular disorders” (“cerebrovascular” “disorders” “cerebrovascular disorders” (“cerebrovascular” AND “disease”) “cerebrovascular disease” |
| Search | (homocysteine) AND (perioperative care) |
| Filters | in the last 10 years, Humans, English, Adult: 19+ years [All Fields] OR [MeSH Subheading] OR [MeSH Terms] FOR: (“homocystein” “homocysteine” “homocysteine” “homocysteine s” “homocysteines”) AND (“perioperative care” (“perioperative” “care”) “perioperative care”) |
| Translations | homocysteine: “homocystein” “homocysteine” “homocysteine” “homocysteine’s” “homocysteines” perioperative care: “perioperative care” (“perioperative” AND “care”) “perioperative care”[All Fields] |
| Search | (((homocysteine) AND (traumatic)) AND (brain)) AND (injury) |
| Filters | in the last 10 years, Humans, English, Adult: 19+ years [All Fields] OR [MeSH Subheading] OR [MeSH Terms] FOR: (“homocystein” “homocysteine” “homocysteine” “homocysteine s” “homocysteines”) AND (“traumatic” “traumatically” “traumatism” “traumatisms” “traumatization” “traumatizations” “traumatize” “traumatized” “traumatizes” “traumatizing”) AND (“brain” “brain” “brains” “brain s”) AND (“injurie” “injuried” “injuries” “injuries” “wounds and injuries” (“wounds” AND “injuries”) “wounds and injuries” “injurious” “injury s” “injuryed” “injurys” “injury”) |
| Translations | homocysteine: “homocystein” “homocysteine” “homocysteine” “homocysteine’s” “homocysteines” traumatic: “traumatic” “traumatically” “traumatism” “traumatisms” “traumatization” “traumatizations” “traumatize” “traumatized” “traumatizes” “traumatizing” brain: “brain” “brain” “brains” “brain’s” injury: “injurie” “injuried” “injuries” “injuries” “wounds and injuries” (“wounds” AND “injuries”) “wounds and injuries” “injurious” “injury’s” “injuryed” “injurys” “injury” |
| Search | (((((homocysteine) AND (intracranial)) AND (haemorrhage)) OR (homocysteine)) AND (subarachnoid)) AND (haemorrhage) |
| Filters | in the last 10 years, Adult: 19+ years [All Fields] OR [MeSH Subheading] OR [MeSH Terms] FOR: ((((“homocystein” “homocysteine” “homocysteine” “homocysteine s” “homocysteines”) AND (“intracranial” “intracranially”) AND (“blood” “blood” “blood” “bloods” “haematology” “haematology” “hematology” “haematoma” “hematoma” “hematoma” “haemorrhage” “hemorrhage” “hemorrhage” “haemorrhages” “hemorrhages” “haemorrhagic” “haemorrhaging” “hematologies” “haematomas” “hematomas” “hematoma s” “hematomae” “hemorrhaged” “hemorrhagic” “hemorrhagical” “hemorrhaging”)) (“homocystein” “homocysteine” “homocysteine” “homocysteine s” “homocysteines”)) AND (“subarachnoid space” (“subarachnoid” AND “space”) “subarachnoid space” “subarachnoid” “subarachnoidal” “subarachnoidally” “subarachnoideal”) AND (“blood” “blood” “blood” “bloods” “haematology” “hematology” “hematology” “haematoma” “hematoma” “hematoma” “haemorrhage” “hemorrhage” “hemorrhage” “haemorrhages” “hemorrhages” “haemorrhagic” “haemorrhaging” “hematologies” “haematomas” “hematomas” “hematoma s” “hematomae”[All Fields] OR “hemorrhaged” “hemorrhagic” “hemorrhagical” “hemorrhaging”)) AND ((y_10[Filter]) AND (alladult[Filter]) AND (2011:2021[pdat])) |
| Translations | homocysteine: “homocystein” “homocysteine” “homocysteine” “homocysteine’s”[All Fields] OR “homocysteines” intracranial: “intracranial” “intracranially” hemorrhage: “blood” “blood” “blood” “bloods” “haematology” “hematology” “hematology” “haematoma” “hematoma” “hematoma” “haemorrhage” “hemorrhage” “hemorrhage” “haemorrhages” “hemorrhages” “haemorrhagic” “haemorrhaging”“hematologies” “haematomas” “hematomas” “hematoma’s” “hematomae” “hemorrhaged” “hemorrhagic” “hemorrhagical” “hemorrhaging” homocysteine: “homocystein” “homocysteine” “homocysteine” “homocysteine’s” “homocysteines” subarachnoid: “subarachnoid space” (“subarachnoid” AND “space”) “subarachnoid space” “subarachnoid” “subarachnoidal” “subarachnoidally” “subarachnoideal” hemorrhage: “blood” “blood” “blood” “bloods” “haematology” “hematology” “hematology” “haematoma” “hematoma” “hematoma” “haemorrhage” “hemorrhage” “hemorrhage” “haemorrhages” “hemorrhages” “haemorrhagic” “haemorrhaging” “hematologies” “haematomas” “hematomas” hematoma’s” “hematomae” “hemorrhaged” “hemorrhagic” “hemorrhagical” “hemorrhaging” |
References
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef]
- Fowler, B. Homocysteine: Overview of biochemistry, molecular biology, and role in disease processes. Semin. Vasc. Med. 2005, 5, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.S.; Wong, P.W.K.; Malinow, M.R. Hyperhomocysteinemia as a risk factor for occlusive vascular disease. Annu. Rev. Nutr. 1992, 12, 279–298. [Google Scholar] [CrossRef]
- Von Eckardstein, A.; Assmann, G. Plasma homocysteine levels and mortality in patients with coronary artery disease. N. Engl. J. Med. 1997, 337, 1632–1633. [Google Scholar] [PubMed]
- Jin, L.; Abou-Mohamed, G.; Caldwell, R.B.; Caldwell, R.W. Endothelial cell dysfunction in a model of oxidative stress. Med. Sci. Monit. 2001, 7, 585–591. [Google Scholar]
- Bagi, Z.; Ungvari, Z.; Koller, A. Xanthine oxidase-derived reactive oxygen species convert flow-induced arteriolar dilation to constriction in hyperhomocysteinemia: Possible role of peroxynitrite. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 28–33. [Google Scholar] [CrossRef][Green Version]
- Poddar, R.; Sivasubramanian, N.; DiBello, P.M.; Robinson, K.; Jacobsen, D.W. Homocysteine induces expression and secretion of monocyte chemoattractant protein-1 and interleukin-8 in human aortic endothelial cells: Implications for vascular disease. Circulation 2001, 103, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment: A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef]
- Ferlazzo, N.; Condello, S.; Currò, M.; Parisi, G.; Ientile, R.; Caccamo, D. NF-kappaB activation is associated with homocysteine-induced injury in Neuro2a cells. BMC Neurosci. 2008, 9, 62. [Google Scholar] [CrossRef]
- Besancon, E.; Guo, S.; Lok, J.; Tymianski, M.; Lo, E.H. Beyond NMDA and AMPA glutamate receptors: Emerging mechanisms for ionic imbalance and cell death in stroke. Trends Pharmacol. Sci. 2008, 29, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Ientile, R.; Curro’, M.; Ferlazzo, N.; Condello, S.; Caccamo, D.; Pisani, F. Homocysteine, vitamin determinants and neurological diseases. Front. Biosci. 2010, 2, 359–372. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Cochrane Review Group. Systematic Review on “Intervention X in Patients Y”; Cochrane: London, UK, 2007; pp. 1–13. [Google Scholar]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-bias Visualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res. Synth. Methods 2021, 12, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.M.; Lee, Y.S.; Bae, H.J.; Kang, D.W. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke 2014, 45, 871–873. [Google Scholar] [CrossRef]
- Shi, Z.; Guan, Y.; Huo, Y.R.; Liu, S.; Zhang, M.; Lu, H.; Yue, W.; Wang, J.; Ji, Y. Elevated total homocysteine levels in acute ischemic stroke are associated with long-term mortality. Stroke 2015, 46, 2419–2425. [Google Scholar] [CrossRef]
- Zhong, C.; Xu, T.; Xu, T.; Peng, Y.; Wang, A.; Wang, J.; Peng, H.; Li, Q.; Geng, D.; Zhang, D.; et al. Plasma homocysteine and prognosis of acute ischemic stroke: A gender-specific analysis from CATIS randomized clinical trial. Mol. Neurobiol. 2017, 54, 2022–2030. [Google Scholar] [CrossRef]
- Yin, J.; Zhong, C.; Zhu, Z.; Bu, X.; Xu, T.; Guo, L.; Wang, X.; Zhang, J.; Cui, Y.; Li, D.; et al. Elevated circulating homocysteine and high-sensitivity C-reactive protein jointly predicts post-stroke depression among Chinese patients with acute ischemic stroke. Clin. Chim. Acta 2018, 479, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhong, C.; Guo, D.; Bu, X.; Xu, T.; Guo, L.; Liu, J.; Zhang, J.; Li, D.; Ju, Z.; et al. Multiple biomarkers covering several pathways improve predictive ability for cognitive impairment among ischemic stroke patients with elevated blood pressure. Atherosclerosis 2019, 287, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, Y.; Li, H.; Zuo, Z.; Lin, J.; Wang, A.; Zhao, X.; Liu, L.; Wang, Y.; for the CHANCE Investigators. Homocysteine level predicts response to dual antiplatelet in women with minor stroke or transient ischemic attack: Subanalysis of the CHANCE Trial. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Scher, A.I.; Wu, H.; Tsao, J.W.; Blom, H.J.; Feit, P.; Nevin, R.L.; Schwab, K.A. MTHFR C677T genotype as a risk factor for epilepsy including post-traumatic epilepsy in a representative military cohort. J. Neurotrauma 2011, 28, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.; Hatefi, M.; Dastjerdi, M.M.; Zare, M.; Imani, A.; Shirazi, D. Correlation between serum homocysteine levels and outcome of patients with severe traumatic brain injury. World Neurosurg. 2016, 87, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Hatefi, M.; Behzadi, S.; Dastjerdi, M.M.; Ghahnavieh, A.A.; Rahmani, A.; Mahdizadeh, F.; Hafezi Ahmadi, M.R.; Asadollahi, K. Correlation of homocysteine with cerebral hemodynamic abnormality, endothelial dysfunction markers, and cognition impairment in patients with traumatic brain injury. World Neurosurg. 2017, 97, 70–79. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, B.; Chen, C.; Huang, J.; Chen, S.; Guo, F.; Hu, Z. Elevated homocysteine levels contribute to larger hematoma volume in patients with intracerebral hemorrhage. J. Stroke Cerebrovasc. Dis. 2015, 24, 784–788. [Google Scholar] [CrossRef]
- Bernstein, J.E.; Savla, P.; Dong, F.; Zampella, B.; Wiginton, J.G., 4th; Miulli, D.E.; Wacker, M.R.; Menoni, R. Inflammatory markers and severity of intracerebral hemorrhage. Cureus 2018, 10, e3529. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Goudihalli, S.; Mukherjee, K.; Dhandapani, S.; Sandhir, R. Methylenetetrahydrofolate reductase C677T variant and hyperhomocysteinemia in subarachnoid hemorrhage patients from India. Metab. Brain. Dis. 2018, 33, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Hendrix, P.; Foreman, P.M.; Harrigan, M.R.; Fisher, W.S., III; Nyas, N.A.; Lipsky, R.H.; Lin, M.; Walters, B.C.; Tubbs, R.S.; Shoja, M.M.; et al. Association of cystathionine beta-synthase polymorphisms and aneurysmal subarachnoid hemorrhage. J. Neurosurg. 2018, 128, 1771–1777. [Google Scholar] [CrossRef]
- Cheng, G.; Chang, F.-J.; Wang, Y.; You, P.-H.; Chen, C.-H.; Han, W.-Q.; Wang, J.-W.; Zhong, N.; Min, Z.-Q. Factors influencing stent sestenosis after percutaneous coronary intervention in patients with coronary heart disease: A clinical trial based on 1-Year follow-up. Med. Sci. Monit. 2019, 25, 240–247. [Google Scholar] [CrossRef]
- Köktürk, N.; Kanbay, A.; Aydoğdu, M.; Özyılmaz, E.; Bukan, N.; Ekim, N. Hyperhomocysteinemia prevalence among patients with venous thromboembolism. Clin. Appl. Thromb. Hemost. 2011, 17, 487–493. [Google Scholar] [CrossRef]
- Santilli, F.; Davì, G.; Patrono, C. Homocysteine, methylenetetrahydrofolate reductase, folate status and atherothrombosis: A mechanistic and clinical perspective. Vascul. Pharmacol. 2016, 78, 1–9. [Google Scholar] [CrossRef]
- Zhao, M.; Wang, X.; He, M.; Qin, X.; Tang, G.; Huo, Y.; Li, J.; Fu, J.; Huang, X.; Chen, X.; et al. Homocysteine and Stroke Risk: Modifying effect of Methylenetetrahydrofolate Reductase C677T Polymorphism and Folic Acid intervention. Stroke 2017, 48, 1183–1190. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Li, P.; Li, J.; Bao, H.; Zhang, Y.; Wang, B.; Sun, N.; Wang, J.; He, M.; et al. Association between percent decline in serum total homocysteine and risk of first stroke. Neurology 2017, 89, 2101–2107. [Google Scholar] [CrossRef] [PubMed]
- Ding, R.; Lin, S.; Chen, D. The association of Cystathionine β Synthase (CBS) T833C polymorphism and the risk of stroke: A meta-analysis. J. Neurol. Sci. 2012, 312, 26–30. [Google Scholar] [CrossRef]
- Gao, Y.; Wen, S.; Song, B.; Qin, J.; Fang, H.; Ji, Y.; Zhang, R.; Sun, S.; Zu, Y. Homocysteine level is associated with white matter hyperintensity locations in patients with acute ischemic stroke. PLoS ONE 2015, 10, e0144431. [Google Scholar] [CrossRef] [PubMed]
- Forti, P.; Maioli, F.; Arnone, G.; Coveri, M.; Pirazzoli, G.L.; Zoli, M.; Procaccinati, G. Homocysteinemia and early outcome of acute ischemic stroke in elderly patients. Brain Behav. 2016, 6, e00460. [Google Scholar] [CrossRef]
- Shu, X.J.; Li, Z.F.; Chang, Y.W.; Liu, S.Y.; Wang, W.H. Effects of folic acid combined with vitamin B12 on DVT in patients with homocysteine cerebral infarction. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 2538–2544. [Google Scholar] [PubMed]
- Wang, B.; Wu, H.; Li, Y.; Ban, Q.; Huang, X.; Chen, L.; Li, J.; Zhang, Y.; Cui, Y.; He, M.; et al. Effect of long-term low-dose folic acid supplementation on degree of total homocysteine-lowering: Major effect modifiers. Br. J. Nutr. 2018, 120, 1122–1130. [Google Scholar] [CrossRef]
- Hankey, G.J.; Eikelboom, J.W.; Yi, Q.; Lees, K.R.; Chen, C.; Xavier, D.; Navarro, J.C.; Uddin, W.; Ricci, S.; Gommans, J.; et al. Antiplatelet therapy and the effects of B vitamins in patients with previous stroke or transient ischaemic attack: A post-hoc subanalysis of VITATOPS, a randomised, placebo-controlled trial. Lancet Neurol. 2012, 11, 512–520. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lauretta, M.P.; Melotti, R.M.; Sangermano, C.; George, A.M.; Badenes, R.; Bilotta, F. Homocysteine Plasmatic Concentration in Brain-Injured Neurocritical Care Patients: Systematic Review of Clinical Evidence. J. Clin. Med. 2022, 11, 394. https://doi.org/10.3390/jcm11020394
Lauretta MP, Melotti RM, Sangermano C, George AM, Badenes R, Bilotta F. Homocysteine Plasmatic Concentration in Brain-Injured Neurocritical Care Patients: Systematic Review of Clinical Evidence. Journal of Clinical Medicine. 2022; 11(2):394. https://doi.org/10.3390/jcm11020394
Chicago/Turabian StyleLauretta, Maria Paola, Rita Maria Melotti, Corinne Sangermano, Anneliya Maria George, Rafael Badenes, and Federico Bilotta. 2022. "Homocysteine Plasmatic Concentration in Brain-Injured Neurocritical Care Patients: Systematic Review of Clinical Evidence" Journal of Clinical Medicine 11, no. 2: 394. https://doi.org/10.3390/jcm11020394
APA StyleLauretta, M. P., Melotti, R. M., Sangermano, C., George, A. M., Badenes, R., & Bilotta, F. (2022). Homocysteine Plasmatic Concentration in Brain-Injured Neurocritical Care Patients: Systematic Review of Clinical Evidence. Journal of Clinical Medicine, 11(2), 394. https://doi.org/10.3390/jcm11020394






