Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome
Abstract
1. Introduction
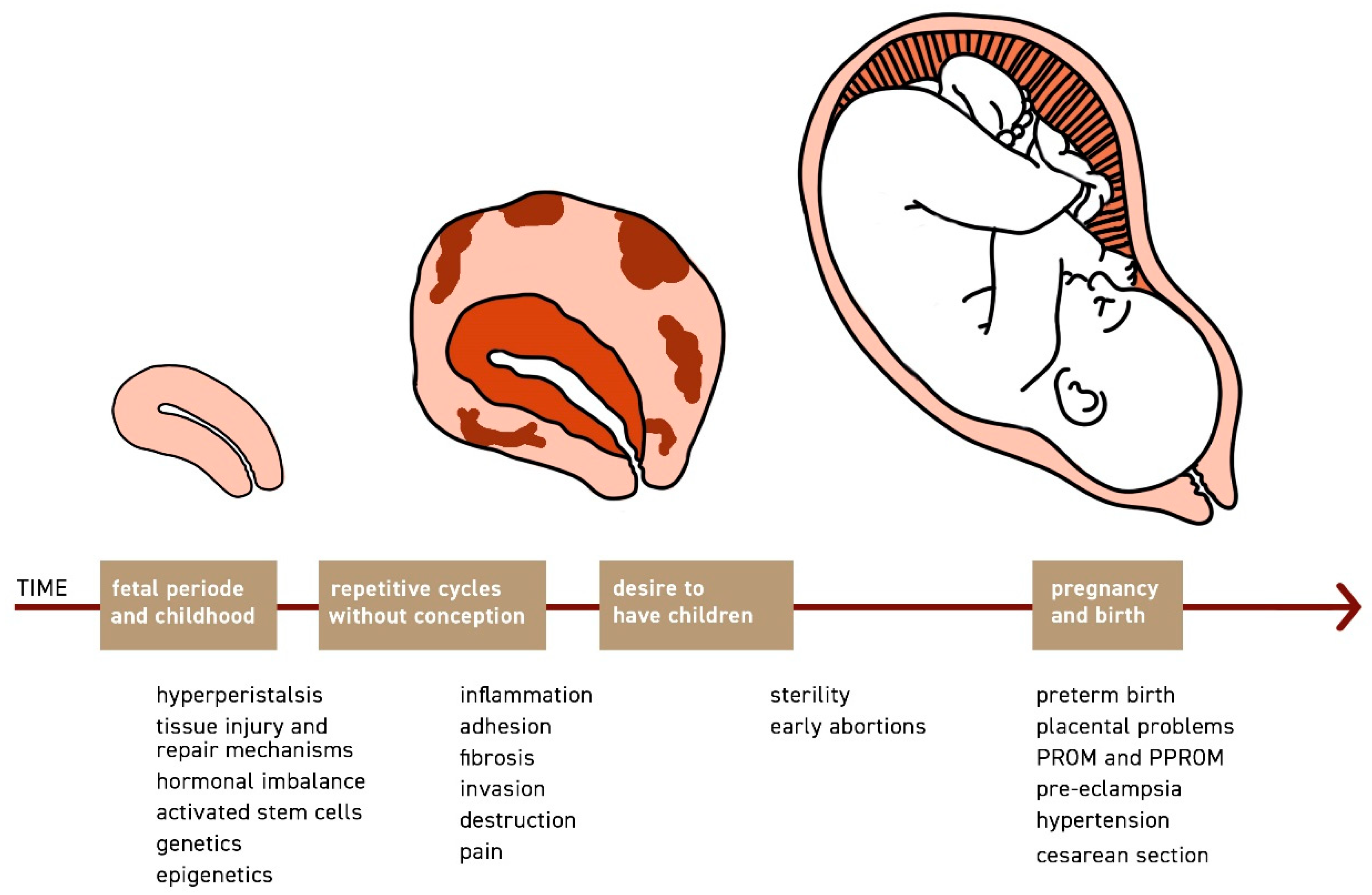
1.1. The Phenomenon Archimetrosis and Its Pathomechanisms
1.2. The Uterus as the Centre of Disease Development
1.3. Archimetrosis and Its Implications on Fertility
1.4. Archimetrosis and Pregnancy
2. Materials and Methods
3. Results
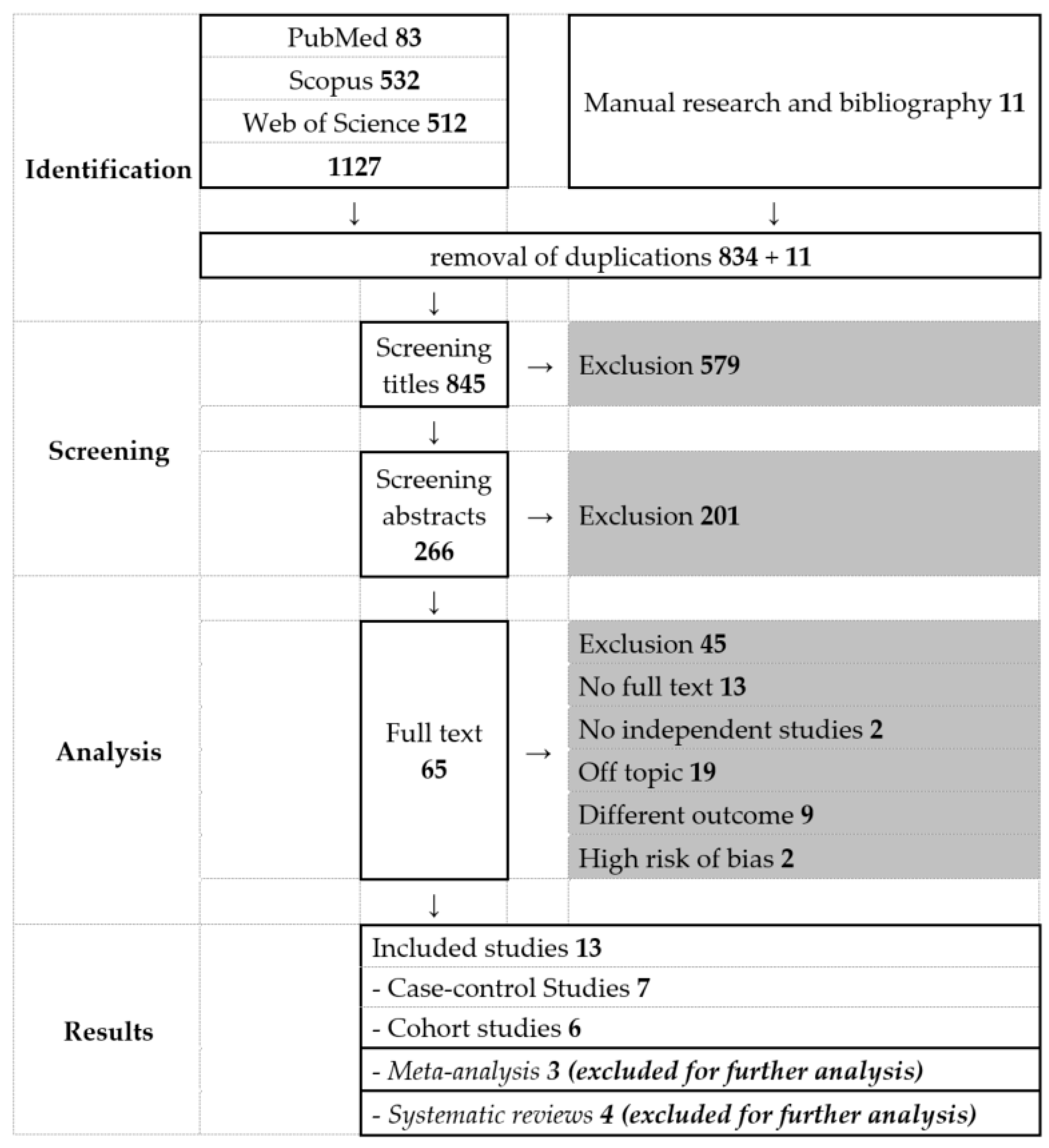
3.1. Literature Search
3.2. Preterm Birth
3.3. Mode of Birth
3.4. Other Complications
4. Discussion
4.1. Archimetrosis and Its Implications for the Mode of Birth
4.2. Underlying Causes of Preterm Birth
4.3. Placental Abnormalities and Its Association with Pregnancy Complications
5. Limitations
5.1. Retrospective Study Design
5.2. Misclassification
5.3. Selection Bias
5.4. Confounding
5.5. Heterogenicity
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Haas, D.; Chvatal, R.; Reichert, B.; Renner, S.; Shebl, O.; Binder, H.; Wurm, P.; Oppelt, P. Endometriosis: A premenopausal disease? Age pattern in 42,079 patients with endometriosis. Arch. Gynecol. Obstet. 2012, 286, 667–670. [Google Scholar] [CrossRef] [PubMed]
- Viganò, P.; Parazzini, F.; Somigliana, E.; Vercellini, P. Endometriosis: Epidemiology and aetiological factors. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 177–200. [Google Scholar] [CrossRef]
- Sampson, J.A. Metastatic or Embolic Endometriosis, due to the Menstrual Dissemination of Endometrial Tissue into the Venous Circulation. Am. J. Pathol. 1927, 3, 93–110. [Google Scholar] [PubMed]
- Koninckx, P.R.; Fernandes, R.; Ussia, A.; Schindler, L.; Wattiez, A.; Al-Suwaidi, S.; Amro, B.; Al-Maamari, B.; Hakim, Z.; Tahlak, M. Pathogenesis Based Diagnosis and Treatment of Endometriosis. Front. Endocrinol. 2021, 12, 745548. [Google Scholar] [CrossRef] [PubMed]
- Chapron, C.; Tosti, C.; Marcellin, L.; Bourdon, M.; Lafay-Pillet, M.C.; Millischer, A.E.; Streuli, I.; Borghese, B.; Petraglia, F.; Santulli, P. Relationship between the magnetic resonance imaging appearance of adenomyosis and endometriosis phenotypes. Hum. Reprod. 2017, 32, 1393–1401. [Google Scholar] [CrossRef]
- Donnez, J.; Dolmans, M.M.; Fellah, L. What if deep endometriotic nodules and uterine adenomyosis were actually two forms of the same disease? Fertil. Steril. 2019, 111, 454–456. [Google Scholar] [CrossRef]
- Leyendecker, G.; Wildt, L.; Mall, G. The pathophysiology of endometriosis and adenomyosis: Tissue injury and repair. Arch. Gynecol. Obstet. 2009, 280, 529–538. [Google Scholar] [CrossRef]
- Kunz, G.; Beil, D.; Huppert, P.; Noe, M.; Kissler, S.; Leyendecker, G. Adenomyosis in endometriosis—Prevalence and impact on fertility. Evidence from magnetic resonance imaging. Hum. Reprod. 2005, 20, 2309–2316. [Google Scholar] [CrossRef]
- Leyendecker, G.; Herbertz, M.; Kunz, G.; Mall, G. Endometriosis results from the dislocation of basal endometrium. Hum. Reprod. 2002, 17, 2725–2736. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Wattiez, A.; Gomel, V.; Martin, D.C. Pathogenesis of endometriosis: The genetic/epigenetic theory. Fertil. Steril. 2019, 111, 327–340. [Google Scholar] [CrossRef]
- Leyendecker, G.; Kunz, G.; Noe, M.; Herbertz, M.; Mall, G. Endometriosis: A dysfunction and disease of the archimetra. Hum. Reprod. Update 1998, 4, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Leyendecker, G.; Bilgicyildirim, A.; Inacker, M.; Stalf, T.; Huppert, P.; Mall, G.; Böttcher, B.; Wildt, L. Adenomyosis and endometriosis. Re-visiting their association and further insights into the mechanisms of auto-traumatisation. An MRI study. Arch. Gynecol. Obstet. 2015, 291, 917–932. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.G.; Sillem, M.; Plendl, J.; Chiantera, V.; Sehouli, J.; Mechsner, S. Myofibroblasts Are Evidence of Chronic Tissue Microtrauma at the Endometrial-Myometrial Junctional Zone in Uteri With Adenomyosis. Reprod. Sci. 2017, 24, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Cousins, F.L.; Dorien, F.O.; Gargett, C.E. Endometrial stem/progenitor cells and their role in the pathogenesis of endometriosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 50, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.M.; Mechsner, S. Pathogenesis of Endometriosis: The Origin of Pain and Subfertility. Cells 2021, 10, 1381. [Google Scholar] [CrossRef] [PubMed]
- Mechsner, S.; Grum, B.; Gericke, C.; Loddenkemper, C.; Dudenhausen, J.W.; Ebert, A.D. Possible roles of oxytocin receptor and vasopressin-1α receptor in the pathomechanism of dysperistalsis and dysmenorrhea in patients with adenomyosis uteri. Fertil. Steril. 2010, 94, 2541–2546. [Google Scholar] [CrossRef]
- Scheerer, C.; Bauer, P.; Chiantera, V.; Sehouli, J.; Kaufmann, A.; Mechsner, S. Characterization of endometriosis-associated immune cell infiltrates (EMaICI). Arch. Gynecol. Obstet. 2016, 294, 657–664. [Google Scholar] [CrossRef]
- Nanda, A.; Kalyani, T.; Banerjee, P.; Dutta, M.; Wangdi, T.; Sharma, P.; Chaudhury, K.; Jana, S.K. Cytokines, Angiogenesis, and Extracellular Matrix Degradation are Augmented by Oxidative Stress in Endometriosis. Ann. Lab. Med. 2020, 40, 390–397. [Google Scholar] [CrossRef]
- Attar, E.; Tokunaga, H.; Imir, G.; Yilmaz, M.B.; Redwine, D.; Putman, M.; Gurates, B.; Attar, R.; Yaegashi, N.; Hales, D.B.; et al. Prostaglandin E2 via steroidogenic factor-1 coordinately regulates transcription of steroidogenic genes necessary for estrogen synthesis in endometriosis. J. Clin. Endocrinol. Metab. 2009, 94, 623–631. [Google Scholar] [CrossRef]
- Kissler, S.; Hamscho, N.; Zangos, S.; Wiegratz, I.; Schlichter, S.; Menzel, C.; Doebert, N.; Gruenwald, F.; Vogl, T.J.; Gaetje, R.; et al. Uterotubal transport disorder in adenomyosis and endometriosis—A cause for infertility. BJOG 2006, 113, 902–908. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Kalaitzopoulos, D.R.; Schwartz, A.S.K.; Spaanderman, M.; Kramer, B.W.; Mueller, M.D.; Mueller, M. Fertility, pregnancy and neonatal outcomes of patients with adenomyosis: A systematic review and meta-analysis. Reprod. BioMed. Online 2021, 42, 185–206. [Google Scholar] [CrossRef]
- Harlow, C.R.; Cahill, D.J.; Maile, L.A.; Talbot, W.M.; Mears, J.; Wardle, P.G.; Hull, M.G. Reduced preovulatory granulosa cell steroidogenesis in women with endometriosis. J. Clin. Endocrinol. Metab. 1996, 81, 426–429. [Google Scholar] [CrossRef][Green Version]
- de Ziegler, D.; Borghese, B.; Chapron, C. Endometriosis and infertility: Pathophysiology and management. Lancet 2010, 376, 730–738. [Google Scholar] [CrossRef]
- Campo, S.; Campo, V.; Benagiano, G. Adenomyosis and infertility. Reprod. BioMed. Online 2012, 24, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Ota, H.; Igarashi, S.; Hatazawa, J.; Tanaka, T. Is adenomyosis an immune disease? Hum. Reprod. Update 1998, 4, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Youm, H.S.; Choi, Y.S.; Han, H.D. In vitro fertilization and embryo transfer outcomes in relation to myometrial thickness. J. Assist. Reprod. Genet. 2011, 28, 1135–1140. [Google Scholar] [CrossRef] [PubMed]
- Maubon, A.; Faury, A.; Kapella, M.; Pouquet, M.; Piver, P. Uterine junctional zone at magnetic resonance imaging: A predictor of in vitro fertilization implantation failure. J. Obstet. Gynaecol. Res. 2010, 36, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Saraswat, L.; Ayansina, D.T.; Cooper, K.G.; Bhattacharya, S.; Miligkos, D.; Horne, A.W.; Bhattacharya, S. Pregnancy outcomes in women with endometriosis: A national record linkage study. BJOG 2017, 124, 444–452. [Google Scholar] [CrossRef]
- Zullo, F.; Spagnolo, E.; Saccone, G.; Acunzo, M.; Xodo, S.; Ceccaroni, M.; Berghella, V. Endometriosis and obstetrics complications: A systematic review and meta-analysis. Fertil. Steril. 2017, 108, 667–672. [Google Scholar] [CrossRef]
- Vercellini, P.; Parazzini, F.; Pietropaolo, G.; Cipriani, S.; Frattaruolo, M.P.; Fedele, L. Pregnancy outcome in women with peritoneal, ovarian and rectovaginal endometriosis: A retrospective cohort study. BJOG 2012, 119, 1538–1543. [Google Scholar] [CrossRef]
- Juang, C.M.; Chou, P.; Yen, M.S.; Twu, N.F.; Horng, H.C.; Hsu, W.L. Adenomyosis and risk of preterm delivery. BJOG 2007, 114, 165–169. [Google Scholar] [CrossRef]
- Petraglia, F.; Arcuri, F.; de Ziegler, D.; Chapron, C. Inflammation: A link between endometriosis and preterm birth. Fertil. Steril. 2012, 98, 36–40. [Google Scholar] [CrossRef]
- Vigano, P.; Corti, L.; Berlanda, N. Beyond infertility: Obstetrical and postpartum complications associated with endometriosis and adenomyosis. Fertil. Steril. 2015, 104, 802–812. [Google Scholar] [CrossRef]
- Brosens, I.; Pijnenborg, R.; Benagiano, G. Defective myometrial spiral artery remodelling as a cause of major obstetrical syndromes in endometriosis and adenomyosis. Placenta 2013, 34, 100–105. [Google Scholar] [CrossRef]
- Nirgianakis, K.; Gasparri, M.L.; Radan, A.P.; Villiger, A.; McKinnon, B.; Mosimann, B.; Papadia, A.; Mueller, M.D. Obstetric complications after laparoscopic excision of posterior deep infiltrating endometriosis: A case-control study. Fertil. Steril. 2018, 110, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.; Sterrenburg, M.; Lane, S.; Maheshwari, A.; Li, T.C.; Cheong, Y. Reproductive, obstetric, and perinatal outcomes of women with adenomyosis and endometriosis: A systematic review and meta-analysis. Hum. Reprod. Update 2019, 25, 592–632. [Google Scholar] [CrossRef] [PubMed]
- Leone Roberti Maggiore, U.; Inversetti, A.; Schimberni, M.; Viganò, P.; Giorgione, V.; Candiani, M. Obstetrical complications of endometriosis, particularly deep endometriosis. Fertil. Steril. 2017, 108, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Soave, I.; Wenger, J.M.; Pluchino, N.; Marci, R. Treatment options and reproductive outcome for adenomyosis-associated infertility. Curr. Med. Res. Opin. 2018, 34, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Johnson, N.P.; Hummelshoj, L.; Adamson, G.D.; Keckstein, J.; Taylor, H.S.; Abrao, M.S.; Bush, D.; Kiesel, L.; Tamimi, R.; Sharpe-Timms, K.L.; et al. World Endometriosis Society consensus on the classification of endometriosis. Hum. Reprod. 2017, 32, 315–324. [Google Scholar] [CrossRef]
- Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010, 25, 603–605. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, A.; Iriyama, T.; Sayama, S.; Nakayama, T.; Komatsu, A.; Miyauchi, A.; Nishii, O.; Nagamatsu, T.; Osuga, Y.; Fujii, T. Adenomyosis and adverse perinatal outcomes: Increased risk of second trimester miscarriage, preeclampsia, and placental malposition. J. Matern. Fetal. Neonatal. Med. 2018, 31, 364–369. [Google Scholar] [CrossRef]
- Yamaguchi, A.; Kyozuka, H.; Fujimori, K.; Hosoya, M.; Yasumura, S.; Yokoyama, T.; Sato, A.; Hashimoto, K. Risk of preterm birth, low birthweight and small-for-gestational-age infants in pregnancies with adenomyosis: A cohort study of the Japan Environment and Children’s Study. Acta. Obstet. Gynecol. Scand. 2019, 98, 359–364. [Google Scholar] [CrossRef]
- Shin, Y.J.; Kwak, D.W.; Chung, J.H.; Kim, M.Y.; Lee, S.W.; Han, Y.J. The Risk of Preterm Births among Pregnant Women with Adenomyosis. J. Ultrasound Med. 2018, 37, 1937–1943. [Google Scholar] [CrossRef]
- Mannini, L.; Sorbi, F.; Noci, I.; Ghizzoni, V.; Perelli, F.; Di Tommaso, M.; Mattei, A.; Fambrini, M. New adverse obstetrics outcomes associated with endometriosis: A retrospective cohort study. Arch. Gynecol. Obstet. 2017, 295, 141–151. [Google Scholar] [CrossRef]
- Exacoustos, C.; Lauriola, I.; Lazzeri, L.; De Felice, G.; Zupi, E. Complications during pregnancy and delivery in women with untreated rectovaginal deep infiltrating endometriosis. Fertil. Steril. 2016, 106, 1129–1135. [Google Scholar] [CrossRef]
- Mochimaru, A.; Aoki, S.; Oba, M.S.; Kurasawa, K.; Takahashi, T.; Hirahara, F. Adverse pregnancy outcomes associated with adenomyosis with uterine enlargement. J. Obstet. Gynaecol. Res. 2015, 41, 529–533. [Google Scholar] [CrossRef]
- Sharma, S.; Bathwal, S.; Agarwal, N.; Chattopadhyay, R.; Saha, I.; Chakravarty, B. Does presence of adenomyosis affect reproductive outcome in IVF cycles? A retrospective analysis of 973 patients. Reprod. BioMed. Online 2019, 38, 13–21. [Google Scholar] [CrossRef]
- Uccella, S.; Manzoni, P.; Cromi, A.; Marconi, N.; Gisone, B.; Miraglia, A.; Biasoli, S.; Zorzato, P.C.; Ferrari, S.; Lanzo, G.; et al. Pregnancy after Endometriosis: Maternal and Neonatal Outcomes according to the Location of the Disease. Am. J. Perinatol. 2019, 36, S91–S98. [Google Scholar] [CrossRef]
- Harada, T.; Taniguchi, F.; Amano, H.; Kurozawa, Y.; Ideno, Y.; Hayashi, K.; Harada, T. Adverse obstetrical outcomes for women with endometriosis and adenomyosis: A large cohort of the Japan Environment and Children’s Study. PLoS ONE 2019, 14, e0220256. [Google Scholar] [CrossRef] [PubMed]
- Porpora, M.G.; Tomao, F.; Ticino, A.; Piacenti, I.; Scaramuzzino, S.; Simonetti, S.; Imperiale, L.; Sangiuliano, C.; Masciullo, L.; Manganaro, L.; et al. Endometriosis and Pregnancy: A Single Institution Experience. Int. J. Environ. Res. Public Health 2020, 17, 401. [Google Scholar] [CrossRef] [PubMed]
- Cantone, D.; Pelullo, C.P.; Cancellieri, M.; Attena, F. Can antenatal classes reduce the rate of cesarean section in southern Italy? Women Birth 2017, 30, e83–e88. [Google Scholar] [CrossRef] [PubMed]
- Thomin, A.; Belghiti, J.; David, C.; Marty, O.; Bornes, M.; Ballester, M.; Roman, H.; Daraï, E. Maternal and neonatal outcomes in women with colorectal endometriosis. BJOG 2018, 125, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Dezee, K.J.; Ahnfeldt, E.P.; Wagner, M. Abdominal wall endometriosis: A surgeon’s perspective and review of 445 cases. Am. J. Surg. 2008, 196, 207–212. [Google Scholar] [CrossRef]
- López Bernal, A.; Watson, S.P.; Phaneuf, S.; Europe-Finner, G.N. Biochemistry and physiology of preterm labour and delivery. Baillieres Clin. Obstet. Gynaecol. 1993, 7, 523–552. [Google Scholar] [CrossRef]
- Razavi, M.; Maleki-Hajiagha, A.; Sepidarkish, M.; Rouholamin, S.; Almasi-Hashiani, A.; Rezaeinejad, M. Systematic review and meta-analysis of adverse pregnancy outcomes after uterine adenomyosis. Int. J. Gynaecol. Obstet. 2019, 145, 149–157. [Google Scholar] [CrossRef]
- Scala, C.; Leone Roberti Maggiore, U.; Barra, F.; Tantari, M.; Ferrero, S. Impact of Endometriomas and Deep Infiltrating Endometriosis on Pregnancy Outcomes and on First and Second Trimester Markers of Impaired Placentation. Medicina 2019, 55, 550. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gruber, T.M.; Ortlieb, L.; Henrich, W.; Mechsner, S. Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome. J. Clin. Med. 2022, 11, 157. https://doi.org/10.3390/jcm11010157
Gruber TM, Ortlieb L, Henrich W, Mechsner S. Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome. Journal of Clinical Medicine. 2022; 11(1):157. https://doi.org/10.3390/jcm11010157
Chicago/Turabian StyleGruber, Teresa Mira, Laura Ortlieb, Wolfgang Henrich, and Sylvia Mechsner. 2022. "Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome" Journal of Clinical Medicine 11, no. 1: 157. https://doi.org/10.3390/jcm11010157
APA StyleGruber, T. M., Ortlieb, L., Henrich, W., & Mechsner, S. (2022). Deep Infiltrating Endometriosis and Adenomyosis: Implications on Pregnancy and Outcome. Journal of Clinical Medicine, 11(1), 157. https://doi.org/10.3390/jcm11010157





