Assessment of Asymptomatic Severe Aortic Regurgitation by Doppler-Derived Echo Indices: Comparison with Magnetic Resonance Quantification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Echocardiography
2.3. Cardiac Magnetic Resonance
2.4. Statistical Analysis
3. Results
3.1. Baseline Clinical and Imaging Characteristics
3.2. Comparison of Quantitative Echo and CMR Parameters
3.3. Comparison of Indirect Echo Indices to Quantitative CMR Parameters
3.4. Echo Parameters Predicting Severe AR by CMR Quantification
4. Discussion
4.1. Quantitative Echocardiographic Parameters
4.2. Indirect Echocardiographic Parameters
4.3. Clinical Implications
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rigolin, V.H.; Bonow, R.O. Hemodynamic Characteristics and Progression to Heart Failure in Regurgitant Lesions. Heart Fail. Clin. 2006, 2, 453–460. [Google Scholar] [CrossRef] [PubMed]
- Tornos, M.; Olona, M.; Permanyer-Miralda, G.; Herrejon, M.; Camprecios, M.; Evangelista, A.; del Castillo, H.G.; Candell, J.; Soler-Soler, J. Clinical outcome of severe asymptomatic chronic aortic regurgitation: A long-term prospective follow-up study. Am. Heart J. 1995, 130, 333–339. [Google Scholar] [CrossRef]
- Chaliki, H.P.; Mohty, D.; Avierinos, J.F.; Scott, C.G.; Schaff, H.V.; Tajik, A.J.; Enriquez-Sarano, M. Outcomes after aortic valve replacement in patients with severe aortic regurgitation and markedly reduced left ventricular function. Circulation 2002, 106, 2687–2693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turina, J.; Milincic, J.; Seifert, B.; Turina, M. Valve replacement in chronic aortic regurgitation. True predictors of survival after extended follow-up. Circulation 1998, 98, 100–107. [Google Scholar]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Lancellotti, P.; Tribouilloy, C.H.; Hagendorff, A. European Association of Echocardiography recommendations for the assessment of valvular regurgitation. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [Green Version]
- Sato, H.; Ohta, T.; Hiroe, K.; Okada, S.; Shimizu, K.; Murakami, R.; Tanabe, K. Severity of aortic regurgitation assessed by area of vena contracta: A clinical two-dimensional and three-dimensional colour. Doppler imaging study. Cardiovasc. Ultrasound 2015, 59, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Ewe, S.H.; Delgado, V.; van der Geest, R.; Westenberg, J.J.; Haeck, M.L.; Witkowski, T.G.; Auger, D.; Marsan, N.A.; Holman, E.R.; de Roos, A.; et al. Accuracy of Three-Dimensional Versus Two-Dimensional Echocardiography for Quantification of Aortic Regurgitation and Validation by Three-Dimensional Three-Directional Velocity-Encoded Magnetic Resonance Imaging. Am. J. Cardiol. 2013, 112, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Grothues, F.; Smith, G.C.; Moon, J.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Lee, J.C.; Branch, K.R.; Hamilton-Craig, C.; Krieger, E.V. Evaluation of aortic regurgitation with cardiac magnetic resonance imaging: A systemic review. Heart 2017, 104, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Søndergaard, L.; Lindvig, K.; Hildebrandt, P.; Thomsen, C.; Ståhlberg, F.; Joen, T.; Henriksen, O. Quantification of aortic regurgitation by magnetic resonance velocity mapping. Am. Heart J. 1993, 125, 1081–1090. [Google Scholar] [CrossRef]
- Myerson, S.G.; d’Arcy, J.; Mohiaddin, R.; Greenwood, J.P.; Karamitsos, T.D.; Francis, J.M.; Banning, A.P.; Christiansen, J.P.; Neubauer, S. Aortic regurgitation quantification using cardiovascular magnetic resonance: Association with clinical outcome. Circulation 2012, 126, 1452–1460. [Google Scholar] [CrossRef] [Green Version]
- Gabriel, R.S.; Renapurkar, R.; Bolen, M.A.; Verhaert, D.; Leiber, M.; Flamm, S.D.; Griffin, B.P.; Desai, M.Y. Comparison of Severity of Aortic Regurgitation by Cardiovascular Magnetic Resonance Versus Transthoracic Echocardiography. Am. J. Cardiol. 2011, 108, 1014–1020. [Google Scholar] [CrossRef]
- Kutty, S.; Whitehead, K.K.; Natarajan, S.; Harris, M.A.; Wernovsky, G.; Fogel, M.A. Qualitative Echocardiographic Assessment of Aortic Valve Regurgitation with Quantitative Cardiac Magnetic Resonance: A Comparative Study. Pediatr. Cardiol. 2009, 30, 971–977. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2015, 28, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for noninvasive evaluation of native valvular regurgitation A report from the American society of echocardiography developed in collaboration with the society for cardiovascular magnetic resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Hsiung, M.C.; Miller, A.P.; Nanda, N.C.; Yin, W.H.; Young, M.S.; Velayudhan, D.E. Assessment of aortic regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area: Usefulness and validation. Echocardiography 2005, 22, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Kočková, R.; Línková, H.; Hlubocká, Z.; Pravečková, A.; Polednová, A.; Súkupová, L.; Bláha, M.; Malý, J.; Honsová, E.; Sedmera, D.; et al. New Imaging Markers of Clinical Outcome in Asymptomatic Patients with Severe Aortic Regurgitation. J. Clin. Med. 2019, 8, 1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzimavroudis, G.P.; Walker, P.G.; Oshinski, J.N.; Franch, R.H.; Pettigrew, R.I.; Yoganathan, A.P. Slice location dependence of aortic regurgitation measurements with MR phase contrast velocity mapping. Magn. Reason. Med. 1997, 37, 545–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Detaint, D.; Messika-Zeitoun, D.; Maalouf, J.; Tribouilloy, C.; Mahoney, D.W.; Tajik, A.J.; Enriquez-Sarano, M. Quantitative Echocardiographic Determinants of Clinical Outcome in Asymptomatic Patients With Aortic Regurgitation: A Prospective Study. JACC Cardiovasc. Imaging 2008, 1, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cawley, P.J.; Hamilton-Craig, C.; Owens, D.S.; Krieger, E.V.; Strugnell, W.E.; Mitsumori, L.; D’Jang, C.L.; Schwaegler, R.G.; Nguyen, K.Q.; Nguyen, B.; et al. Prospective comparison of valve regurgitation quantitation by cardiac magnetic resonance imaging and transthoracic echocardiography. Circ. Cardiovasc. Imaging 2013, 6, 48–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelfand, E.V.; Hughes, S.; Hauser, T.H.; Yeon, S.B.; Goepfert, L.; Kissinger, K.V.; Rofsky, N.M.; Manning, W.J. Severity of Mitral and Aortic Regurgitation as Assessed by Cardiovascular Magnetic Resonance: Optimizing Correlation with Doppler Echocardiography. J. Cardiovasc. Magn. Reson. 2006, 8, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.F.; Kuo, L.C.; Nelson, J.G.; Limacher, M.C.; Quinones, M.A. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: Clinical validation of two new methods using the apical window. Circulation 1984, 70, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, A.; Hamilton-Craig, C.; Cawley, P.J.; Mitsumori, L.M.; Otto, C.M.; Maki, J.H. Quantitating aortic regurgitation by cardiovascular magnetic resonance: Significant variations due to slice location and breath holding. Eur. Radiol. 2016, 26, 3180–3189. [Google Scholar] [CrossRef] [PubMed]
- Nayak, K.S.; Nielsen, J.-F.; Bernstein, M.A.; Markl, M.; Gatehouse, P.D.; Botnar, R.M.; Saloner, D.; Lorenz, C.H.; Wen, H.; Hu, B.S.; et al. Cardiovascular magnetic resonance phase contrast imaging. J. Cardiovasc. Magn. Reson. 2015, 17, 71. [Google Scholar] [CrossRef] [Green Version]
- Messika-Zeitoun, D.; Detaint, D.; Leye, M.; Tribouilloy, C.; Michelena, H.I.; Pislaru, S.; Brochet, E.; Iung, B.; Vahanian, A.; Enriquez-Sarano, M. Comparison of Semiquantitative and Quantitative Assessment of Severity of Aortic Regurgitation: Clinical Implications. J. Am. Soc. Echocardiogr. 2011, 24, 1246–1252. [Google Scholar] [CrossRef]
- Bolen, M.A.; Popovic, Z.B.; Rajiah, P. Cardiac MR assessment of aortic regurgitation: Holodiastolic flow reversal in the de-scending aorta helps stratify severity. Radiology 2011, 260, 98–104. [Google Scholar] [CrossRef]
- Evangelista, A.; Tornos, P.; Sambola, A.; Permanyer-Miralda, G.; Soler-Soler, J. Long-Term Vasodilator Therapy in Patients with Severe Aortic Regurgitation. N. Engl. J. Med. 2005, 353, 1342–1349. [Google Scholar] [CrossRef] [PubMed]
- Kammerlander, A.A.; Wiesinger, M.; Duca, F.; Aschauer, S.; Binder, C.; Tufaro, C.Z.; Nitsche, C.; Badre-Eslam, R.; Schönbauer, R.; Bartko, P.; et al. Diagnostic and Prognostic Utility of Cardiac Magnetic Resonance Imaging in Aortic Regurgitation. JACC Cardiovasc. Imaging 2019, 12, 1474–1483. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.; Levine, R.A.; Hua, L.; Morris, E.L.; Kang, Y.; Flaherty, M.; Morgan, N.V.; Hung, J. Diagnostic value of vena contracta area in the quantification of mitral regurgitation severity by colour Doppler 3D echocardiography. Circ. Cardiovasc. Imaging 2011, 4, 506–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abudiab, M.M.; Chao, C.J.; Liu, S.; Naqvi, T.Z. Quantitation of valve regurgitation severity by three-dimensional vena contracta area is superior to flow convergence method of quantification on transoesophageal echocardiography. Echocardiography 2017, 34, 992–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
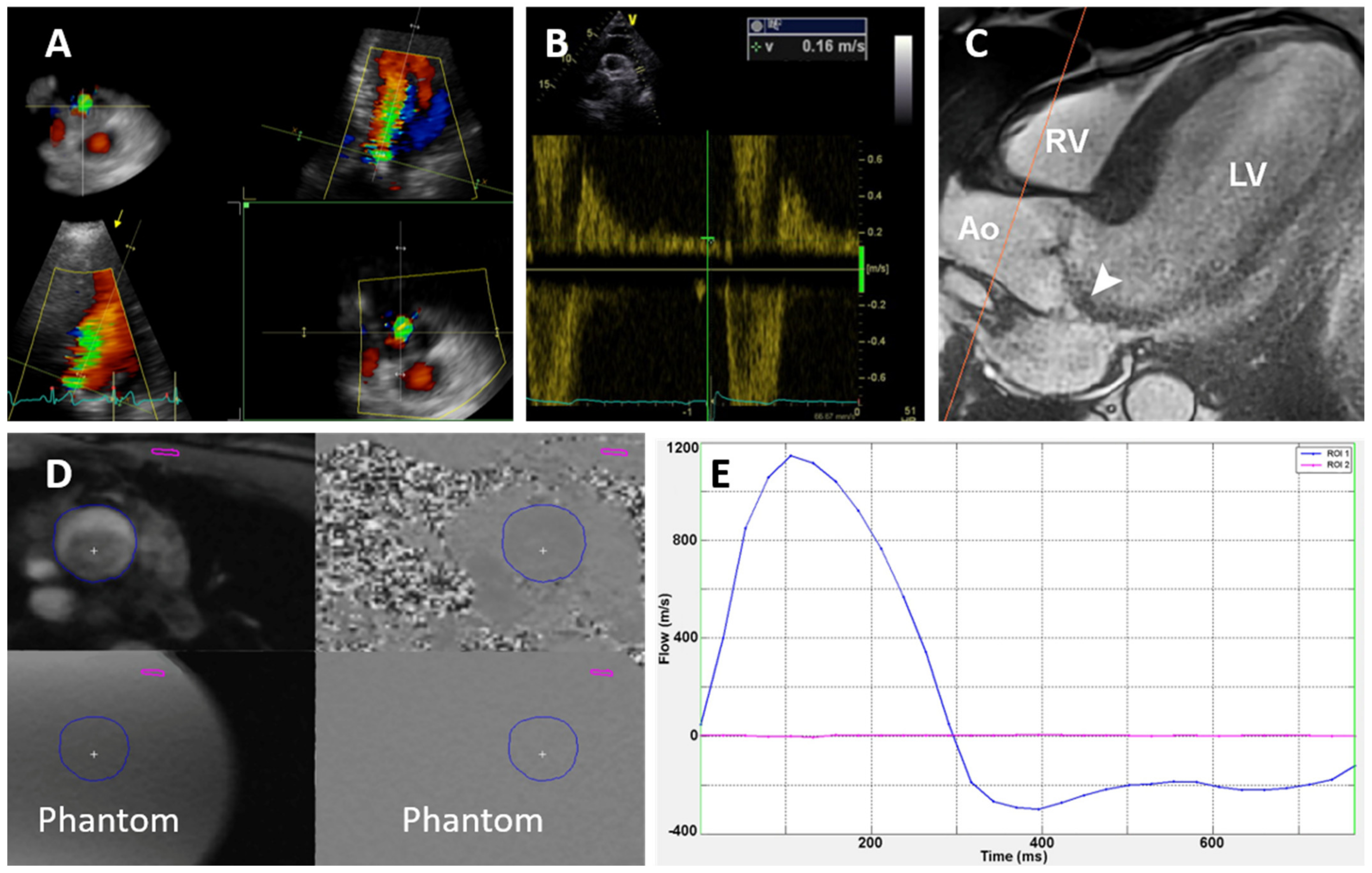
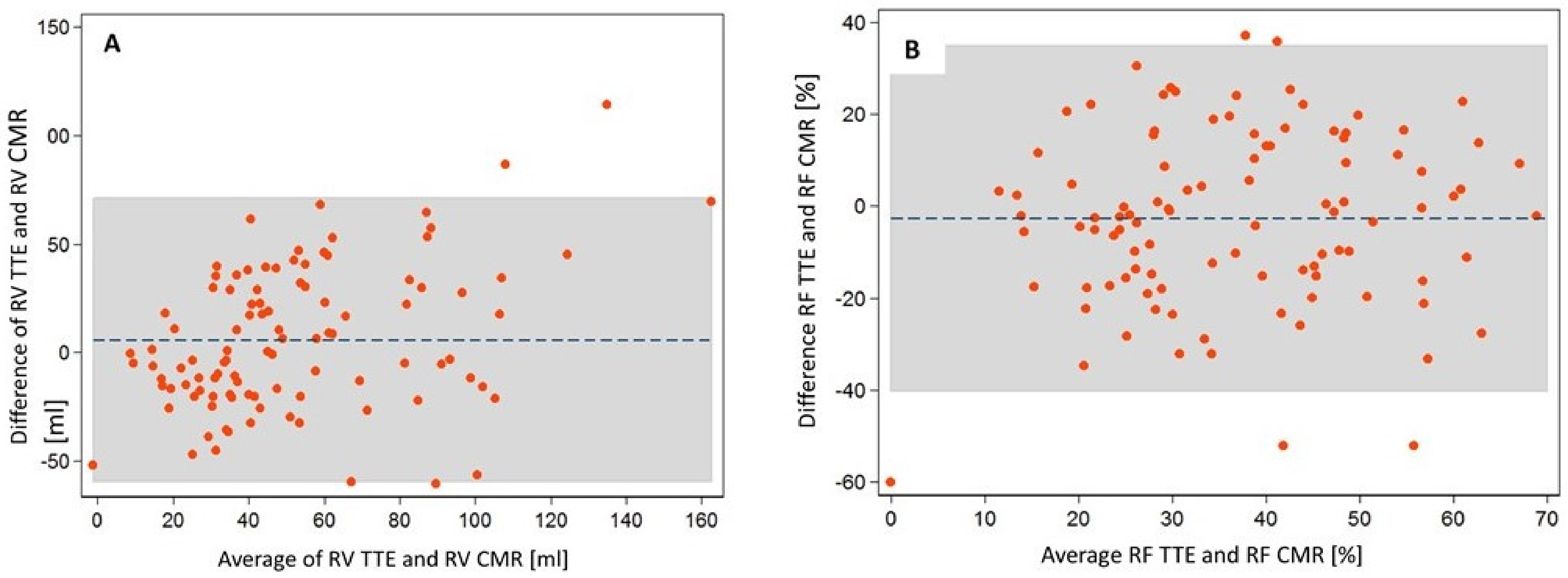
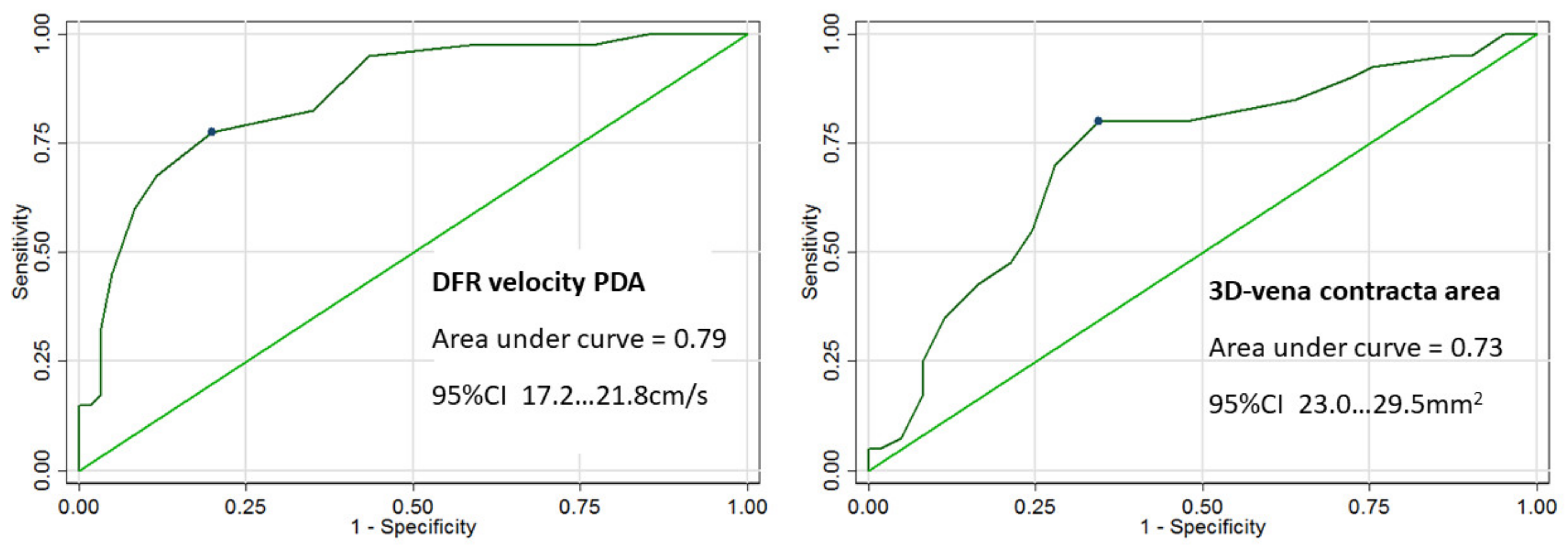
| Variable | Value (%) |
|---|---|
| Age, years | 44 ± 13 |
| Male gender, N (%) | 89 (86) |
| Hypertension, N (%) | 50 (48) |
| Diabetes mellitus, N (%) | 6 (6) |
| Hyperlipidemia, N (%) | 29 (28) |
| Smoker, N (%) | 14 (13) |
| Coronary artery disease, N (%) | 4 (4) |
| Previous cardiac surgery, N (%) | 4 (4) |
| Stroke, N (%) | 1 (1) |
| NYHA Class I, N (%) | 104 (100) |
| Height, cm | 180 ± 9 |
| Weight, kg | 85 ± 14 |
| Systolic blood pressure, mmHg | 136 ± 16 |
| Diastolic blood pressure, mmHg | 70 ± 12 |
| Heart rate, beats per min | 64 ± 10 |
| B natriuretic peptide, ng/L | 27 (42) |
| Hemoglobin, g/L | 153 ± 13 |
| Variable | Value |
|---|---|
| Aortic valve morphology | |
| Trileaflet, N (%) | 14 (13.6) |
| Bicuspid, N (%) | 79 (76.7) |
| Unicuspid/quadricuspid, N (%) | 4 (4) |
| Unknown, N (%) | 6 (6) |
| Aortic regurgitation assessment | |
| Integrative approach | |
| Moderate-to-severe AR, N (%) | 56 (54) |
| Severe AR, N (%) | 48 (46) |
| 2D echo vena contracta width, mm | 6.5 ± 1.5 |
| Diastolic flow reversal velocity, cm/s | 19.4 ± 4.3 |
| 2D echo regurgitant volume, mL | 52 ± 48 |
| 2D echo regurgitant fraction, % | 36 ± 18 |
| 3D echo vena contracta area, mm2 | 29 ± 13 |
| CMR regurgitation volume, mL | 50 ± 28 |
| CMR regurgitation fraction, % | 38 ± 17 |
| Left ventricle assessment | |
| 2D echo end-diastolic diameter, mm | 58 ± 6 |
| 2D echo end-systolic diameter, mm | 37 ± 5 |
| 2D echo end-systolic diameter index, mm/m2 | 18 ± 3 |
| 2D echo end-diastolic volume, mL | 158 ± 68.0 |
| 2D echo end-systolic volume, mL | 56 ± 32 |
| 2D echo ejection fraction, % | 64 ± 6 |
| 3D echo end-diastolic volume, mL | 177 ± 51 |
| 3D echo end-diastolic volume index, mL/m2 | 86 ± 23 |
| 3D echo end-systolic volume, mL | 69 ± 24 |
| 3D echo end-systolic volume index, mL/m2 | 33 ± 11 |
| 3D echo ejection fraction, % | 62 ± 5 |
| CMR end-diastolic volume, mL | 234 ± 81 |
| CMR end-diastolic volume index, mL/m2 | 118 ± 30 |
| CMR end-systolic volume, mL | 88 ± 51 |
| CMR end-systolic volume index, mL/m2 | 43 ± 23 |
| CMR ejection fraction, % | 61 ± 6 |
| Variable | rs | p Value |
|---|---|---|
| Regurgitant volume by CMR | ||
| vena contracta width | 0.18 | 0.07 |
| 3D VCA | 0.48 | <0.001 |
| DFR velocity in descending aorta | 0.62 | <0.001 |
| RV by TTE volumetric method | 0.50 | <0.001 |
| RF by TTE volumetric method | 0.44 | <0.001 |
| Regurgitant fraction by CMR | ||
| vena contracta width | 0.05 | 0.62 |
| 3D VCA | 0.38 | <0.001 |
| DFR velocity in descending aorta | 0.50 | <0.001 |
| RV by TTE volumetric method | 0.43 | <0.001 |
| RF by TTE volumetric method | 0.40 | <0.001 |
| LV EDV by CMR and by TTE | 0.78 | <0.001 |
| LV ESV by CMR and by TTE | 0.73 | <0.001 |
| LV ejection fraction by CMR and by TTE | 0.44 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hlubocká, Z.; Kočková, R.; Línková, H.; Pravečková, A.; Hlubocký, J.; Dostálová, G.; Bláha, M.; Pěnička, M.; Linhart, A. Assessment of Asymptomatic Severe Aortic Regurgitation by Doppler-Derived Echo Indices: Comparison with Magnetic Resonance Quantification. J. Clin. Med. 2022, 11, 152. https://doi.org/10.3390/jcm11010152
Hlubocká Z, Kočková R, Línková H, Pravečková A, Hlubocký J, Dostálová G, Bláha M, Pěnička M, Linhart A. Assessment of Asymptomatic Severe Aortic Regurgitation by Doppler-Derived Echo Indices: Comparison with Magnetic Resonance Quantification. Journal of Clinical Medicine. 2022; 11(1):152. https://doi.org/10.3390/jcm11010152
Chicago/Turabian StyleHlubocká, Zuzana, Radka Kočková, Hana Línková, Alena Pravečková, Jaroslav Hlubocký, Gabriela Dostálová, Martin Bláha, Martin Pěnička, and Aleš Linhart. 2022. "Assessment of Asymptomatic Severe Aortic Regurgitation by Doppler-Derived Echo Indices: Comparison with Magnetic Resonance Quantification" Journal of Clinical Medicine 11, no. 1: 152. https://doi.org/10.3390/jcm11010152
APA StyleHlubocká, Z., Kočková, R., Línková, H., Pravečková, A., Hlubocký, J., Dostálová, G., Bláha, M., Pěnička, M., & Linhart, A. (2022). Assessment of Asymptomatic Severe Aortic Regurgitation by Doppler-Derived Echo Indices: Comparison with Magnetic Resonance Quantification. Journal of Clinical Medicine, 11(1), 152. https://doi.org/10.3390/jcm11010152






