Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Schedule of the Study Visits
2.3. Diagnostic Polysomnography
2.4. CPAP Titration under PSG Surveillance
2.5. Compliance Assessment of Long-Term CPAP Treatment
2.6. Data Management and Statistical Analysis
3. Results
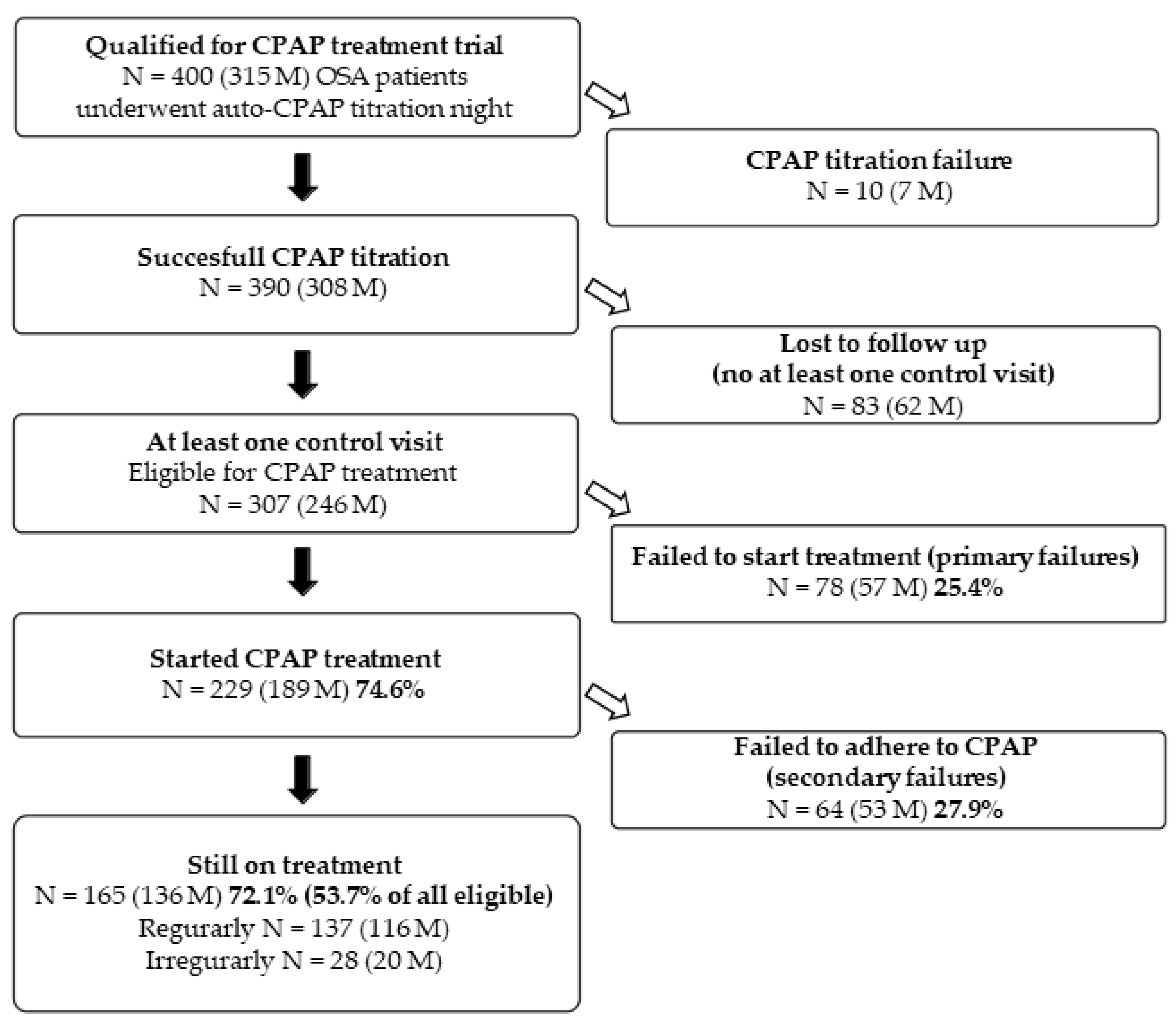
3.1. Study Population
3.2. Characteristic of Patients
3.3. Causes of CPAP Titration, Primary and Secondary Failures
3.4. Characteristic of Patients That Initiated CPAP, Primary Failures, Patients on Treatment and Secondary Failures
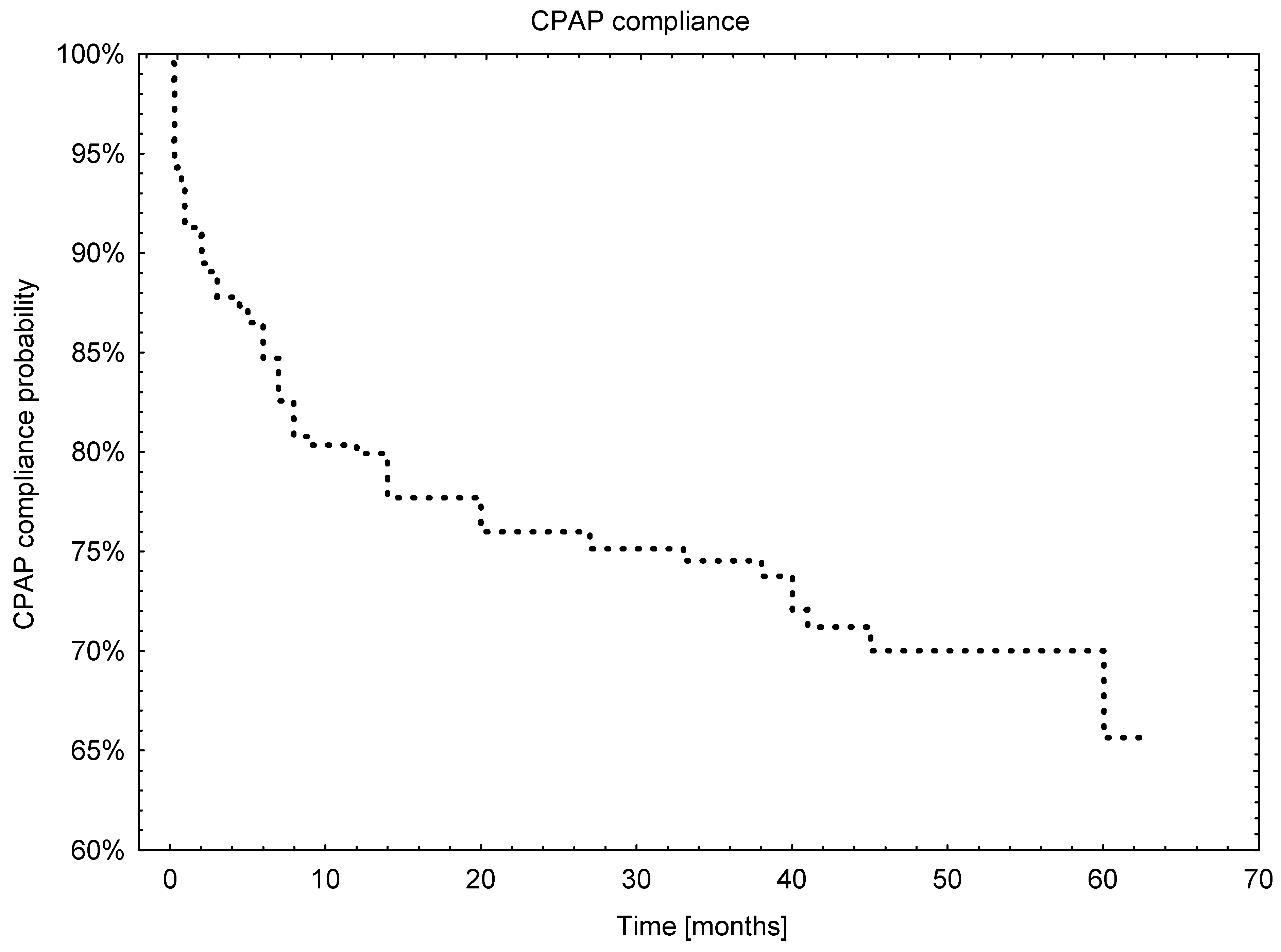
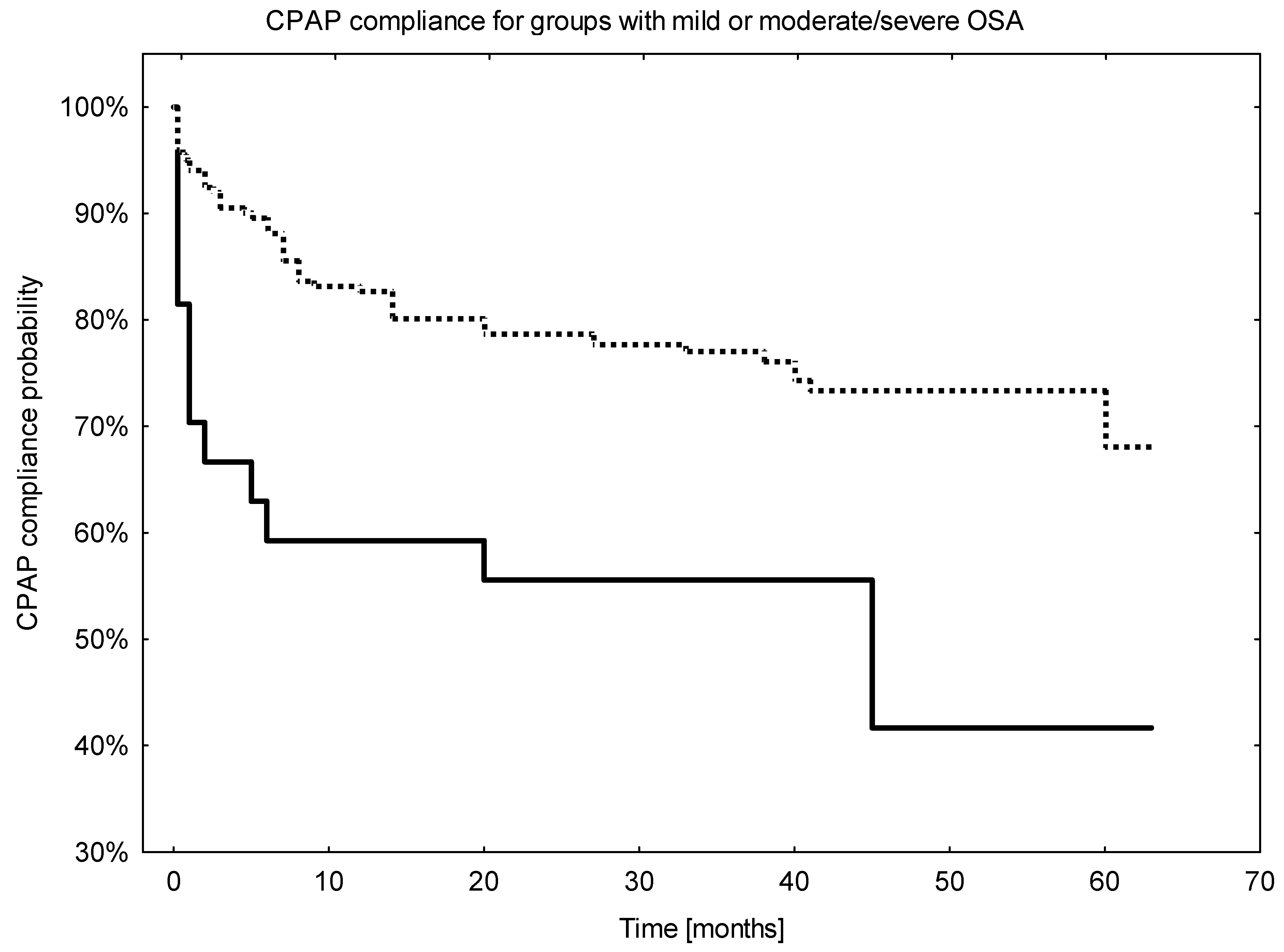
3.5. Analysis of Long-Term Compliance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Patil, S.P.; Ayappa, I.A.; Caples, S.M.; John Kimoff, R.; Patel, S.R.; Harrod, C.G. Treatment of Adult Obstructive Sleep Apnea with Positive Airway Pressure: An American Academy of Sleep Medicine Systematic Review, Meta-Analysis, and GRADE Assessment. J. Clin. Sleep Med. 2019, 15, 301–334. [Google Scholar] [CrossRef] [PubMed]
- Ardle, N.M.C.; Devereux, G.; Heidarnejad, H.; Engleman, H.M.; Mackay, T.W.; Douglas, N.J. Long-Term Use of CPAP Therapy for Sleep Apnea/Hypopnea Syndrome. Am. J. Respir. Crit. Care Med. 1999, 159, 1108–1114. [Google Scholar]
- Pépin, J.L.; Bailly, S.; Rinder, P.; Adler, D.; Szeftel, D.; Malhotra, A.; Cistulli, P.A.; Benjafield, A.; Lavergne, F.; Josseran, A.; et al. Cpap Therapy Termination Rates by Osa Phenotype: A French Nationwide Database Analysis. J. Clin. Med. 2021, 10, 936. [Google Scholar] [CrossRef] [PubMed]
- Cistulli, P.A.; Armitstead, J.; Pepin, J.L.; Woehrle, H.; Nunez, C.M.; Benjafield, A.; Malhotra, A. Short-Term CPAP Adherence in Obstructive Sleep Apnea: A Big Data Analysis Using Real World Data. Sleep Med. 2019, 59, 114–116. [Google Scholar] [CrossRef] [PubMed]
- Rotenberg, B.W.; Murariu, D.; Pang, K.P. Trends in CPAP Adherence over Twenty Years of Data Collection: A Flattened Curve. J. Otolaryngol. —Head Neck Surg. 2016, 45, 1–9. [Google Scholar] [CrossRef]
- Borker, P.V.; Carmona, E.; Essien, U.R.; Saeed, G.J.; Nouraie, S.M.; Bakker, J.P.; Stitt, C.J.; Aloia, M.S.; Patel, S.R. Neighborhoods with Greater Prevalence of Minority Residents Have Lower Continuous Positive Airway Pressure Adherence. Am. J. Respir. Crit. Care Med. 2021, 204, 339–346. [Google Scholar] [CrossRef]
- Sabil, A.; le Vaillant, M.; Stitt, C.; Goupil, F.; Pigeanne, T.; Leclair-Visonneau, L.; Masson, P.; Bizieux-Thaminy, A.; Humeau, M.-P.; Meslier, N.; et al. A CPAP Data–Based Algorithm for Automatic Early Prediction of Therapy Adherence. Sleep Breath. 2021, 25, 957–962. [Google Scholar] [CrossRef]
- Chai-Coetzer, C.L.; Luo, Y.M.; Antic, N.A.; Zhang, X.L.; Chen, B.Y.; He, Q.Y.; Heeley, E.; Huang, S.G.; Anderson, C.; Zhong, N.S.; et al. Predictors of Long-Term Adherence to Continuous Positive Airway Pressure Therapy in Patients with Obstructive Sleep Apnea and Cardiovascular Disease in the SAVE Study. Sleep 2013, 36, 1929–1937. [Google Scholar] [CrossRef]
- Hu, Y.; Su, Y.; Hu, S.; Ma, J.; Zhang, Z.; Fang, F.; Guan, J. Effects of Telemedicine Interventions in Improving Continuous Positive Airway Pressure Adherence in Patients with Obstructive Sleep Apnoea: A Meta-Analysis of Randomised Controlled Trials. Sleep Breath. 2021, 25, 1761–1771. [Google Scholar] [CrossRef]
- Garmendia, O.; Farré, R.; Ruiz, C.; Suarez-Girón, M.; Torres, M.; Cebrian, R.; Saura, L.; Monasterio, C.; Negrín, M.A.; Montserrat, J.M. Telemedicine Strategy to Rescue Cpap Therapy in Sleep Apnea Patients with Low Treatment Adherence: A Pilot Study. J. Clin. Med. 2021, 10, 4123. [Google Scholar] [CrossRef]
- Parmaksız, E.T. Can We Enhance Compliance to Treatment by Performing a Continuous Positive Airway Pressure Trial in Obstructive Sleep Apnea? Sleep Breath. 2021, 25, 2039–2043. [Google Scholar] [CrossRef] [PubMed]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Silber, M.H.; Ancoli-Israel, S.; Bonnet, M.H.; Chokroverty, S.; Grigg-Damberger, M.M.; Hirshkowitz, M.; Kapen, S.; Keenan, S.A.; Kryger, M.H.; Penzel, T.; et al. The Visual Scoring of Sleep in Adults. J. Clin. Sleep Med. 2007, 03, 121–131. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for Scoring Respiratory Events in Sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Pépin, J.L.; Krieger, J.; Rodenstein, D.; Cornette, A.; Sforza, E.; Delguste, P.; Deschaux, C.; Grillier, V.; Lévy, P. Effective Compliance during the First 3 Months of Continuous Positive Airway Pressure A European Prospective Study of 121 Patients. Am. J. Respir. Crit. Care Med. 1999, 160, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, A.R.; Eriksen, F.; Hansen, R.W.; Erlandsen, M.; Thorup, L.; Damgård, M.B.; Kirkegaard, M.G.; Hansen, K.W. Determinants for Adherence to Continuous Positive Airway Pressure Therapy in Obstructive Sleep Apnea. PLoS ONE 2017, 12, e0189614. [Google Scholar] [CrossRef]
- van Ryswyk, E.; Anderson, C.S.; Antic, N.A.; Barbe, F.; Bittencourt, L.; Freed, R.; Heeley, E.; Liu, Z.; Loffler, K.A.; Lorenzi-Filho, G.; et al. Predictors of Long-Term Adherence to Continuous Positive Airway Pressure in Patients with Obstructive Sleep Apnea and Cardiovascular Disease. Sleep 2019, 42, 1929–1937. [Google Scholar] [CrossRef]
- Krieger, J.; Kurtz, D.; Petiau, C.; Sforza, E.; Trautmann, D. Long-Term Compliance With CPAP Therapy in Obstructive Sleep Apnea Patients and in Snorers. Sleep 1996, 19 (Suppl. 9), S136–S143. [Google Scholar] [CrossRef]
- Morrone, E.; Giordano, A.; Carli, S.; Visca, D.; Rossato, F.; Godio, M.; Paracchini, E.; Rossi, S.; Balbi, B.; Sacco, C.; et al. Something Is Changing in Adherence to CPAP Therapy: Real World Data after 1 Year of Treatment in Patients with Obstructive Sleep Apnoea. Eur. Respir. J. 2020, 55, 1901419. [Google Scholar] [CrossRef]
- Chiu, H.-Y.; Chen, P.-Y.; Chuang, L.-P.; Chen, N.-H.; Tu, Y.-K.; Hsieh, Y.-J.; Wang, Y.-C.; Guilleminault, C. Diagnostic Accuracy of the Berlin Questionnaire, STOP-BANG, STOP, and Epworth Sleepiness Scale in Detecting Obstructive Sleep Apnea: A Bivariate Meta-Analysis. Sleep Med. Rev. 2017, 36, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, K.; James, K.T.; Weaver, E.M. Predicting CPAP Use and Treatment Outcomes Using Composite Indices of Sleep Apnea Severity. J. Clin. Sleep Med. 2016, 12, 849–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kushida, C.A.; Berry, R.B.; Blau, A.; Crabtree, T.; Fietze, I.; Kryger, M.H.; Kuna, S.T.; Pegram, G.V.; Penzel, T. Positive Airway Pressure Initiation: A Randomized Controlled Trial to Assess the Impact of Therapy Mode and Titration Process on Efficacy, Adherence, and Outcomes. Sleep 2011, 34, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
| All Subjects N = 307 | Males N = 246 | Females N = 61 | p | |
|---|---|---|---|---|
| Age [years] | 56.9 ± 10.8 | 55.9 ± 11.3 | 61.0 ± 7.3 | <0.001 |
| BMI [kg/m2] | 33.2 ± 5.5 | 33.2 ± 5.5 | 33.0 ± 6.1 | 0.790 |
| AHI | 35.0, 21.0–56.4 | 37.8, 21.0–58.3 | 29.9, 21.2–46.0 | 0.068 |
| AHI ≥ 15 | 40.6, 25.3–60.0 N = 265 | 43.0, 26.0–61.0 N = 214 | 33.0, 23.0–53.4 N = 51 | 0.081 |
| AHI < 15 | 10.9, 8.2–13.0 N = 42 | 11.0, 8.1–13.1 N = 32 | 9.1, 8.3–13.0 N = 10 | 0.525 |
| AHI CPAP | 4.0, 2.0–11.0 | 5.0, 2.0–12.0 | 3.0, 1.0–6.0 | 0.005 |
| 90% CPAP pressure [mbar] | 11.3 ± 2.3 | 11.5 ± 2.4 | 10.7 ± 2.4 | 0.033 |
| ESS | 9.5, 6.0–13.0 | 10.0, 6.0–13.0 | 8.0, 5.0–12.0 | 0.047 |
| Causes of CPAP Failure | Titration Failure N = 10 out of 400 | Primary Failure N = 78 out of 307 | Secondary Failure N = 64 out of 229 |
|---|---|---|---|
| CPAP titration pressure not effective | 5 (3 M) | NA | NA |
| Predominant central apnoea | 1 M | NA | NA |
| CPAP was not tolerated | 4 (3 M) | 15 (9 M) | 31 (23 M) |
| CPAP was too expensive | NA | 24 (19 M) | NA |
| Preferred other treatment * | NA | 18 (16 M) | 11 (10 M) |
| Afraid of CPAP treatment | - | 4 (2 M) | - |
| Treatment of comorbidities was more important | NA | 3 (1 M) | - |
| Did not feel benefit | - | - | 2 (1 M) |
| Spontaneous improvement | NA | NA | 8 (7 M) |
| Other reasons or declined to answer | NA | 14 (10 M) | 12 M |
| Total N | 10 (7 M) | 78 (57 M) | 64 (53 M) |
| Initiated CPAP N = 229 | Primary Failures N = 78 | p | |
|---|---|---|---|
| Age [years] | 57.8 ± 10.2 | 54.2 ± 12.1 | 0.013 |
| BMI [kg/m2] | 33.2 ± 5.2 | 33.2 ± 6.2 | 0.960 |
| Sex—M (%) | 189 (82.5%) | 57 (73.1%) | 0.071 |
| AHI | 38.0, 22.0–58.3 | 28.9, 17.8–51.0 | 0.028 |
| AHI CPAP | 4.0, 2.0–11.0 | 4.0, 1.3–9.0 | 0.511 |
| 90% CPAP pressure [mbar] | 11.4 ± 2.5 | 11.1 ± 2.3 | 0.307 |
| AHI ≥ 15 [N] | 202 (88.2%) | 63 (80.9%) | 0.099 |
| Positional OSA | 109/185 (59%) * | 42/60 (70%) * | 0.167 |
| ESS | 10.0, 7.0–13.0 | 8.0, 5.0–12.0 | 0.085 |
| Still on CPAP N = 165 | Secondary Failures N = 64 | p | All Failures N = 142 | p | |
|---|---|---|---|---|---|
| Age [years] | 57.6 ± 10.0 | 58.2 ± 9.1 | 0.68 | 56.0 ± 11.0 | 0.208 |
| BMI [kg/m2] | 33.2 ± 5.6 | 33.1 ± 4.1 | 0.87 | 32.7 ± 5.3 | 0.932 |
| Sex—M (%) | 136 (82.4%) | 53 (82.5%) | 0.94 | 110 (77.5%) | 0.346 |
| AHI | 40.6, 24.6–60.9 | 30.7, 16.5–52.1 | 0.017 | 30.4, 17.0–52.0 | 0.001 |
| AHI CPAP | 4.0, 2.0–11.0 | 4.0, 1.5–11.0 | 0.804 | 4.0, 1.3–10.0 | 0.826 |
| 90% CPAP pressure [mbar] | 11.0, 10.0–13.0 | 11.0, 10.0–12.0 | 0.214 | 11.0, 10.0–12.0 | 0.079 |
| AHI ≥ 15 | 151 (91.5%) | 51 (79.7%) | 0.024 | 114 (80.3%) | 0.007 |
| Positional OSA | 74/129 (57%) * | 35/56 (63%) * | 0.624 | 77/116 (66%) * | 0.188 |
| ESS | 10.0, 7.0–13.0 | 9.0, 6.0–13.0 | 0.706 | 9.0, 6.0–12.5 | 0.163 |
| Beta | p | HR (95% CI) | |
|---|---|---|---|
| age | 0.006 | 0.630 | 1.01 (0.98–1.03) |
| sex | −0.044 | 0.896 | 0.96 (0.49–1.85) |
| BMI | 0.017 | 0.509 | 1.02 (0.97–1.07) |
| AHI ≥ 15 | −0.947 | 0.005 | 0.39 (0.20–0.75) |
| Positional OSA | 0.053 | 0.871 | 1.05 (0.56–2.00) |
| ESS | −0.009 | 0.741 | 0.99 (0.94–1.05) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gabryelska, A.; Sochal, M.; Wasik, B.; Szczepanowski, P.; Białasiewicz, P. Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience. J. Clin. Med. 2022, 11, 139. https://doi.org/10.3390/jcm11010139
Gabryelska A, Sochal M, Wasik B, Szczepanowski P, Białasiewicz P. Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience. Journal of Clinical Medicine. 2022; 11(1):139. https://doi.org/10.3390/jcm11010139
Chicago/Turabian StyleGabryelska, Agata, Marcin Sochal, Bartosz Wasik, Przemysław Szczepanowski, and Piotr Białasiewicz. 2022. "Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience" Journal of Clinical Medicine 11, no. 1: 139. https://doi.org/10.3390/jcm11010139
APA StyleGabryelska, A., Sochal, M., Wasik, B., Szczepanowski, P., & Białasiewicz, P. (2022). Factors Affecting Long-Term Compliance of CPAP Treatment—A Single Centre Experience. Journal of Clinical Medicine, 11(1), 139. https://doi.org/10.3390/jcm11010139






