Cardiac Autonomic Dysfunction Is Associated with Severity of REM Sleep without Atonia in Isolated REM Sleep Behavior Disorder
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Clinical Assessments
2.3. Polysomnographic Recording
2.4. 123I-Metaiodobenzylguanidine Cardiac Scintigraphy
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DLB | Dementia with Lewy bodies |
| EMG | Electromyography |
| HMR | Heart-to-mediastinum ratio |
| iRBD | Isolated REM sleep behavior disorder |
| MIBG | Metaiodobenzylguanidine |
| MSA | Multiple system atrophy |
| PD | Parkinson’s disease |
| PSG | Polysomnography |
| RBDQ-KR | The Korean version of RBD questionnaire |
| REM | Rapid eye movement |
| RWA | REM sleep without atonia |
| SCOPA-AUT | Scales for Outcomes in Parkinson’s Disease for Autonomic Symptoms |
References
- International Classification of Sleep Disorders, 3rd ed.; American Academy of Sleep Medicine: Darien, IL, USA, 2014.
- Schenck, C.H.; Bundlie, S.R.; Ettinger, M.G.; Mahowald, M.W. Chronic behavioral disorders of human REM sleep: A new category of parasomnia. Sleep 1986, 9, 293–308. [Google Scholar] [CrossRef]
- Schenck, C.H.; Boeve, B.F.; Mahowald, M.W. Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: A 16-year update on a previously reported series. Sleep Med. 2013, 14, 744–748. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Fernandez-Arcos, A.; Tolosa, E.; Serradell, M.; Molinuevo, J.L.; Valldeoriola, F.; Gelpi, E.; Vilaseca, I.; Sanchez-Valle, R.; Llado, A.; et al. Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: Study in 174 patients. PLoS ONE 2014, 9, e89741. [Google Scholar] [CrossRef]
- Boeve, B.F.; Silber, M.H.; Ferman, T.J.; Lin, S.C.; Benarroch, E.E.; Schmeichel, A.M.; Ahlskog, J.E.; Caselli, R.J.; Jacobson, S.; Sabbagh, M.; et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep Med. 2013, 14, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Boeve, B.F.; Silber, M.H.; Saper, C.B.; Ferman, T.J.; Dickson, D.W.; Parisi, J.E.; Benarroch, E.E.; Ahlskog, J.E.; Smith, G.E.; Caselli, R.C.; et al. Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease. Brain 2007, 130, 2770–2788. [Google Scholar] [CrossRef]
- Högl, B.; Stefani, A.; Videnovic, A. Idiopathic REM sleep behaviour disorder and neurodegeneration—An update. Nat. Rev. Neurol. 2018, 14, 40–55. [Google Scholar] [CrossRef]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Högl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef]
- Arnaldi, D.; Chincarini, A.; Hu, M.T.; Sonka, K.; Boeve, B.; Miyamoto, T.; Puligheddu, M.; De Cock, V.C.; Terzaghi, M.; Plazzi, G.; et al. Dopaminergic imaging and clinical predictors for phenoconversion of REM sleep behaviour disorder. Brain 2021, 144, 278–287. [Google Scholar] [CrossRef]
- Iranzo, A.; Santamaria, J.; Tolosa, E. Idiopathic rapid eye movement sleep behaviour disorder: Diagnosis, management, and the need for neuroprotective interventions. Lancet Neurol. 2016, 15, 405–419. [Google Scholar] [CrossRef]
- Barber, T.R.; Lawton, M.; Ben-Shlomo, Y.; Hu, M.T.M. Considerations in the Use of MDS Research Criteria for Prodromal Parkinson’s in Rapid Eye Movement Sleep Behaviour Disorder and Population Cohorts. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.; Amino, T.; Uchihara, T.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Decreased cardiac uptake of MIBG is a potential biomarker for the presence of Lewy bodies. J. Neurol. 2007, 254, IV21–IV28. [Google Scholar] [CrossRef]
- Orimo, S. The clinical significance of MIBG myocardial scintigraphy in Parkinson disease. Brain Nerve Shinkei Kenkyu No Shinpo 2012, 64, 403–412. [Google Scholar] [PubMed]
- Chung, E.J.; Kim, S.J. 123I-Metaiodobenzylguanidine Myocardial Scintigraphy in Lewy Body-Related Disorders: A Literature Review. JMD 2015, 8, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Köllensperger, M.; Seppi, K.; Liener, C.; Boesch, S.; Heute, D.; Mair, K.J.; Mueller, J.; Sawires, M.; Scherfler, C.; Schocke, M.F.; et al. Diffusion weighted imaging best discriminates PD from MSA-P: A comparison with tilt table testing and heart MIBG scintigraphy. Mov. Disord. 2007, 22, 1771–1776. [Google Scholar] [CrossRef]
- Druschky, A.; Hilz, M.J.; Platsch, G.; Radespiel-Troger, M.; Druschky, K.; Kuwert, T.; Neundorfer, B. Differentiation of Parkinson’s disease and multiple system atrophy in early disease stages by means of I-123-MIBG-SPECT. J. Neurol. Sci. 2000, 175, 3–12. [Google Scholar] [CrossRef]
- Lee, P.H.; Yeo, S.H.; Kim, H.J.; Youm, H.Y. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson’s disease and multiple system atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2006, 21, 1975–1977. [Google Scholar] [CrossRef]
- Orimo, S.; Kanazawa, T.; Nakamura, A.; Uchihara, T.; Mori, F.; Kakita, A.; Wakabayashi, K.; Takahashi, H. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol. 2007, 113, 81–86. [Google Scholar] [CrossRef]
- Nagayama, H.; Ueda, M.; Yamazaki, M.; Nishiyama, Y.; Hamamoto, M.; Katayama, Y. Abnormal cardiac [123I]-meta-iodobenzylguanidine uptake in multiple system atrophy. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1744–1747. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Ferman, T.J.; Thomas, A.J.; Blanc, F.; Boeve, B.F.; Fujishiro, H.; Kantarci, K.; Muscio, C.; O’Brien, J.T.; Postuma, R.B.; et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020, 94, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Miyamoto, M.; Inoue, Y.; Usui, Y.; Suzuki, K.; Hirata, K. Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder. Neurology 2006, 67, 2236–2238. [Google Scholar] [CrossRef]
- Miyamoto, T.; Miyamoto, M.; Suzuki, K.; Nishibayashi, M.; Iwanami, M.; Hirata, K. 123I-MIBG cardiac scintigraphy provides clues to the underlying neurodegenerative disorder in idiopathic REM sleep behavior disorder. Sleep 2008, 31, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Salsone, M.; Labate, A.; Quattrone, A. Cardiac denervation precedes nigrostriatal damage in idiopathic rapid eye movement sleep behavior disorder. Mov. Disord. Off. J. Mov. Disord. Soc. 2012, 27, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Labate, A.; Salsone, M.; Novellino, F.; Morelli, M.; Sturniolo, M.; Gambardella, A.; Quattrone, A. Combined use of cardiac m-i123-iodobenzylguanidine scintigraphy and 123I-fp-cit single photon emission computed tomography in older adults with rapid eye movement sleep behavior disorder. J. Am. Geriatr. Soc. 2011, 59, 928–929. [Google Scholar] [CrossRef]
- Knudsen, K.; Fedorova, T.D.; Hansen, A.K.; Sommerauer, M.; Otto, M.; Svendsen, K.B.; Nahimi, A.; Stokholm, M.G.; Pavese, N.; Beier, C.P.; et al. In-vivo staging of pathology in REM sleep behaviour disorder: A multimodality imaging case-control study. Lancet. Neurol. 2018, 17, 618–628. [Google Scholar] [CrossRef]
- Sakakibara, R.; Tateno, F.; Kishi, M.; Tsuyusaki, Y.; Terada, H.; Inaoka, T. MIBG myocardial scintigraphy in pre-motor Parkinson’s disease: A review. Parkinsonism Relat. Disord. 2014, 20, 267–273. [Google Scholar] [CrossRef]
- Lim, S.Y.; Lee, E.J.; Jeong, S.W.; Kim, H.C.; Jeong, C.H.; Jeon, T.Y.; Yi, M.S.; Kim, J.M.; Jo, H.J.; Kim, J.B. The Validation Study of Beck Depression Scale 2 in Korean Version. Anxiety Mood 2011, 7, 48–53. [Google Scholar]
- Oh, H.; Park, K.; Yoon, S.; Kim, Y.; Lee, S.H.; Choi, Y.Y.; Choi, K.H. Clinical Utility of Beck Anxiety Inventory in Clinical and Nonclinical Korean Samples. Front. Psychiatry 2018, 9, 666. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.W.; Song, M.L.; Morin, C.M. Validation of a Korean version of the insomnia severity index. J. Clin. Neurol. (Seoul Korea) 2014, 10, 210–215. [Google Scholar] [CrossRef]
- Cho, Y.W.; Lee, J.H.; Son, H.K.; Lee, S.H.; Shin, C.; Johns, M.W. The reliability and validity of the Korean version of the Epworth sleepiness scale. Sleep Breath. Schlaf Atm. 2011, 15, 377–384. [Google Scholar] [CrossRef]
- Sohn, S.I.; Kim, D.H.; Lee, M.Y.; Cho, Y.W. The reliability and validity of the Korean version of the Pittsburgh Sleep Quality Index. Sleep Breath. Schlaf Atm. 2012, 16, 803–812. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Moon, H.J.; Do, S.Y.; Wing, Y.K.; Sunwoo, J.S.; Jung, K.Y.; Cho, Y.W. The REM Sleep Behavior Disorder Screening Questionnaire: Validation Study of the Korean Version (RBDQ-KR). J. Clin. Sleep Med. 2017, 13, 1429–1433. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, I.U.; Koh, S.B.; Ahn, T.B.; Kim, S.J.; Cheon, S.M.; Cho, J.W.; Kim, Y.J.; Ma, H.I.; Park, M.Y.; et al. Validation of the Korean Version of the Scale for Outcomes in Parkinson’s Disease-Autonomic. J. Mov. Disord. 2017, 10, 29–34. [Google Scholar] [CrossRef]
- Choi, W.R.; Jeong, H.Y.; Kim, J.H. Reliability and validity of the Korean version of the Questionnaire of Olfactory Disorders. Int. Forum Allergy Rhinol. 2018, 8, 1481–1485. [Google Scholar] [CrossRef]
- Frauscher, B.; Iranzo, A.; Gaig, C.; Gschliesser, V.; Guaita, M.; Raffelseder, V.; Ehrmann, L.; Sola, N.; Salamero, M.; Tolosa, E.; et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep 2012, 35, 835–847. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.; Harding, S.M.; Lloyd, R.M.; Quan, S.F.; Troester, M.T.; Vaughn, B.V. AASM Scoring Manual Updates for 2017 (Version 2.4). J. Clin. Sleep Med. 2017, 13, 665–666. [Google Scholar] [CrossRef]
- Nakajima, K.; Yamada, M. 123I-Meta-iodobenzylguanidine Sympathetic Imaging: Standardization and Application to Neurological Diseases. Chonnam Med. J. 2016, 52, 145–150. [Google Scholar] [CrossRef]
- Sakakibara, R.; Tateno, F.; Aiba, Y.; Ogata, T.; Kishi, M.; Terada, H.; Inaoka, T.; Nakatsuka, T.; Matsuoka, K. MIBG Myocardial Scintigraphy Identifies Premotor PD/DLB During a Negative DAT Scan Period: Second Report. Mov. Disord. Clin. Pract. 2019, 6, 46–50. [Google Scholar] [CrossRef]
- Seto, M.; Nakata, R.; Yuasa, T.; Nakao, Y.; Ichinose, K.; Tomita, I.; Satoh, H.; Satoh, A.; Ochi, M.; Tsujihata, M.; et al. Observed similar findings of 123i-mibg myocardial scintigraphy in dlb/pd and rem sleep behavior disorder (rbd), but not in other neurodegenerative disorders. Alzheimer’s Dement. J. Alzheimer’s Assoc. 2016, 12, P913. [Google Scholar] [CrossRef]
- Nomura, T.; Kishi, M.; Nakashima, K. Differences in clinical characteristics when REM sleep behavior disorder precedes or comes after the onset of Parkinson’s disease. J. Neurol. Sci. 2017, 382, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, H.E.; Oh, Y.S.; Lee, S.H.; Park, J.W.; Son, B.C.; Lee, K.S. Orthostatic hypotension and cardiac sympathetic denervation in Parkinson disease patients with REM sleep behavioral disorder. J. Neurol. Sci. 2016, 362, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kim, J.S.; Ryu, D.W.; Oh, Y.S.; Yoo, I.R.; Lee, K.S. Cardiac Sympathetic Denervation Can Predict the Wearing-off Phenomenon in Patients with Parkinson Disease. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2018, 59, 1728–1733. [Google Scholar] [CrossRef]
- Langston, J.W.; Wiley, J.C.; Tagliati, M. Optimizing Parkinson’s disease diagnosis: The role of a dual nuclear imaging algorithm. NPJ Parkinsons Dis. 2018, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Postuma, R.B.; Adler, C.H.; Dugger, B.N.; Hentz, J.G.; Shill, H.A.; Driver-Dunckley, E.; Sabbagh, M.N.; Jacobson, S.A.; Belden, C.M.; Sue, L.I.; et al. REM sleep behavior disorder and neuropathology in Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2015, 30, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Miyamoto, M.; Iwanami, M.; Hirata, K. Idiopathic REM Sleep Behavior Disorder: Implications for the Pathogenesis of Lewy Body Diseases. Parkinson’s Disease 2011, 2011, 941268. [Google Scholar] [CrossRef]
- Tateno, F.; Sakakibara, R.; Kishi, M.; Ogawa, E.; Takada, N.; Hosoe, N.; Suzuki, Y.; Takahashi, M.; Uchiyama, T.; Yamamoto, T. Constipation and metaiodobenzylguanidine myocardial scintigraphy abnormality. J. Am. Geriatr. Soc. 2012, 60, 185–187. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.F.; Rompré, S.; Montplaisir, J.Y. Severity of REM atonia loss in idiopathic REM sleep behavior disorder predicts Parkinson disease. Neurology 2010, 74, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Linn-Evans, M.E.; Petrucci, M.N.; Amundsen Huffmaster, S.L.; Chung, J.W.; Tuite, P.J.; Howell, M.J.; Videnovic, A.; MacKinnon, C.D. REM sleep without atonia is associated with increased rigidity in patients with mild to moderate Parkinson’s disease. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2020, 131, 2008–2016. [Google Scholar] [CrossRef]
- Dijkstra, F.; Van den Bossche, K.; de Bruyn, B.; Reyn, N.; Viaene, M.; De Volder, I.; Cras, P.; Crosiers, D. REM sleep without atonia and the relation with Lewy body disease. Parkinsonism Relat Disord 2019, 67, 90–98. [Google Scholar] [CrossRef]
- McCarter, S.J.; Sandness, D.J.; McCarter, A.R.; Feemster, J.C.; Teigen, L.N.; Timm, P.C.; Boeve, B.F.; Silber, M.H.; St Louis, E.K. REM sleep muscle activity in idiopathic REM sleep behavior disorder predicts phenoconversion. Neurology 2019, 93, e1171–e1179. [Google Scholar] [CrossRef]
- Nepozitek, J.; Dostalova, S.; Dusek, P.; Kemlink, D.; Prihodova, I.; Ibarburu Lorenzo, Y.L.V.; Friedrich, L.; Bezdicek, O.; Nikolai, T.; Perinova, P.; et al. Simultaneous tonic and phasic REM sleep without atonia best predicts early phenoconversion to neurodegenerative disease in idiopathic REM sleep behavior disorder. Sleep 2019, 42. [Google Scholar] [CrossRef]
- Palma, J.A. Autonomic dysfunction in sleep disorders: Introduction to the series. Clin. Auton Res. 2018, 28, 507–508. [Google Scholar] [CrossRef] [PubMed]
- McCarter, S.J.; St Louis, E.K.; Boeve, B.F. REM sleep behavior disorder and REM sleep without atonia as an early manifestation of degenerative neurological disease. Curr. Neurol. Neurosci. Rep. 2012, 12, 182–192. [Google Scholar] [CrossRef]
- Bedard, M.A.; Aghourian, M.; Legault-Denis, C.; Postuma, R.B.; Soucy, J.P.; Gagnon, J.F.; Pelletier, A.; Montplaisir, J. Brain cholinergic alterations in idiopathic REM sleep behaviour disorder: A PET imaging study with 18F-FEOBV. Sleep Med. 2019, 58, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Valencia Garcia, S.; Brischoux, F.; Clément, O.; Libourel, P.A.; Arthaud, S.; Lazarus, M.; Luppi, P.H.; Fort, P. Ventromedial medulla inhibitory neuron inactivation induces REM sleep without atonia and REM sleep behavior disorder. Nat. Commun. 2018, 9, 504. [Google Scholar] [CrossRef]
- Braune, S.; Reinhardt, M.; Schnitzer, R.; Riedel, A.; Lücking, C.H. Cardiac uptake of 123I-MIBG separates Parkinson’s disease from multiple system atrophy. Neurology 1999, 53, 1020–1025. [Google Scholar] [CrossRef]
- Postuma, R.B.; Gagnon, J.-F.; Bertrand, J.-A.; Génier Marchand, D.; Montplaisir, J.Y. Parkinson risk in idiopathic REM sleep behavior disorder: Preparing for neuroprotective trials. Neurology 2015, 84, 1104–1113. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Arcos, A.; Iranzo, A.; Serradell, M.; Gaig, C.; Guaita, M.; Salamero, M.; Santamaria, J. Diagnostic Value of Isolated Mentalis Versus Mentalis Plus Upper Limb Electromyography in Idiopathic REM Sleep Behavior Disorder Patients Eventually Developing a Neurodegenerative Syndrome. Sleep 2017, 40. [Google Scholar] [CrossRef] [PubMed]
- Schenck, C.H. Clinical and research implications of a validated polysomnographic scoring method for REM sleep behavior disorder. Sleep 2005, 28, 917–919. [Google Scholar] [CrossRef][Green Version]
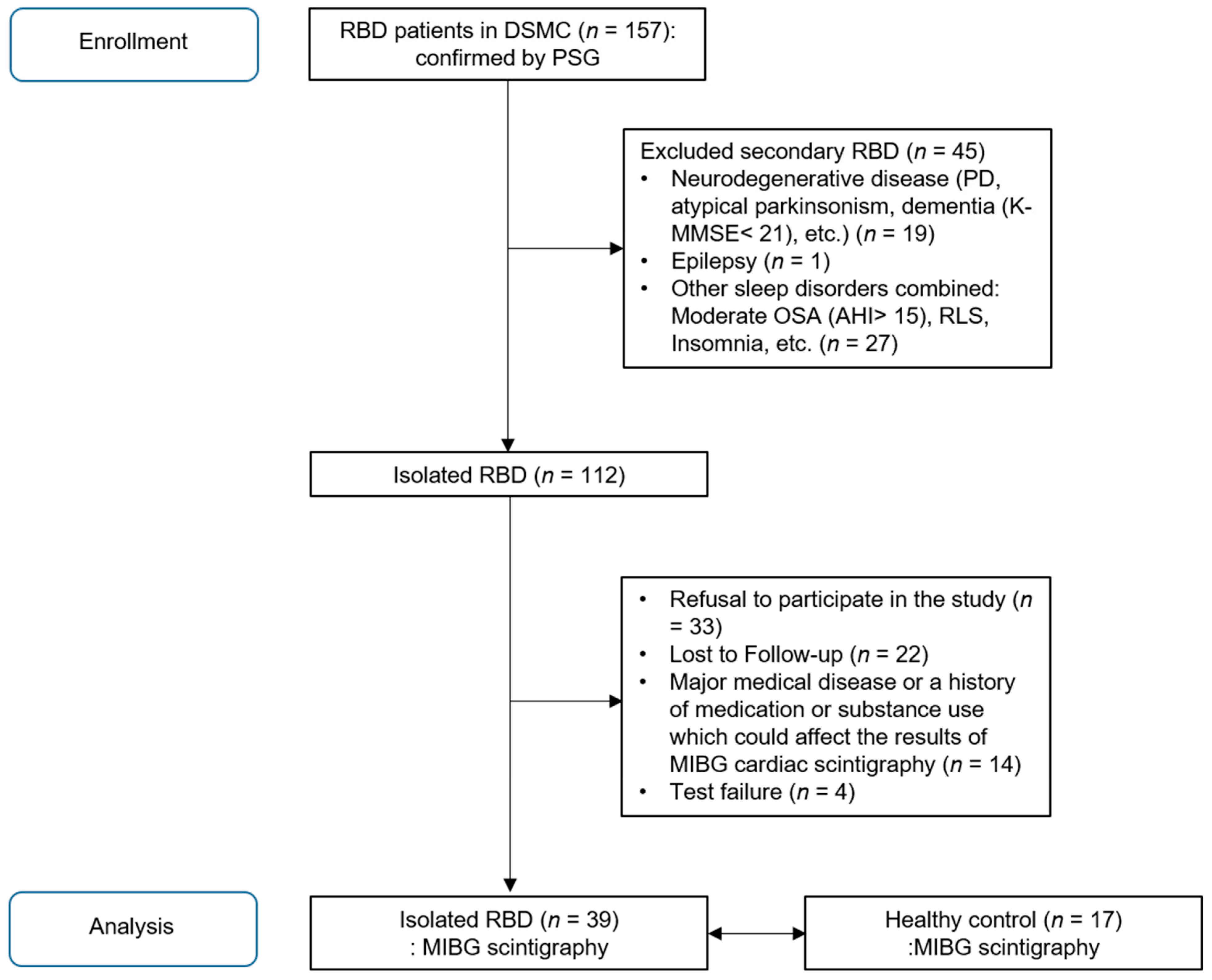
| iRBD (n = 39) | Healthy Control (n = 17) | p-Value | |
|---|---|---|---|
| Age | 65.4 ± 6.5 | 66.0 ± 7.4 | 0.954 |
| Sex (male, %) | 25 (64.1) | 6 (35.3) | 0.044 # * |
| RBD disease duration | 6.5 ± 4.8 (0.25–13.0) | - | - |
| (range, years) | |||
| H & Y stage | 0 | 0 | 1 |
| UPDRS part III | 0.3 ± 1.0 | 0.6 ± 1.5 | 0.258 |
| Sleep questionnaire | |||
| ISI | 6.1 ± 5.8 | 7.1 ± 7.2 | 0.961 |
| ESS | 4.8 ± 4.2 | 2.8 ± 2.8 | 0.065 |
| PSQI | 6.5 ± 3.6 | 6.2 ± 7.3 | 0.349 |
| RBDQ-KR | 42.8 ± 18.2 | 14.7 ± 19.4 | <0.001 * |
| BDI | 12.0 ± 10.8 | 11.5 ± 9.2 | 0.842 |
| BAI | 7.2 ± 7.9 | 6.3 ± 7.3 | 0.828 |
| MMSE | 27.1 ± 2.4 | 26.1 ± 2.8 | 0.184 |
| SCOPA-AUT, total | 8.9 ± 4.7 | 6.7 ± 5.3 | 0.054 |
| Gastrointestinal | 2.6 ± 2.1 | 1.2 ± 1.4 | 0.014 * |
| Urinary | 4.7 ± 2.9 | 4.1 ± 3.0 | 0.415 |
| Cardiovascular | 0.5 ± 0.8 | 0.7 ± 1.3 | 0.911 |
| Pupilomotor | 0.4 ± 0.7 | 0.2 ± 0.7 | 0.293 |
| Thermoregulatory | 0.4 ± 0.9 | 0.2 ± 0.4 | 1 |
| Sexual | 0.4 ± 0.9 | 0.2 ± 0.8 | 0.279 |
| KVSS test | 4.2 ± 2.8 | 5.5 ± 2.2 | 0.185 |
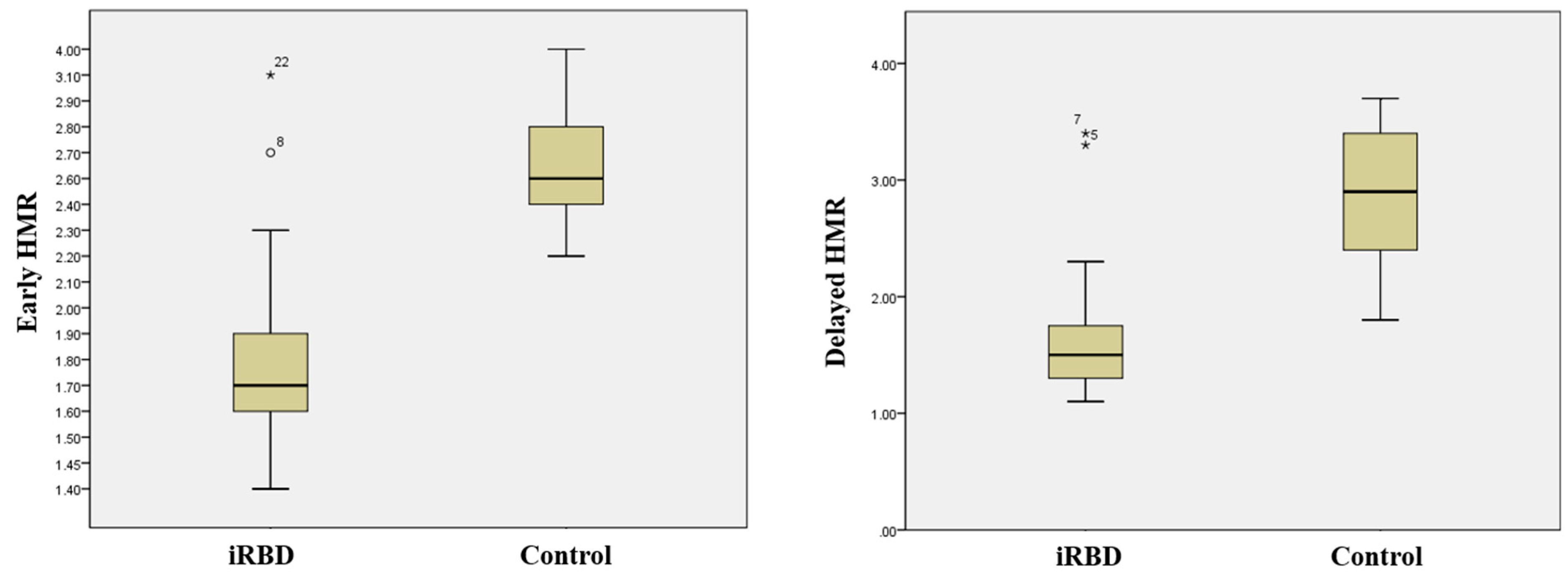
| Early HMR | 1.8 ± 0.3 | 2.7 ± 0.4 | <0.001 * |
| Delayed HMR | 1.6 ± 0.5 | 2.8 ± 0.6 | <0.001 * |
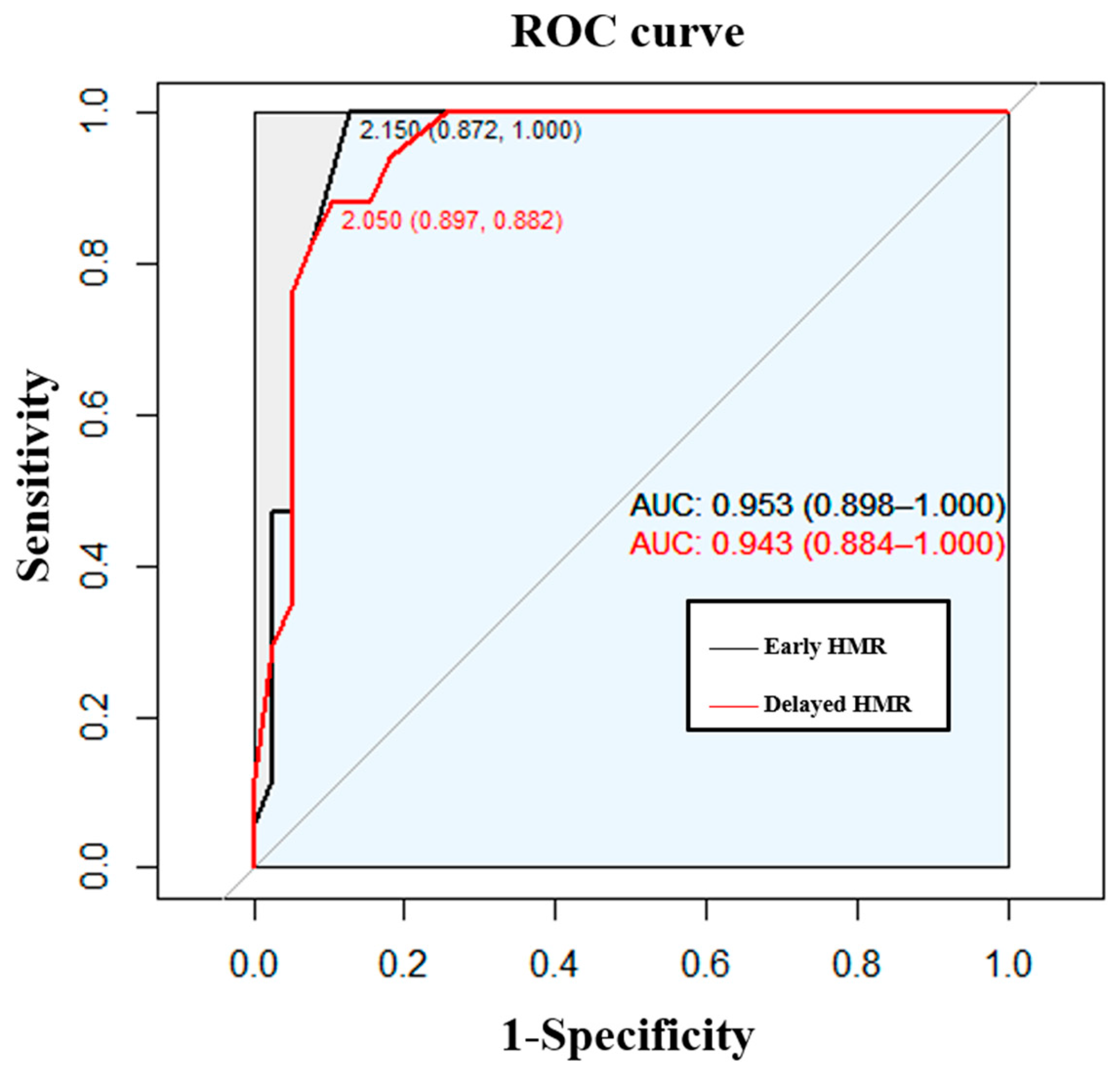
| ≥2.15 (n = 5) | <2.15 (n = 34) | p-Value | |
|---|---|---|---|
| Age | 63.6 ± 7.9 | 65.6 ± 6.3 | 0.752 |
| Sex (male, %) | 3 (60.0) | 22 (64.7) | 1.000 # |
| UPDRS part III | 0.8 ± 1.8 | 0.2 ± 0.9 | 0.243 |
| Onset age, year | 54.6 ± 2.6 | 60.2 ± 7.0 | 0.269 |
| Duration, year Interval of symptoms to PSG, year | 11.6 ± 10.7 9.8 ± 4.4 | 5.7 ± 2.8 3.0 ± 0.5 | 0.304 0.186 |
| ISI | 6.4 ± 6.9 | 6.0 ± 5.7 | 0.909 |
| ESS | 2.2 ± 2.2 | 5.2 ± 4.4 | 0.120 |
| BDI | 9.2 ± 10.6 | 12.4 ± 11.0 | 0.646 |
| BAI | 5.2 ± 7.1 | 7.4 ± 8.1 | 0.532 |
| PSQI | 6.4 ± 3.0 | 6.6 ± 3.3 | 0.925 |
| MMSE | 26.2 ± 3.1 | 27.2 ± 2.3 | 0.527 |
| RBDQ-KR Factor 1 Factor 2 | 35.4 ± 18.5 10.6 ± 7.4 25.2 ± 17.1 | 43.9 ± 18.2 13.3 ± 5.7 31.0 ± 14.6 | 0.467 0.360 0.424 |
| SCOPA-AUT total | 6.8 ± 4.3 | 9.2 ± 4.7 | 0.289 |
| Gastrointestinal | 2.4 ± 1.7 | 2.6 ± 2.1 | 0.963 |
| Urinary | 3.4 ± 3.1 | 4.9 ± 2.9 | 0.248 |
| Cardiovascular | 0.2 ± 0.5 | 0.6 ± 0.8 | 0.483 |
| Pupilomotor | 0.4 ± 0.6 | 0.4 ± 0.7 | 0.609 |
| Thermoreulatory | 0.2 ± 0.5 | 0.3 ± 0.5 | 1.000 |
| Sexual | 0.2 ± 0.5 | 0.5 ± 0.9 | 0.794 |
| KVSS test | 4.6 ± 3.1 | 4.1 ± 2.7 | 0.745 |
| MIBG | |||
| Early HMR | 2.5 ± 0.4 | 1.7 ± 0.2 | <0.001 * |
| Delayed HMR | 2.6 ± 0.7 | 1.5 ± 0.2 | <0.001 * |
| Total sleep time (min) | 355.3 ± 80.8 | 384.8 ± 55.4 | 0.522 |
| N1 (%) | 13.9 ± 4.9 | 13.8 ± 6.1 | 0.669 |
| N2 (%) | 47.6 ± 10.1 | 50.0 ± 8.4 | 0.419 |
| N3 (%) | 20.1 ± 3.4 | 15.0 ± 9.1 | 0.205 |
| REM (%) | 18.4 ± 6.3 | 21.4 ± 7.4 | 0.358 |
| Latency to sleep onset | 48.4 ± 84.1 | 12.6 ± 13.2 | 0.592 |
| Latency to sleep stage 2 (min) | 55.7 ± 82.8 | 18.1 ± 15.5 | 0.481 |
| Latency to REM sleep stage (min) | 110.1 ± 87.5 | 118.5 ± 70.0 | 0.967 |
| RWA (%) | 11.0 ± 5.6 | 29.3 ± 23.2 | 0.018 * |
| Sleep efficiency (%) | 73.8 ± 16.5 | 79.17 ± 10.7 | 0.557 |
| AHI, Total PLM index, Total | 3.7 ± 5.2 8.8 ± 19.7 | 4.3 ± 6.5 14.6 ± 22.9 | 0.859 0.557 |
| Arousal index, Total | 11.4 ± 4.7 | 12.3 ± 8.0 | 0.974 |
| ≥2.05 (n = 4) | <2.05 (n = 35) | p-Value | |
|---|---|---|---|
| Age | 63.5 ± 9.1 | 65.6 ± 6.2 | 0.831 |
| Sex (male, %) | 3 (75.0) | 22 (62.9) | 0.545 # |
| UPDRS part III | 1.00 ± 2.0 | 0.2 ± 0.9 | 0.197 |
| Onset age, year | 53.0 ± 13.9 | 60.2 ± 6.9 | 0.166 |
| Duration, year Interval of symptoms to PSG, year | 13.8 ± 11.0 12.5 ± 10.3 | 5.6 ± 2.8 3.6 ± 3.0 | 0.078 0.179 |
| ISI | 6.3 ± 7.9 | 6.0 ± 5.6 | 0.902 |
| ESS | 2.0 ± 2.5 | 2.1 ± 4.3 | 0.122 |
| BDI | 7.8 ± 11.6 | 12.5 ± 10.8 | 0.426 |
| BAI | 4.3 ± 7.9 | 7.5 ± 8.0 | 0.293 |
| PSQI | 5.5 ± 2.5 | 6.7 ± 3.8 | 0.644 |
| MMSE | 25.5 ± 3.1 | 27.3 ± 2.3 | 0.242 |
| RBDQ-KR Factor 1 Factor 2 | 38.3 ± 20.1 9.8 ± 8.3 29.0 ± 17.2 | 43.3 ± 18.2 13.3 ± 5.6 30.4 ± 14.8 | 0.815 0.268 0.865 |
| SCOPA-AUT total | 7.0 ± 5.0 | 9.1 ± 4.7 | 0.455 |
| Gastrointestinal | 2.0 ± 1.6 | 2.6 ± 2.1 | 0.659 |
| Urinary | 4.3 ± 2.9 | 4.8 ± 3.0 | 0.664 |
| Cardiovascular | 0.0 ± 0.0 | 0.6 ± 0.8 | 0.251 |
| Pupilomotor | 0.3 ± 0.5 | 0.4 ± 0.7 | 0.920 |
| Thermoreulatory | 0.3 ± 0.5 | 0.3 ± 0.4 | 1.000 |
| Sexual | 0.3 ± 0.5 | 0.5 ± 0.9 | 0.822 |
| KVSS test | 4.0 ± 3.3 | 4.2 ± 2.7 | 0.827 |
| MIBG | |||
| Early HMR | 2.6 ± 0.4 | 1.7 ± 0.2 | <0.001 * |
| Delayed HMR | 2.8 ± 0.7 | 1.5 ± 0.2 | <0.001 * |
| Total sleep time (min) | 354.8 ± 93.3 | 384.0 ± 54.8 | 0.656 |
| N1 (%) | 14.2 ± 5.5 | 13.6 ± 6.0 | 0.725 |
| N2 (%) | 49.1 ± 11.0 | 49.8 ± 8.4 | 0.795 |
| N3 (%) | 20.05 ± 3.90 | 15.1 ± 9.1 | 0.272 |
| REM (%) | 16.6 ± 5.7 | 21.6 ± 7.3 | 0.141 |
| Latency to sleep onset | 54.5 ± 95.8 | 12.9 ± 13.1 | 1.000 |
| Latency to sleep stage 2 (min) | 57.4 ± 95.6 | 19.0 ± 16.1 | 0.991 |
| Latency to REM sleep stage (min) | 114.4 ± 89.9 | 117.7 ± 69.1 | 0.945 |
| RWA (%) | 9.1 ± 4.3 | 30.0 ± 22.9 | 0.011 * |
| Sleep efficiency (%) | 72.9 ± 18.9 | 79.1 ± 10.5 | 0.697 |
| AHI, Total PLM index, Total | 4.7 ± 5.5 11.0 ± 22.0 | 4.2 ± 6.4 14.2 ± 22.7 | 0.733 0.731 |
| Arousal index total | 12.7 ± 4.4 | 12.2 ± 7.9 | 0.528 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
You, S.; Won, K.S.; Kim, K.T.; Lee, H.W.; Cho, Y.W. Cardiac Autonomic Dysfunction Is Associated with Severity of REM Sleep without Atonia in Isolated REM Sleep Behavior Disorder. J. Clin. Med. 2021, 10, 5414. https://doi.org/10.3390/jcm10225414
You S, Won KS, Kim KT, Lee HW, Cho YW. Cardiac Autonomic Dysfunction Is Associated with Severity of REM Sleep without Atonia in Isolated REM Sleep Behavior Disorder. Journal of Clinical Medicine. 2021; 10(22):5414. https://doi.org/10.3390/jcm10225414
Chicago/Turabian StyleYou, Sooyeoun, Kyoung Sook Won, Keun Tae Kim, Hyang Woon Lee, and Yong Won Cho. 2021. "Cardiac Autonomic Dysfunction Is Associated with Severity of REM Sleep without Atonia in Isolated REM Sleep Behavior Disorder" Journal of Clinical Medicine 10, no. 22: 5414. https://doi.org/10.3390/jcm10225414
APA StyleYou, S., Won, K. S., Kim, K. T., Lee, H. W., & Cho, Y. W. (2021). Cardiac Autonomic Dysfunction Is Associated with Severity of REM Sleep without Atonia in Isolated REM Sleep Behavior Disorder. Journal of Clinical Medicine, 10(22), 5414. https://doi.org/10.3390/jcm10225414






