Real-Time Brain Monitoring by Near-Infrared Spectroscopy Predicts Neurological Outcome after Cardiac Arrest and Resuscitation in Rats: A Proof of Concept Study of a Novel Prognostic Measure after Cardiac Arrest
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Monitoring by Near-Infrared Spectroscopy
2.3. Definition of Tnadir as a Predictive Marker for Neurological Outcome after CA/CPR
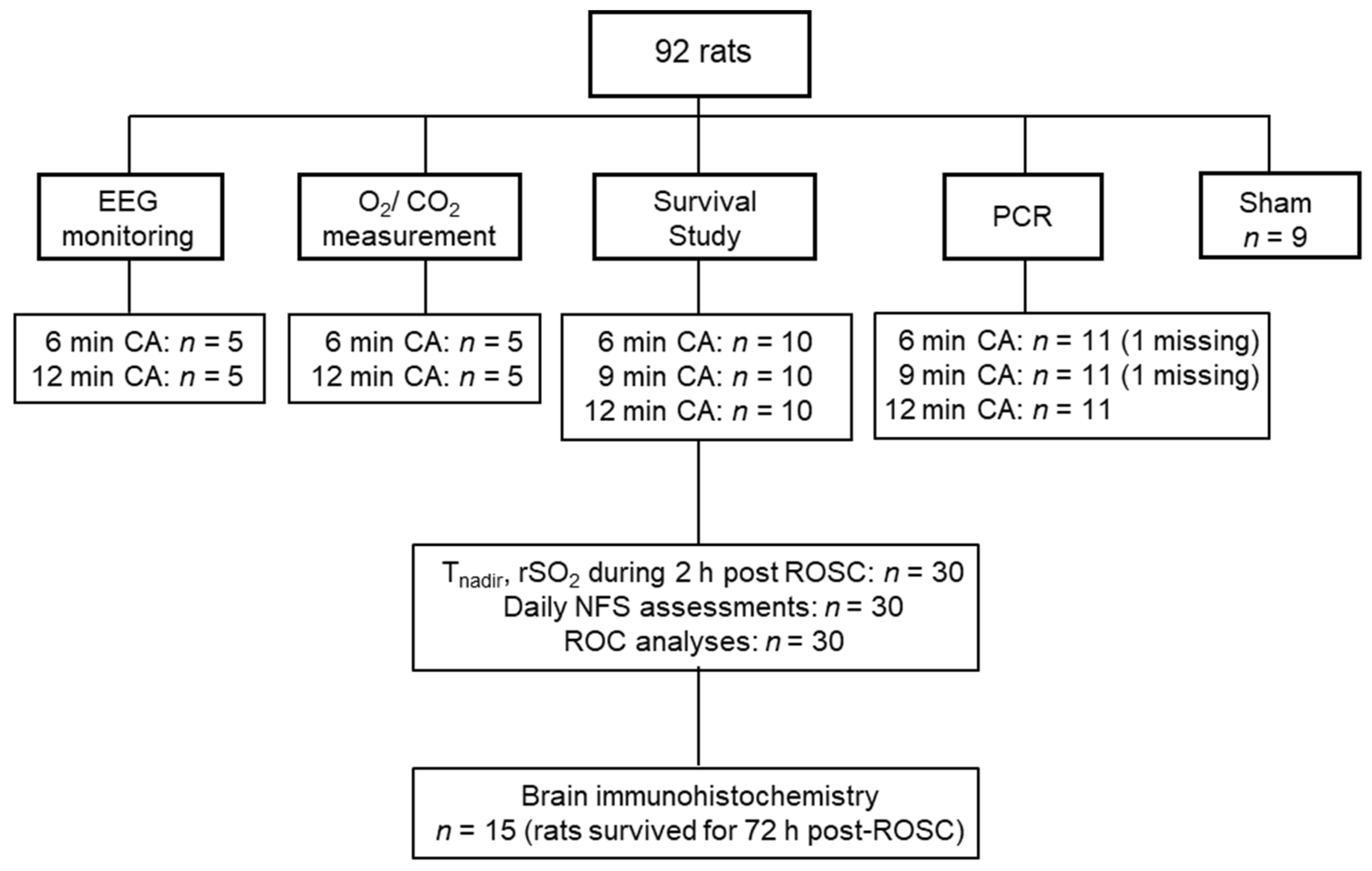
2.4. Study Design
2.5. Rat Model of Asphyxial CA and CPR
2.6. Electroencephalography Monitoring
2.7. Assessment of Oxygen Consumption and Carbon Dioxide Production in the Brain
2.8. Assessment of Neurological Function
2.9. Measurement of Interleukin (IL)-6 Levels in the Plasma
2.10. Brain Immunohistochemistry
2.11. Measurements of Gene Expressions
2.12. Statistical Analysis
3. Results
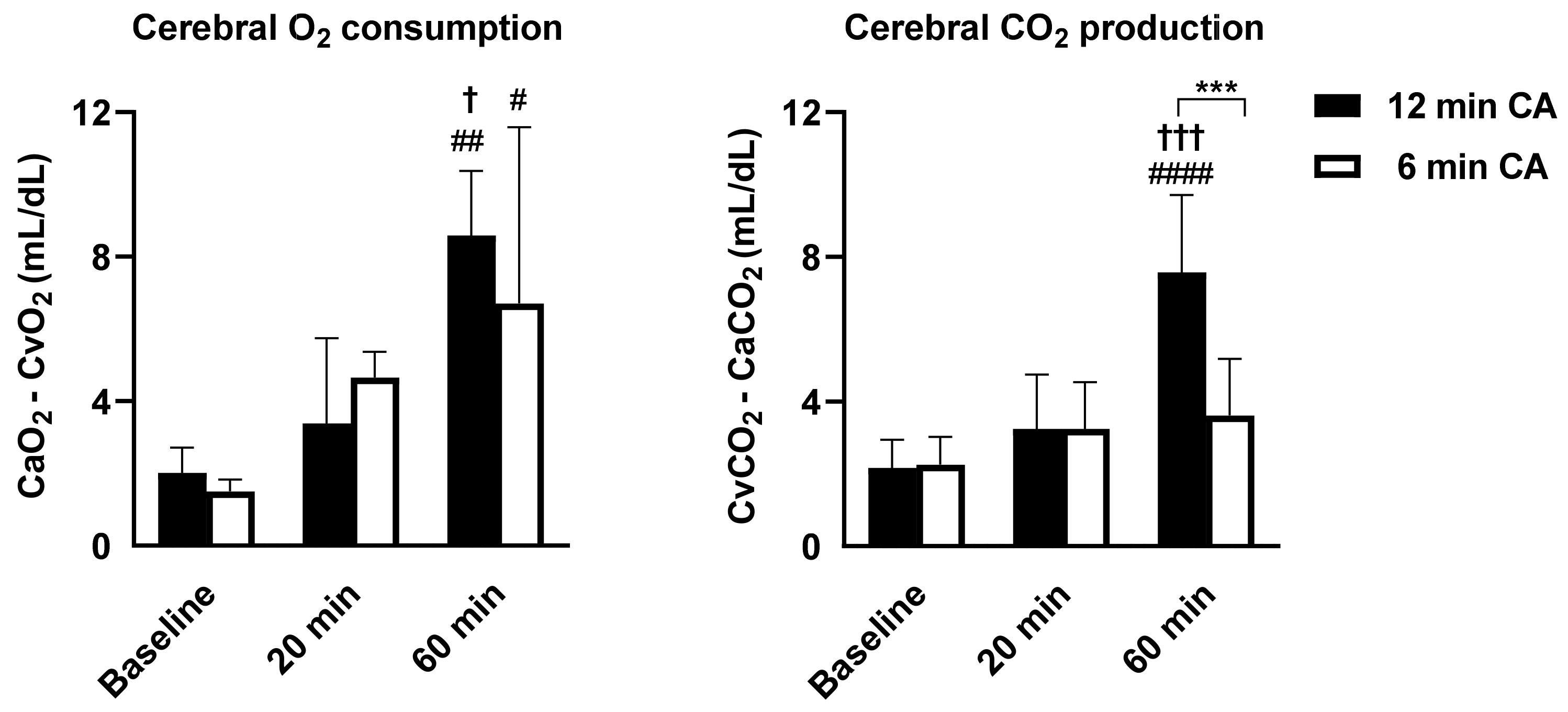
3.1. The Impact of Different CA Durations on Brain Recovery Time and Cerebral Metabolism after ROSC
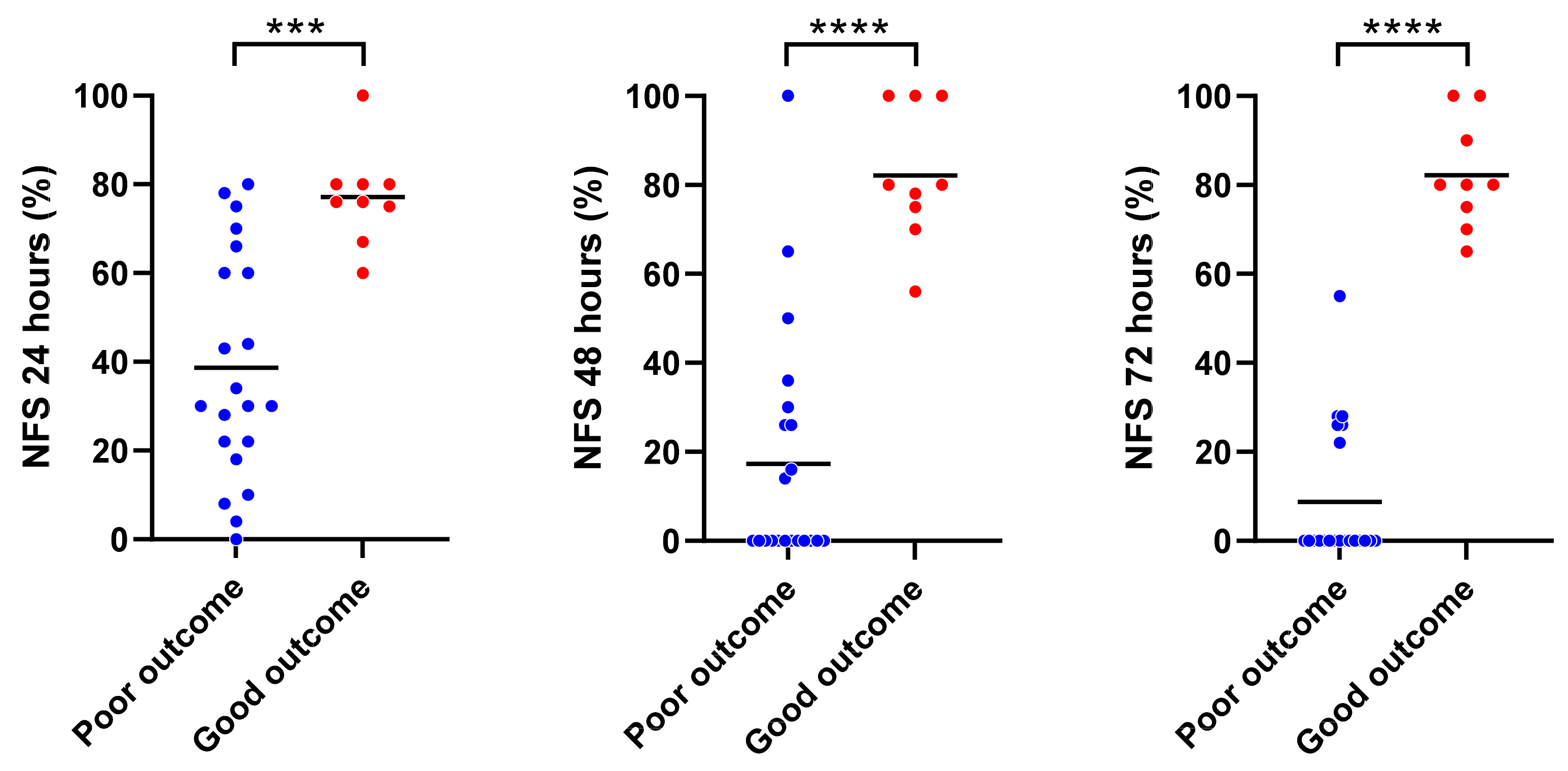
3.2. Tnadir by Real-Time NIRS Monitoring Predicted a Short-Term Neurofunctional Status after CA/CPR
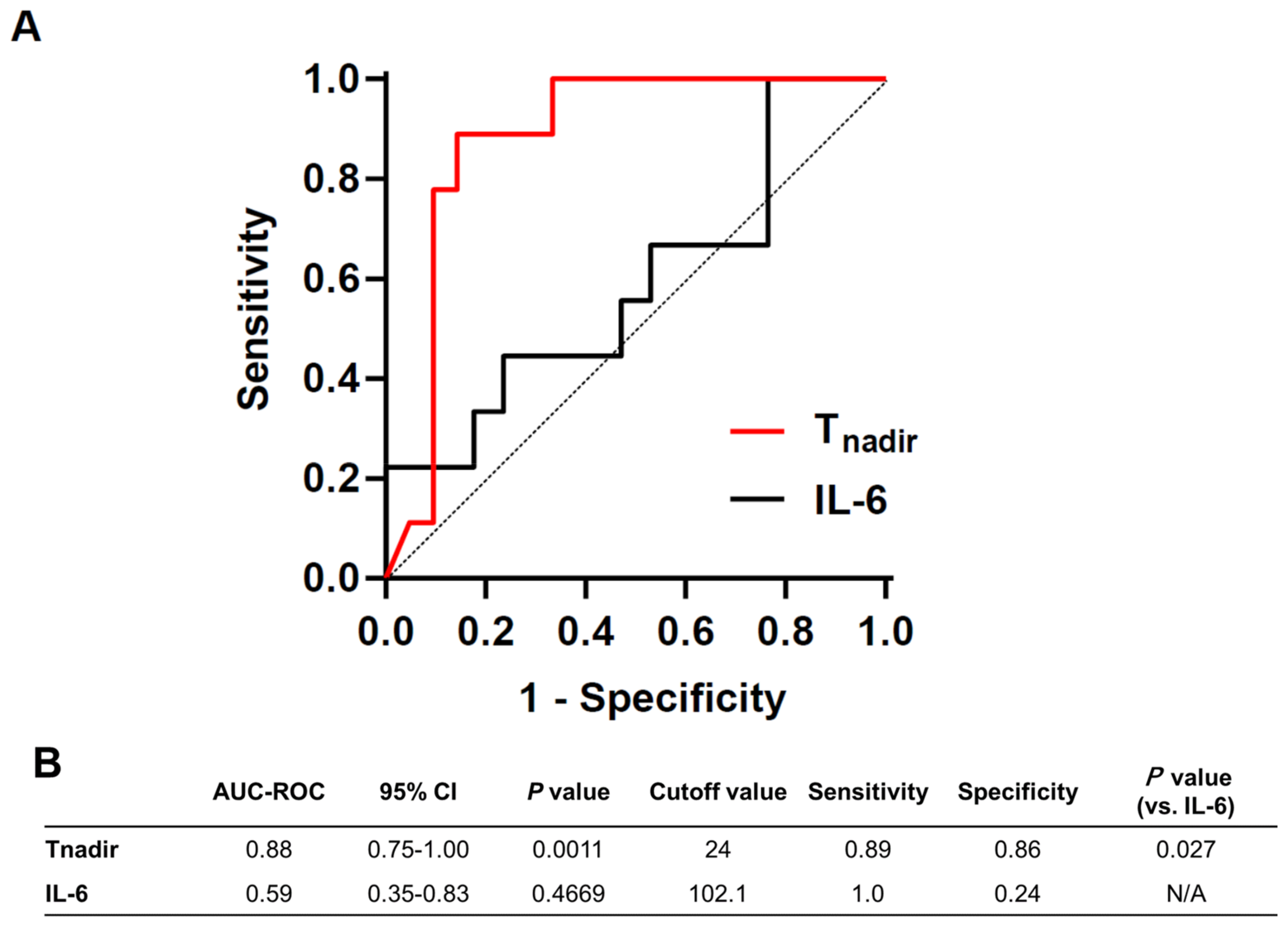
3.3. Predictive Value of Tnadir on Good Neurological Outcome Was Superior to That of Plasma IL-6 Level at 1 h after ROSC
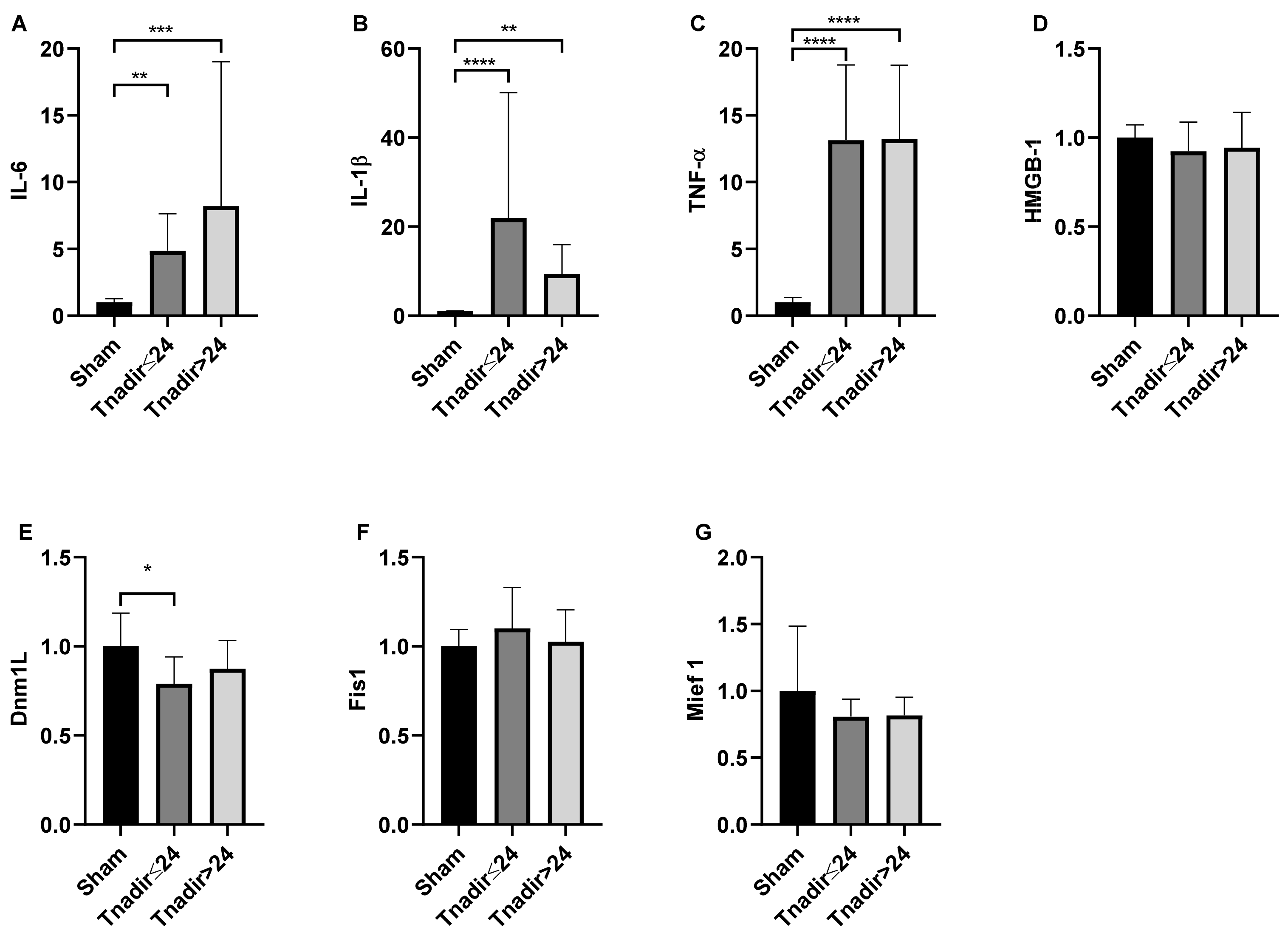
3.4. Tnadir Was Associated with Alterations in Proinflammatory Cytokines in the Brain Immediately after ROSC
3.5. Tnadir Was Associated with Neuronal Degeneration Immediately after ROSC
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Gan, Y.; Jiang, N.; Wang, R.; Chen, Y.; Luo, Z.; Zong, Q.; Chen, S.; Lv, C. The global survival rate among adult out-of-hospital cardiac arrest patients who received cardiopulmonary resuscitation: A systematic review and meta-analysis. Crit. Care 2020, 24, 61. [Google Scholar] [CrossRef] [PubMed]
- Okubo, M.; Kiyohara, K.; Iwami, T.; Callaway, C.W.; Kitamura, T. Nationwide and regional trends in survival from out-of-hospital cardiac arrest in Japan: A 10-year cohort study from 2005 to 2014. Resuscitation 2017, 115, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Soar, J.; Donnino, M.W.; Maconochie, I.; Aickin, R.; Atkins, D.L.; Andersen, L.W.; Berg, K.M.; Bingham, R.; Bottiger, B.W.; Callaway, C.W.; et al. 2018 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations Summary. Circulation 2018, 138, e714–e730. [Google Scholar] [CrossRef]
- Li, Y.; Tang, Q.; Wang, P.; Qin, J.; Wu, H.; Lin, J.; Huang, Z. Dynamic Changes of Mitochondrial Fusion and Fission in Brain Injury after Cardiac Arrest in Rats. Biomed. Res. Int. 2017, 2017, 1948070. [Google Scholar] [CrossRef]
- Neumar, R.W.; Nolan, J.P.; Adrie, C.; Aibiki, M.; Berg, R.A.; Bottiger, B.W.; Callaway, C.; Clark, R.S.; Geocadin, R.G.; Jauch, E.C.; et al. Post-cardiac arrest syndrome: Epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation 2008, 118, 2452–2483. [Google Scholar]
- Cournoyer, A.; Iseppon, M.; Chauny, J.M.; Denault, A.; Cossette, S.; Notebaert, E. Near-infrared Spectroscopy Monitoring during Cardiac Arrest: A Systematic Review and Meta-analysis. Acad. Emerg. Med. 2016, 23, 851–862. [Google Scholar] [CrossRef]
- Schnaubelt, S.; Sulzgruber, P.; Menger, J.; Skhirtladze-Dworschak, K.; Sterz, F.; Dworschak, M. Regional cerebral oxygen saturation during cardiopulmonary resuscitation as a predictor of return of spontaneous circulation and favourable neurological outcome—A review of the current literature. Resuscitation 2018, 125, 39–47. [Google Scholar] [CrossRef]
- Tobias, J.D. Cerebral oxygenation monitoring: Near-infrared spectroscopy. Expert Rev. Med. Devices 2006, 3, 235–243. [Google Scholar] [CrossRef]
- Thavasothy, M.; Broadhead, M.; Elwell, C.; Peters, M.; Smith, M. A comparison of cerebral oxygenation as measured by the NIRO 300 and the INVOS 5100 Near-Infrared Spectrophotometers. Anaesthesia 2002, 57, 999–1006. [Google Scholar] [CrossRef]
- Parnia, S.; Nasir, A.; Shah, C.; Patel, R.; Mani, A.; Richman, P. A feasibility study evaluating the role of cerebral oximetry in predicting return of spontaneous circulation in cardiac arrest. Resuscitation 2012, 83, 982–985. [Google Scholar] [CrossRef]
- Ehara, N.; Hirose, T.; Shiozaki, T.; Wakai, A.; Nishimura, T.; Mori, N.; Ohnishi, M.; Sadamitsu, D.; Shimazu, T. The relationship between cerebral regional oxygen saturation during extracorporeal cardiopulmonary resuscitation and the neurological outcome in a retrospective analysis of 16 cases. J. Intensive Care 2017, 5, 20. [Google Scholar] [CrossRef][Green Version]
- Takegawa, R.; Hayashida, K.; Rolston, D.M.; Li, T.; Miyara, S.J.; Ohnishi, M.; Shiozaki, T.; Becker, L.B. Near-Infrared Spectroscopy Assessments of Regional Cerebral Oxygen Saturation for the Prediction of Clinical Outcomes in Patients with Cardiac Arrest: A Review of Clinical Impact, Evolution, and Future Directions. Front. Med. 2020, 7, 587930. [Google Scholar] [CrossRef]
- Takegawa, R.; Shiozaki, T.; Furuya, S.; Hirose, T.; Mori, N.; Ohnishi, M.; Shimazu, T. Biphasic changes of rSO2 after cardio–pulmonary resuscitation preceded the good neurological outcome: A case report. J. Jpn. Assoc. Acute Med. 2018, 29, 138–142. [Google Scholar]
- Okuma, Y.; Aoki, T.; Miyara, S.J.; Hayashida, K.; Nishikimi, M.; Takegawa, R.; Yin, T.; Kim, J.; Becker, L.B.; Shinozaki, K. The evaluation of pituitary damage associated with cardiac arrest: An experimental rodent model. Sci. Rep. 2021, 11, 629. [Google Scholar] [CrossRef]
- Geocadin, R.G.; Sherman, D.L.; Christian Hansen, H.; Kimura, T.; Niedermeyer, E.; Thakor, N.V.; Hanley, D.F. Neurological recovery by EEG bursting after resuscitation from cardiac arrest in rats. Resuscitation 2002, 55, 193–200. [Google Scholar] [CrossRef]
- Shinozaki, K.; Becker, L.B.; Saeki, K.; Kim, J.; Yin, T.; Da, T.; Lampe, J.W. Dissociated Oxygen Consumption and Carbon Dioxide Production in the Post-Cardiac Arrest Rat: A Novel Metabolic Phenotype. J. Am. Heart Assoc. 2018, 7, e007721. [Google Scholar] [CrossRef]
- Nishikimi, M.; Yagi, T.; Shoaib, M.; Takegawa, R.; Rasul, R.; Hayashida, K.; Okuma, Y.; Yin, T.; Choudhary, R.C.; Becker, L.B.; et al. Phospholipid Screening Postcardiac Arrest Detects Decreased Plasma Lysophosphatidylcholine: Supplementation as a New Therapeutic Approach. Crit. Care Med. 2021. [Google Scholar] [CrossRef]
- Young, L.M.; Choudhary, R.; Jia, X. Multimodel quantitative analysis of somatosensory evoked potentials after cardiac arrest with graded hypothermia. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society IEEE Engineering in Medicine and Biology Society Annual International Conference, Orlando, FL, USA, 16–20 August 2016; Volume 2016, pp. 1846–1849. [Google Scholar]
- Chen, B.; Chen, G.; Dai, C.; Wang, P.; Zhang, L.; Huang, Y.; Li, Y. Comparison of Quantitative Characteristics of Early Post-resuscitation EEG Between Asphyxial and Ventricular Fibrillation Cardiac Arrest in Rats. Neurocrit. Care 2018, 28, 247–256. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, L.; Zhu, Z.; Seal, R.; McQuillan, P.M. Effects of Hypothermic Cardiopulmonary Bypass on Internal Jugular Bulb Venous Oxygen Saturation, Cerebral Oxygen Saturation, and Bispectral Index in Pediatric Patients Undergoing Cardiac Surgery: A Prospective Study. Medicine 2016, 95, e2483. [Google Scholar] [CrossRef]
- Plötz, F.B.; van Lingen, R.A.; Bos, A.P. Venous oxygen measurements in the inferior vena cava in neonates with respiratory failure. Crit. Care 1998, 2, 57. [Google Scholar] [CrossRef]
- Neviere, R.; Mathieu, D.; Riou, Y.; Guimez, P.; Renaud, N.; Chagnon, J.L.; Wattel, F. Carbon dioxide rebreathing method of cardiac output measurement during acute respiratory failure in patients with chronic obstructive pulmonary disease. Crit. Care Med. 1994, 22, 81–85. [Google Scholar] [CrossRef]
- Neumar, R.W.; Bircher, N.G.; Sim, K.M.; Xiao, F.; Zadach, K.S.; Radovsky, A.; Katz, L.; Ebmeyer, E.; Safar, P. Epinephrine and sodium bicarbonate during CPR following asphyxial cardiac arrest in rats. Resuscitation 1995, 29, 249–263. [Google Scholar] [CrossRef]
- Schmued, L.C.; Hopkins, K.J. Fluoro-Jade B: A high affinity fluorescent marker for the localization of neuronal degeneration. Brain Res. 2000, 874, 123–130. [Google Scholar] [CrossRef]
- Hayashida, K.; Bagchi, A.; Miyazaki, Y.; Hirai, S.; Seth, D.; Silverman, M.G.; Rezoagli, E.; Marutani, E.; Mori, N.; Magliocca, A.; et al. Improvement in Outcomes After Cardiac Arrest and Resuscitation by Inhibition of S-Nitrosoglutathione Reductase. Circulation 2019, 139, 815–827. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology 1983, 148, 839–843. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Kjaergaard, J.; Stammet, P.; Wise, M.P.; Hovdenes, J.; Åneman, A.; Horn, J.; Devaux, Y.; Erlinge, D.; Gasche, Y.; et al. Predictive value of interleukin-6 in post-cardiac arrest patients treated with targeted temperature management at 33 °C or 36 °C. Resuscitation 2016, 98, 1–8. [Google Scholar] [CrossRef]
- Peberdy, M.A.; Andersen, L.W.; Abbate, A.; Thacker, L.R.; Gaieski, D.; Abella, B.S.; Grossestreuer, A.V.; Rittenberger, J.C.; Clore, J.; Ornato, J.; et al. Inflammatory markers following resuscitation from out-of-hospital cardiac arrest-A prospective multicenter observational study. Resuscitation 2016, 103, 117–124. [Google Scholar] [CrossRef]
- Oda, Y.; Tsuruta, R.; Kasaoka, S.; Inoue, T.; Maekawa, T. The cutoff values of intrathecal interleukin 8 and 6 for predicting the neurological outcome in cardiac arrest victims. Resuscitation 2009, 80, 189–193. [Google Scholar] [CrossRef]
- Bro-Jeppesen, J.; Kjaergaard, J.; Wanscher, M.; Nielsen, N.; Friberg, H.; Bjerre, M.; Hassager, C. Systemic Inflammatory Response and Potential Prognostic Implications after Out-of-Hospital Cardiac Arrest: A Substudy of the Target Temperature Management Trial. Crit. Care Med. 2015, 43, 1223–1232. [Google Scholar] [CrossRef]
- Jou, C.; Shah, R.; Figueroa, A.; Patel, J.K. The Role of Inflammatory Cytokines in Cardiac Arrest. J. Intensive Care Med. 2020, 35, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Sharp, W.W.; Beiser, D.G.; Fang, Y.H.; Han, M.; Piao, L.; Varughese, J.; Archer, S.L. Inhibition of the mitochondrial fission protein dynamin-related protein 1 improves survival in a murine cardiac arrest model. Crit. Care Med. 2015, 43, e38–e47. [Google Scholar] [CrossRef] [PubMed]
- GenePisano, A.; Di Fraja, D.; Palmieri, C. Monitoring Cerebral Oximetry by Near-Infrared Spectroscopy (NIRS) in Anesthesia and Critical Care: Progress and Perspectives. In General Anesthesia Research; Cascella, M., Ed.; Springer: New York, NY, USA, 2020; pp. 75–96. [Google Scholar]
- Murkin, J.M.; Arango, M. Near-infrared spectroscopy as an index of brain and tissue oxygenation. Br. J. Anaesth. 2009, 103 (Suppl. 1), i3–i13. [Google Scholar] [CrossRef] [PubMed]
- Maconochie, I.K.; Aickin, R.; Hazinski, M.F.; Atkins, D.L.; Bingham, R.; Couto, T.B.; Guerguerian, A.-M.; Nadkarni, V.M.; Ng, K.-C.; Nuthall, G.A.; et al. Pediatric Life Support: 2020 International Consensus on Cardiopulmonary Resuscitation and Emergency Cardiovascular Care Science with Treatment Recommendations. Circulation 2020, 142, S140–S184. [Google Scholar] [CrossRef]
- Bickler, P.E.; Feiner, J.R.; Rollins, M.D. Factors affecting the performance of 5 cerebral oximeters during hypoxia in healthy volunteers. Anesth. Analg. 2013, 117, 813–823. [Google Scholar] [CrossRef]
- Andresen, B.; Greisen, G.; Hyttel-Sorensen, S. Comparison of INVOS 5100C and Nonin SenSmart X-100 oximeter performance in preterm infants with spontaneous apnea. Pediatric Res. 2020, 87, 1244–1250. [Google Scholar] [CrossRef]
- Davie, S.N.; Grocott, H.P. Impact of extracranial contamination on regional cerebral oxygen saturation: A comparison of three cerebral oximetry technologies. Anesthesiology 2012, 116, 834–840. [Google Scholar] [CrossRef]
- Zaouter, C.; Arbeid, E. Influence of ambient light on cerebral oximeters. Br. J. Anaesth. 2010, 105, 873–874. [Google Scholar] [CrossRef][Green Version]
- Vaahersalo, J.; Skrifvars, M.B.; Pulkki, K.; Stridsberg, M.; Røsjø, H.; Hovilehto, S.; Tiainen, M.; Varpula, T.; Pettilä, V.; Ruokonen, E. Admission interleukin-6 is associated with post resuscitation organ dysfunction and predicts long-term neurological outcome after out-of- hospital ventricular fibrillation. Resuscitation 2014, 85, 1573–1579. [Google Scholar] [CrossRef]




| Poor Neurological Outcome Group (n = 21) | Good Neurological Outcome Group (n = 9) | p Value | |
|---|---|---|---|
| Weight, g | 441.0 ± 31.5 | 432.7 ± 27.6 | 0.50 |
| MAP baseline, mmHg | 87.7 ± 13.2 | 82.0 ± 17.0 | 0.12 |
| HR baseline, bpm | 294.4 ± 35.4 | 262.6 ± 24.5 | 0.02 |
| BT baseline, °C | 36.4 ± 0.2 | 36.6 ± 0.2 | 0.004 |
| EtCO2 baseline, mmHg | 38.9 ± 4.0 | 39.8 ± 2.9 | 0.29 |
| rSO2 value baseline, % | 66.6 ± 2.1 | 66.4 ± 1.9 | 0.77 |
| Time to CA, s | 186.3 ± 22.7 | 165.9 ± 26.9 | 0.04 |
| Time to ROSC, s | 80.1 ± 22.5 | 67.7 ± 9.0 | 0.13 |
| MAP 20 min after ROSC, mmHg | 123.2 ± 23.7 | 116.0 ± 14.2 | 0.41 |
| HR 20 min after ROSC, bpm | 335.1 ± 44.2 | 390.1 ± 39.4 | 0.003 |
| BT 20 min after ROSC, | 36.8 ± 0.3 | 36.8 ± 0.3 | 0.69 |
| EtCO2 20 min after ROSC, mmHg | 40.5 ± 4.1 | 43.0 ± 5.9 | 0.19 |
| rSO2 value 20 min after ROSC, % | 62.6 ± 5.3 | 61.0 ± 2.1 | 0.05 |
| MAP 90 min after ROSC, mmHg | 101.2 ± 12.9 | 108.4 ± 8.6 | 0.14 |
| HR 90 min after ROSC, bpm | 331.4 ± 35.7 | 354.4 ± 50.8 | 0.17 |
| BT 90 min after ROSC, | 36.7 ± 0.3 | 36.7 ± 0.4 | 0.96 |
| EtCO2 90 min after ROSC, mmHg | 38.7 ± 10.1 | 37.6 ± 4.4 | 0.66 |
| rSO2 value 90 min after ROSC, % | 60.8 ± 2.8 | 62.7 ± 2.5 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takegawa, R.; Hayashida, K.; Yin, T.; Choudhary, R.C.; Miyara, S.J.; Khalili, H.; Shoaib, M.; Endo, Y.; Molmenti, E.P.; Becker, L.B. Real-Time Brain Monitoring by Near-Infrared Spectroscopy Predicts Neurological Outcome after Cardiac Arrest and Resuscitation in Rats: A Proof of Concept Study of a Novel Prognostic Measure after Cardiac Arrest. J. Clin. Med. 2022, 11, 131. https://doi.org/10.3390/jcm11010131
Takegawa R, Hayashida K, Yin T, Choudhary RC, Miyara SJ, Khalili H, Shoaib M, Endo Y, Molmenti EP, Becker LB. Real-Time Brain Monitoring by Near-Infrared Spectroscopy Predicts Neurological Outcome after Cardiac Arrest and Resuscitation in Rats: A Proof of Concept Study of a Novel Prognostic Measure after Cardiac Arrest. Journal of Clinical Medicine. 2022; 11(1):131. https://doi.org/10.3390/jcm11010131
Chicago/Turabian StyleTakegawa, Ryosuke, Kei Hayashida, Tai Yin, Rishabh C. Choudhary, Santiago J. Miyara, Houman Khalili, Muhammad Shoaib, Yusuke Endo, Emesto P. Molmenti, and Lance B. Becker. 2022. "Real-Time Brain Monitoring by Near-Infrared Spectroscopy Predicts Neurological Outcome after Cardiac Arrest and Resuscitation in Rats: A Proof of Concept Study of a Novel Prognostic Measure after Cardiac Arrest" Journal of Clinical Medicine 11, no. 1: 131. https://doi.org/10.3390/jcm11010131
APA StyleTakegawa, R., Hayashida, K., Yin, T., Choudhary, R. C., Miyara, S. J., Khalili, H., Shoaib, M., Endo, Y., Molmenti, E. P., & Becker, L. B. (2022). Real-Time Brain Monitoring by Near-Infrared Spectroscopy Predicts Neurological Outcome after Cardiac Arrest and Resuscitation in Rats: A Proof of Concept Study of a Novel Prognostic Measure after Cardiac Arrest. Journal of Clinical Medicine, 11(1), 131. https://doi.org/10.3390/jcm11010131







