Differential Effectiveness of Hypothermic Targeted Temperature Management According to the Severity of Post-Cardiac Arrest Syndrome
Abstract
1. History of RCT for the Setting Temperature during TTM
2. Possible Reasons for the Discrepant Results among the RCTs
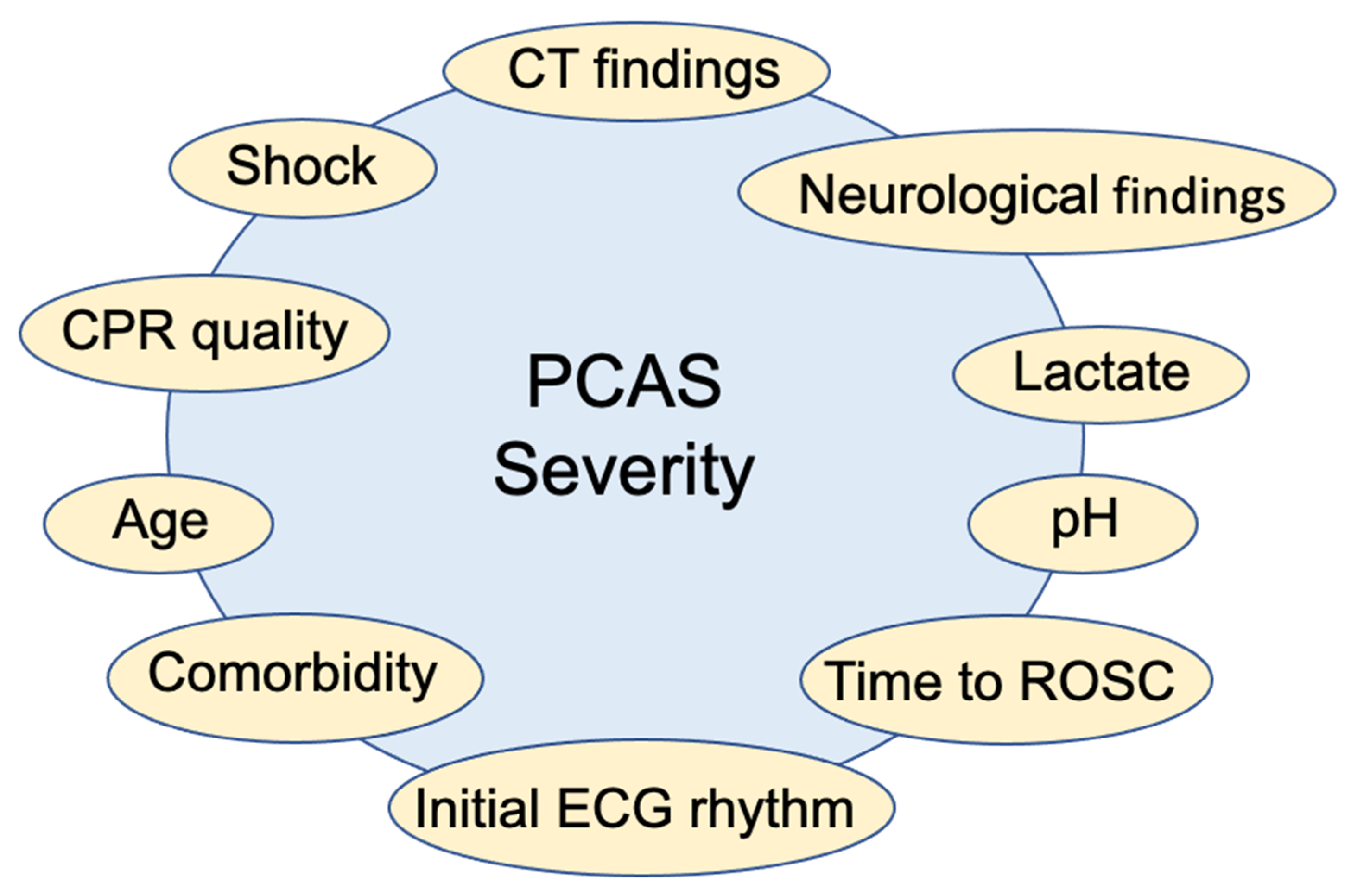
3. Risk Classification for Estimating the PCAS Severity
4. Differential Effectiveness of Hypothermic TTM According to the Severity of PCAS as Assessed by a Single Clinical Factor
5. Differential Effects of Hypothermic TTM According to the Severity of PCAS as Assessed Using a Risk Score Based on Multiple Clinical Factors
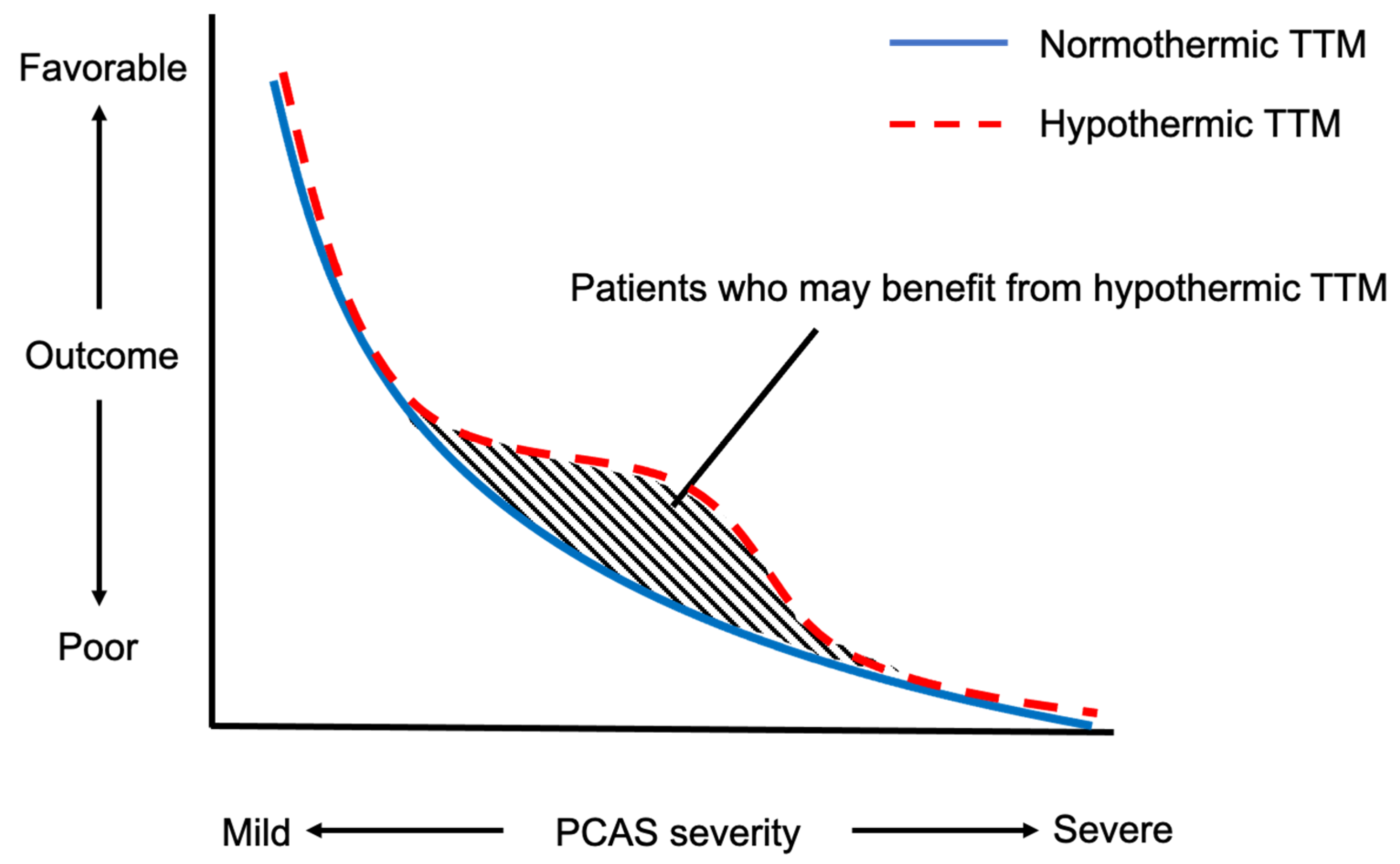
6. Who Are the Most Suitable Candidates for Hypothermic TTM?
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gebhardt, K.; Guyette, F.X.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C.; Post Cardiac Arrest, S. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation 2013, 84, 1062–1067. [Google Scholar] [CrossRef]
- Stub, D.; Bernard, S.; Duffy, S.J.; Kaye, D.M. Post cardiac arrest syndrome: A review of therapeutic strategies. Circulation 2011, 123, 1428–1435. [Google Scholar] [CrossRef]
- Zeiner, A.; Holzer, M.; Sterz, F.; Schorkhuber, W.; Eisenburger, P.; Havel, C.; Kliegel, A.; Laggner, A.N. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch. Intern. Med. 2001, 161, 2007–2012. [Google Scholar] [CrossRef] [PubMed]
- Hypothermia after Cardiac Arrest Study, G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N. Engl. J. Med. 2002, 346, 549–556. [Google Scholar] [CrossRef]
- Bernard, S.A.; Gray, T.W.; Buist, M.D.; Jones, B.M.; Silvester, W.; Gutteridge, G.; Smith, K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N. Engl. J. Med. 2002, 346, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.; Wetterslev, J.; Cronberg, T.; Erlinge, D.; Gasche, Y.; Hassager, C.; Horn, J.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; et al. Targeted temperature management at 33 °C versus 36 °C after cardiac arrest. N. Engl. J. Med. 2013, 369, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Panchal, A.R.; Bartos, J.A.; Cabanas, J.G.; Donnino, M.W.; Drennan, I.R.; Hirsch, K.G.; Kudenchuk, P.J.; Kurz, M.C.; Lavonas, E.J.; Morley, P.T.; et al. Part 3: Adult Basic and Advanced Life Support: 2020 American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation 2020, 142, S366–S468. [Google Scholar] [CrossRef]
- Bray, J.E.; Stub, D.; Bloom, J.E.; Segan, L.; Mitra, B.; Smith, K.; Finn, J.; Bernard, S. Changing target temperature from 33 °C to 36 °C in the ICU management of out-of-hospital cardiac arrest: A before and after study. Resuscitation 2017, 113, 39–43. [Google Scholar] [CrossRef]
- Salter, R.; Bailey, M.; Bellomo, R.; Eastwood, G.; Goodwin, A.; Nielsen, N.; Pilcher, D.; Nichol, A.; Saxena, M.; Shehabi, Y.; et al. Changes in Temperature Management of Cardiac Arrest Patients Following Publication of the Target Temperature Management Trial. Crit. Care Med. 2018, 46, 1722–1730. [Google Scholar] [CrossRef]
- Lascarrou, J.B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.F.; Cariou, A.; Boulain, T.; et al. Targeted Temperature Management for Cardiac Arrest with Nonshockable Rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef]
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullén, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus Normothermia after Out-of-Hospital Cardiac Arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef] [PubMed]
- Le May, M.; Osborne, C.; Russo, J.; So, D.; Chong, A.Y.; Dick, A.; Froeschl, M.; Glover, C.; Hibbert, B.; Marquis, J.F.; et al. Effect of Moderate vs Mild Therapeutic Hypothermia on Mortality and Neurologic Outcomes in Comatose Survivors of Out-of-Hospital Cardiac Arrest: The CAPITAL CHILL Randomized Clinical Trial. JAMA 2021, 326, 1494–1503. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Frisch, A.; Rittenberger, J.C.; Callaway, C.W. Duration of resuscitation efforts and functional outcome after out-of-hospital cardiac arrest: When should we change to novel therapies? Circulation 2013, 128, 2488–2494. [Google Scholar] [CrossRef]
- Wibrandt, I.; Norsted, K.; Schmidt, H.; Schierbeck, J. Predictors for outcome among cardiac arrest patients: The importance of initial cardiac arrest rhythm versus time to return of spontaneous circulation, a retrospective cohort study. BMC Emerg. Med. 2015, 15, 3. [Google Scholar] [CrossRef]
- Grunau, B.; Reynolds, J.; Scheuermeyer, F.; Stenstom, R.; Stub, D.; Pennington, S.; Cheskes, S.; Ramanathan, K.; Christenson, J. Relationship between Time-to-ROSC and Survival in Out-of-hospital Cardiac Arrest ECPR Candidates: When is the Best Time to Consider Transport to Hospital? Prehosp. Emerg. Care 2016, 20, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Momiyama, Y.; Yamada, W.; Miyata, K.; Miura, K.; Fukuda, T.; Fuse, J.; Kikuno, T. Prognostic values of blood pH and lactate levels in patients resuscitated from out-of-hospital cardiac arrest. Acute Med. Surg. 2017, 4, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Al Assil, R.; Singer, J.; Heidet, M.; Fordyce, C.B.; Scheuermeyer, F.; Diepen, S.V.; Sekhon, M.; Leung, K.H.B.; Stenstrom, R.; Christenson, J.; et al. The association of pH values during the first 24h with neurological status at hospital discharge and futility among patients with out-of-hospital cardiac arrest. Resuscitation 2021, 159, 105–114. [Google Scholar] [CrossRef]
- Starodub, R.; Abella, B.S.; Grossestreuer, A.V.; Shofer, F.S.; Perman, S.M.; Leary, M.; Gaieski, D.F. Association of serum lactate and survival outcomes in patients undergoing therapeutic hypothermia after cardiac arrest. Resuscitation 2013, 84, 1078–1082. [Google Scholar] [CrossRef]
- During, J.; Dankiewicz, J.; Cronberg, T.; Hassager, C.; Hovdenes, J.; Kjaergaard, J.; Kuiper, M.; Nielsen, N.; Pellis, T.; Stammet, P.; et al. Lactate, lactate clearance and outcome after cardiac arrest: A post-hoc analysis of the TTM-Trial. Acta Anaesthesiol. Scand. 2018, 62, 1436–1442. [Google Scholar] [CrossRef]
- Choi, S.P.; Park, H.K.; Park, K.N.; Kim, Y.M.; Ahn, K.J.; Choi, K.H.; Lee, W.J.; Jeong, S.K. The density ratio of grey to white matter on computed tomography as an early predictor of vegetative state or death after cardiac arrest. Emerg. Med. J. 2008, 25, 666–669. [Google Scholar] [CrossRef]
- Cristia, C.; Ho, M.L.; Levy, S.; Andersen, L.W.; Perman, S.M.; Giberson, T.; Salciccioli, J.D.; Saindon, B.Z.; Cocchi, M.N.; Donnino, M.W. The association between a quantitative computed tomography (CT) measurement of cerebral edema and outcomes in post-cardiac arrest-a validation study. Resuscitation 2014, 85, 1348–1353. [Google Scholar] [CrossRef] [PubMed]
- Torbey, M.T.; Selim, M.; Knorr, J.; Bigelow, C.; Recht, L. Quantitative analysis of the loss of distinction between gray and white matter in comatose patients after cardiac arrest. Stroke 2000, 31, 2163–2167. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Matsui, K.; Takahashi, K.; Fukaya, K.; Liu, K.; Morita, H.; Nakamura, M.; Matsui, S.; Matsuda, N. Accuracy of the first interpretation of early brain CT images for predicting the prognosis of post-cardiac arrest syndrome patients at the emergency department. J. Intensive Care 2018, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, L.G.; Rovira, A.; Portela, L.A.; Leite Cda, C.; Lucato, L.T. CT and MR in non-neonatal hypoxic-ischemic encephalopathy: Radiological findings with pathophysiological correlations. Neuroradiology 2010, 52, 949–976. [Google Scholar] [CrossRef]
- Lee, K.S.; Lee, S.E.; Choi, J.Y.; Gho, Y.R.; Chae, M.K.; Park, E.J.; Choi, M.H.; Hong, J.M. Useful Computed Tomography Score for Estimation of Early Neurologic Outcome in Post-Cardiac Arrest Patients With Therapeutic Hypothermia. Circ. J. 2017, 81, 1628–1635. [Google Scholar] [CrossRef]
- Kim, W.Y.; Ahn, S.; Hong, J.S.; Cho, G.C.; Seo, D.W.; Jeung, K.W.; Kim, Y.M.; Park, K.N.; Berg, K.; Donnino, M.W. The impact of downtime on neurologic intact survival in patients with targeted temperature management after out-of-hospital cardiac arrest: National multicenter cohort study. Resuscitation 2016, 105, 203–208. [Google Scholar] [CrossRef]
- Kiehl, E.L.; Amuthan, R.; Adams, M.P.; Love, T.E.; Enfield, K.B.; Gimple, L.W.; Cantillon, D.J.; Menon, V. Initial arterial pH as a predictor of neurologic outcome after out-of-hospital cardiac arrest: A propensity-adjusted analysis. Resuscitation 2019, 139, 76–83. [Google Scholar] [CrossRef]
- Adrie, C.; Cariou, A.; Mourvillier, B.; Laurent, I.; Dabbane, H.; Hantala, F.; Rhaoui, A.; Thuong, M.; Monchi, M. Predicting survival with good neurological recovery at hospital admission after successful resuscitation of out-of-hospital cardiac arrest: The OHCA score. Eur. Heart J. 2006, 27, 2840–2845. [Google Scholar] [CrossRef]
- Maupain, C.; Bougouin, W.; Lamhaut, L.; Deye, N.; Diehl, J.L.; Geri, G.; Perier, M.C.; Beganton, F.; Marijon, E.; Jouven, X.; et al. The CAHP (Cardiac Arrest Hospital Prognosis) score: A tool for risk stratification after out-of-hospital cardiac arrest. Eur. Heart J. 2016, 37, 3222–3228. [Google Scholar] [CrossRef] [PubMed]
- Coppler, P.J.; Elmer, J.; Calderon, L.; Sabedra, A.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C.; Dezfulian, C.; Post Cardiac Arrest, S. Validation of the Pittsburgh Cardiac Arrest Category illness severity score. Resuscitation 2015, 89, 86–92. [Google Scholar] [CrossRef]
- Rittenberger, J.C.; Tisherman, S.A.; Holm, M.B.; Guyette, F.X.; Callaway, C.W. An early, novel illness severity score to predict outcome after cardiac arrest. Resuscitation 2011, 82, 1399–1404. [Google Scholar] [CrossRef]
- Gue, Y.X.; Sayers, M.; Whitby, B.T.; Kanji, R.; Adatia, K.; Smith, R.; Davies, W.R.; Perperoglou, A.; Potpara, T.S.; Lip, G.Y.H.; et al. Usefulness of the NULL-PLEASE Score to Predict Survival in Out-of-Hospital Cardiac Arrest. Am. J. Med. 2020, 133, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Kiehl, E.L.; Parker, A.M.; Matar, R.M.; Gottbrecht, M.F.; Johansen, M.C.; Adams, M.P.; Griffiths, L.A.; Dunn, S.P.; Bidwell, K.L.; Menon, V.; et al. C-GRApH: A Validated Scoring System for Early Stratification of Neurologic Outcome After Out-of-Hospital Cardiac Arrest Treated With Targeted Temperature Management. J. Am. Heart Assoc. 2017, 6, e003821. [Google Scholar] [CrossRef]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G.; et al. CAST: A new score for early prediction of neurological outcomes after cardiac arrest before therapeutic hypothermia with high accuracy. Intensive Care Med. 2016, 42, 2106–2107. [Google Scholar] [CrossRef][Green Version]
- Nishikimi, M.; Matsuda, N.; Matsui, K.; Takahashi, K.; Ejima, T.; Liu, K.; Ogura, T.; Higashi, M.; Umino, H.; Makishi, G.; et al. A novel scoring system for predicting the neurologic prognosis prior to the initiation of induced hypothermia in cases of post-cardiac arrest syndrome: The CAST score. Scand. J. Trauma Resusc. Emerg. Med. 2017, 25, 49. [Google Scholar] [CrossRef]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Takahashi, K.; Nakamura, M.; Matsui, S.; Matsuda, N.; Iwami, T. External validation of a risk classification at the emergency department of post-cardiac arrest syndrome patients undergoing targeted temperature management. Resuscitation 2019, 140, 135–141. [Google Scholar] [CrossRef]
- Kaneko, T.; Kasaoka, S.; Nakahara, T.; Sawano, H.; Tahara, Y.; Hase, M.; Nishioka, K.; Shirai, S.; Hazui, H.; Arimoto, H.; et al. Effectiveness of lower target temperature therapeutic hypothermia in post-cardiac arrest syndrome patients with a resuscitation interval of ≤30 min. J. Intensive Care 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Okazaki, T.; Hifumi, T.; Kawakita, K.; Kuroda, Y.; Japanese Association for Acute Medicine out-of-hospital cardiac arrest registry. Targeted temperature management guided by the severity of hyperlactatemia for out-of-hospital cardiac arrest patients: A post hoc analysis of a nationwide, multicenter prospective registry. Ann. Intensive Care 2019, 9, 127. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Takahashi, K.; Fukaya, K.; Liu, K.; Nakamura, M.; Matsui, S.; Matsuda, N. Differential effect of mild therapeutic hypothermia depending on the findings of hypoxic encephalopathy on early CT images in patients with post-cardiac arrest syndrome. Resuscitation 2018, 128, 11–15. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Nielsen, N.; Winther-Jensen, M.; Wanscher, M.; Pellis, T.; Kuiper, M.; Hartvig Thomsen, J.; Wetterslev, J.; Cronberg, T.; Bro-Jeppesen, J.; et al. Impact of time to return of spontaneous circulation on neuroprotective effect of targeted temperature management at 33 or 36° in comatose survivors of out-of hospital cardiac arrest. Resuscitation 2015, 96, 310–316. [Google Scholar] [CrossRef]
- Callaway, C.W.; Coppler, P.J.; Faro, J.; Puyana, J.S.; Solanki, P.; Dezfulian, C.; Doshi, A.A.; Elmer, J.; Frisch, A.; Guyette, F.X.; et al. Association of Initial Illness Severity and Outcomes After Cardiac Arrest With Targeted Temperature Management at 36 °C or 33 °C. JAMA Netw. Open 2020, 3, e208215. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Ogura, T.; Nishida, K.; Hayashida, K.; Emoto, R.; Matsui, S.; Matsuda, N.; Iwami, T. Outcome Related to Level of Targeted Temperature Management in Postcardiac Arrest Syndrome of Low, Moderate, and High Severities: A Nationwide Multicenter Prospective Registry. Crit. Care Med. 2021, 49, e741–e750. [Google Scholar] [CrossRef] [PubMed]
- Nozari, A.; Safar, P.; Stezoski, S.W.; Wu, X.; Kostelnik, S.; Radovsky, A.; Tisherman, S.; Kochanek, P.M. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation 2006, 113, 2690–2696. [Google Scholar] [CrossRef] [PubMed]
- Janata, A.; Bayegan, K.; Sterz, F.; Weihs, W.; Holzer, M.; Sipos, W.; Springler, G.; Behringer, W. Limits of conventional therapies after prolonged normovolemic cardiac arrest in swine. Resuscitation 2008, 79, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients. Available online: https://ClinicalTrials.gov/show/NCT04217551 (accessed on 18 November 2021).
- Engsig, M.; Soholm, H.; Folke, F.; Gadegaard, P.J.; Wiis, J.T.; Molin, R.; Mohr, T.; Engsig, F.N. Similar long-term survival of consecutive in-hospital and out-of-hospital cardiac arrest patients treated with targeted temperature management. Clin. Epidemiol. 2016, 8, 761–768. [Google Scholar] [CrossRef]
- Andersen, L.W.; Holmberg, M.J.; Berg, K.M.; Donnino, M.W.; Granfeldt, A. In-Hospital Cardiac Arrest: A Review. JAMA 2019, 321, 1200–1210. [Google Scholar] [CrossRef] [PubMed]
| Name | Reference | Variables | No of Variables | Simplified or Not (Do Not Need Electric Devices) | Risk Classification (More Than 3 Risk Groups) |
|---|---|---|---|---|---|
| OHCA score | [24] | Initial rhythm, no flow time, low flow time, lactate, creatinine | 5 | Not | Not shown |
| CAHP score | [25] | Age, location of cardiac arrest, initial rhythm, no flow time, low flow time, pH, epinephrin dose | 7 | Not | Low risk (score ≤ 150), Medium risk (score 150–200) High-risk group (score ≥ 200) |
| PCAC | [26,27] | Motor and brain stem scale of FOUR score, cardiovascular and respiratory scale of SOFA score | 4 | Simplified | Mild coma: PCAC 1 Moderate coma and mild cardiopulmonary failure: PCAC 2 Moderate coma and severe cardiopulmonary failure: PCAC 3 Severe coma: PCAC 4 |
| NULL-PLEASE score | [28] | Initial rhythm, age, presence of witness, no flow time, low flow time, pH, lactate, past medical history of ESRD, still resuscitation, extra-cardiac cause | 10 | Simplified | Not shown |
| C-GRApH | [29] | Past medical history of CAD, glucose, initial rhythm, age, pH | 5 | Simplified | Low severity group: ≤1 Medium severity group: 2 and 3 High severity group: ≥4 |
| CAST score | [30,31] | Initial rhythm, presence of witness and time to ROSC, motor scale of GCS, albumin, hemoglobin, pH, lactate, GWR | 8 | Not | Not shown |
| rCAST score | [32] | Initial rhythm, presence of witness and time to ROSC, motor scale of GCS, pH, lactate | 5 | Simplified | Low severity group: ≤5.5 Moderate severity group: between 6.0 and 14.0 High severity group: ≥14.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kikutani, K.; Nishikimi, M.; Shimatani, T.; Kyo, M.; Ohshimo, S.; Shime, N. Differential Effectiveness of Hypothermic Targeted Temperature Management According to the Severity of Post-Cardiac Arrest Syndrome. J. Clin. Med. 2021, 10, 5643. https://doi.org/10.3390/jcm10235643
Kikutani K, Nishikimi M, Shimatani T, Kyo M, Ohshimo S, Shime N. Differential Effectiveness of Hypothermic Targeted Temperature Management According to the Severity of Post-Cardiac Arrest Syndrome. Journal of Clinical Medicine. 2021; 10(23):5643. https://doi.org/10.3390/jcm10235643
Chicago/Turabian StyleKikutani, Kazuya, Mitsuaki Nishikimi, Tatsutoshi Shimatani, Michihito Kyo, Shinichiro Ohshimo, and Nobuaki Shime. 2021. "Differential Effectiveness of Hypothermic Targeted Temperature Management According to the Severity of Post-Cardiac Arrest Syndrome" Journal of Clinical Medicine 10, no. 23: 5643. https://doi.org/10.3390/jcm10235643
APA StyleKikutani, K., Nishikimi, M., Shimatani, T., Kyo, M., Ohshimo, S., & Shime, N. (2021). Differential Effectiveness of Hypothermic Targeted Temperature Management According to the Severity of Post-Cardiac Arrest Syndrome. Journal of Clinical Medicine, 10(23), 5643. https://doi.org/10.3390/jcm10235643






