Cardiovascular and Pre-Frailty Risk Assessment during Shelter-In-Place Measures Based on Multimodal Biomarkers Collected from Smart Telemedical Wearables
Abstract
1. Introduction
2. Clinical Point of View
2.1. Resting Heart Rate
2.2. Sleep Duration and Quality
2.3. Number of Daily Steps
2.4. A Wearable Device for Health Tracking
3. Materials and Methods
3.1. Cardiovascular and Pre-Frailty Risk Assessment (CFRA)
3.2. Study Design
3.3. Methods
3.4. Statistical Analysis
3.5. Definitions
4. Results
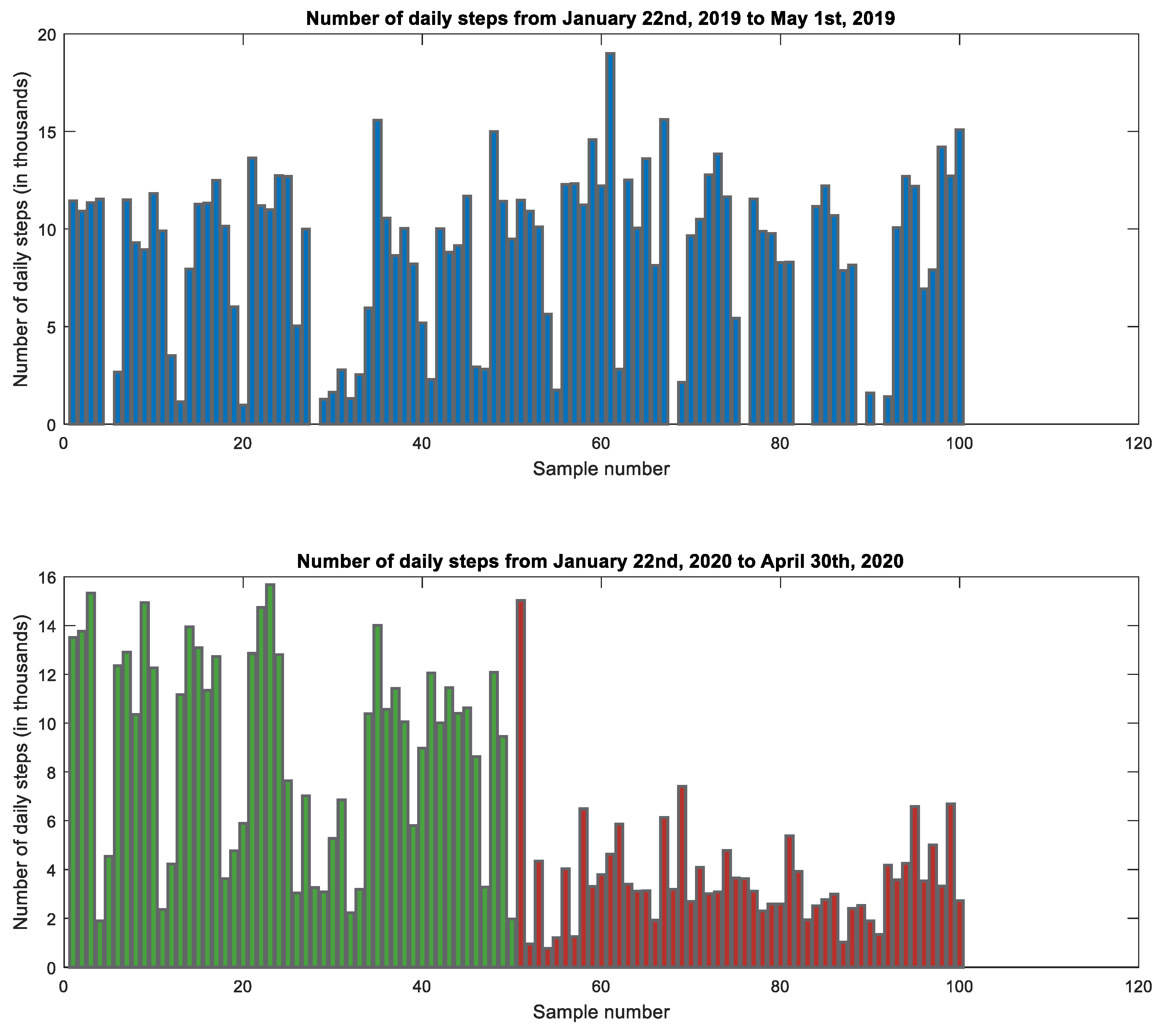
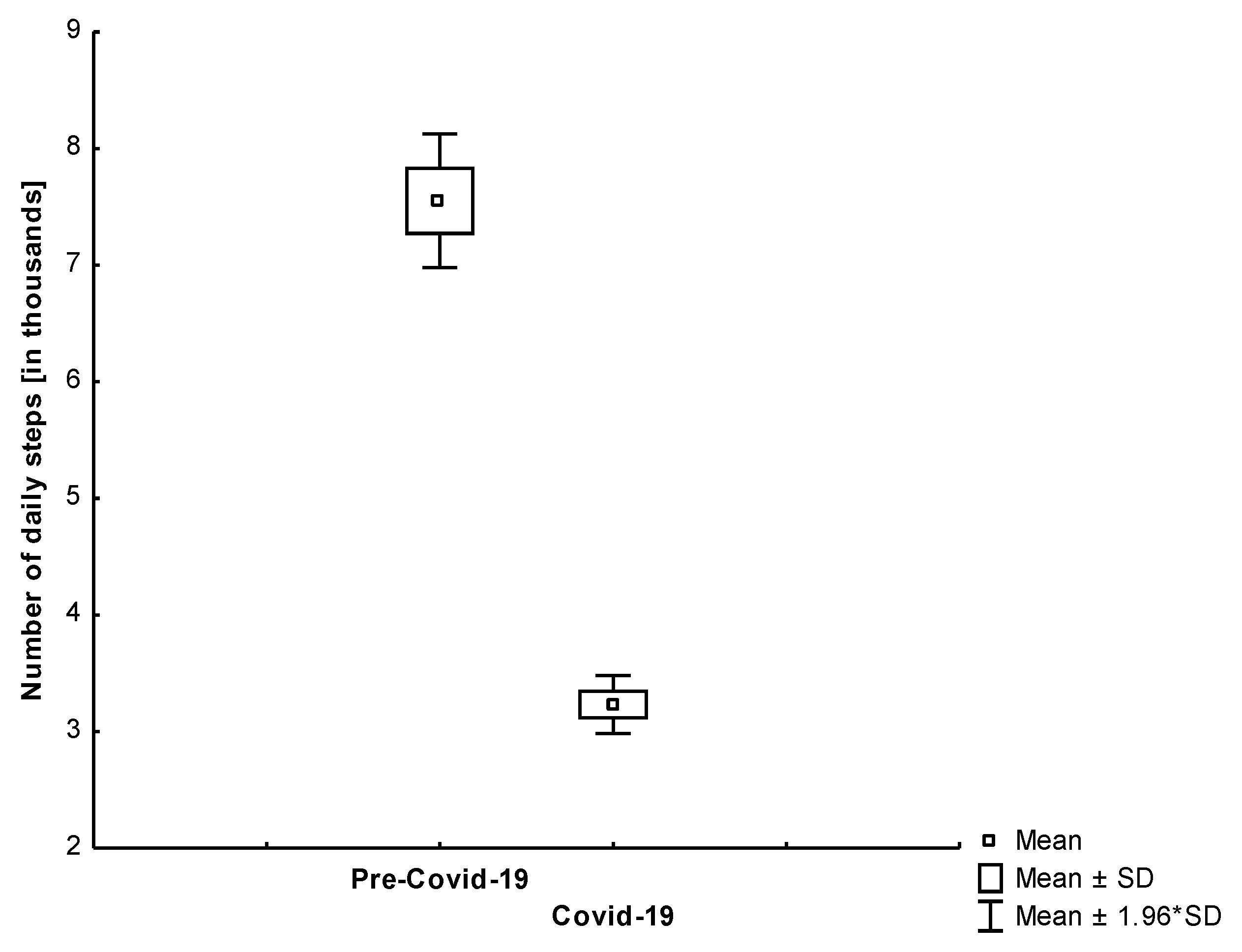
4.1. Number of Daily Steps
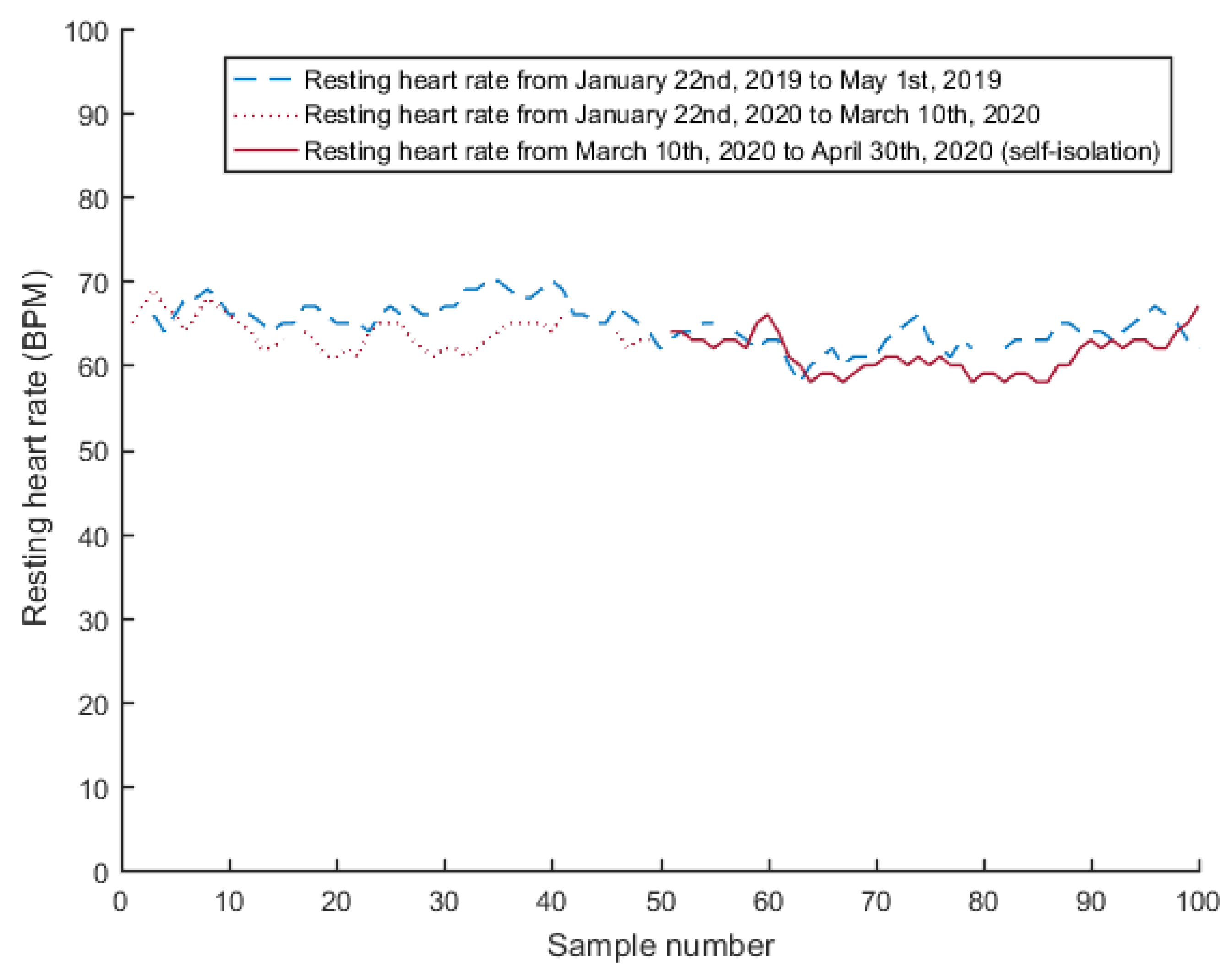
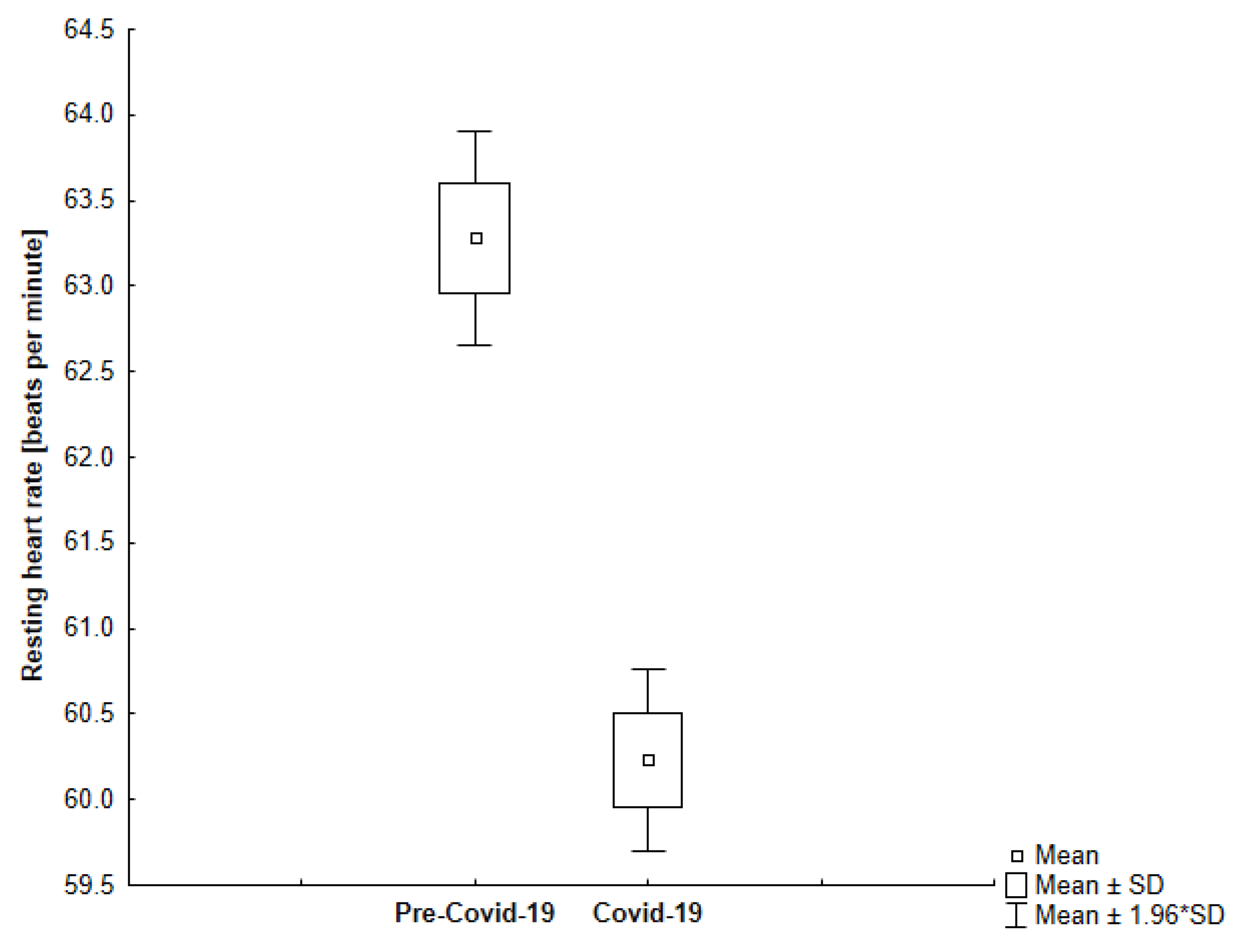
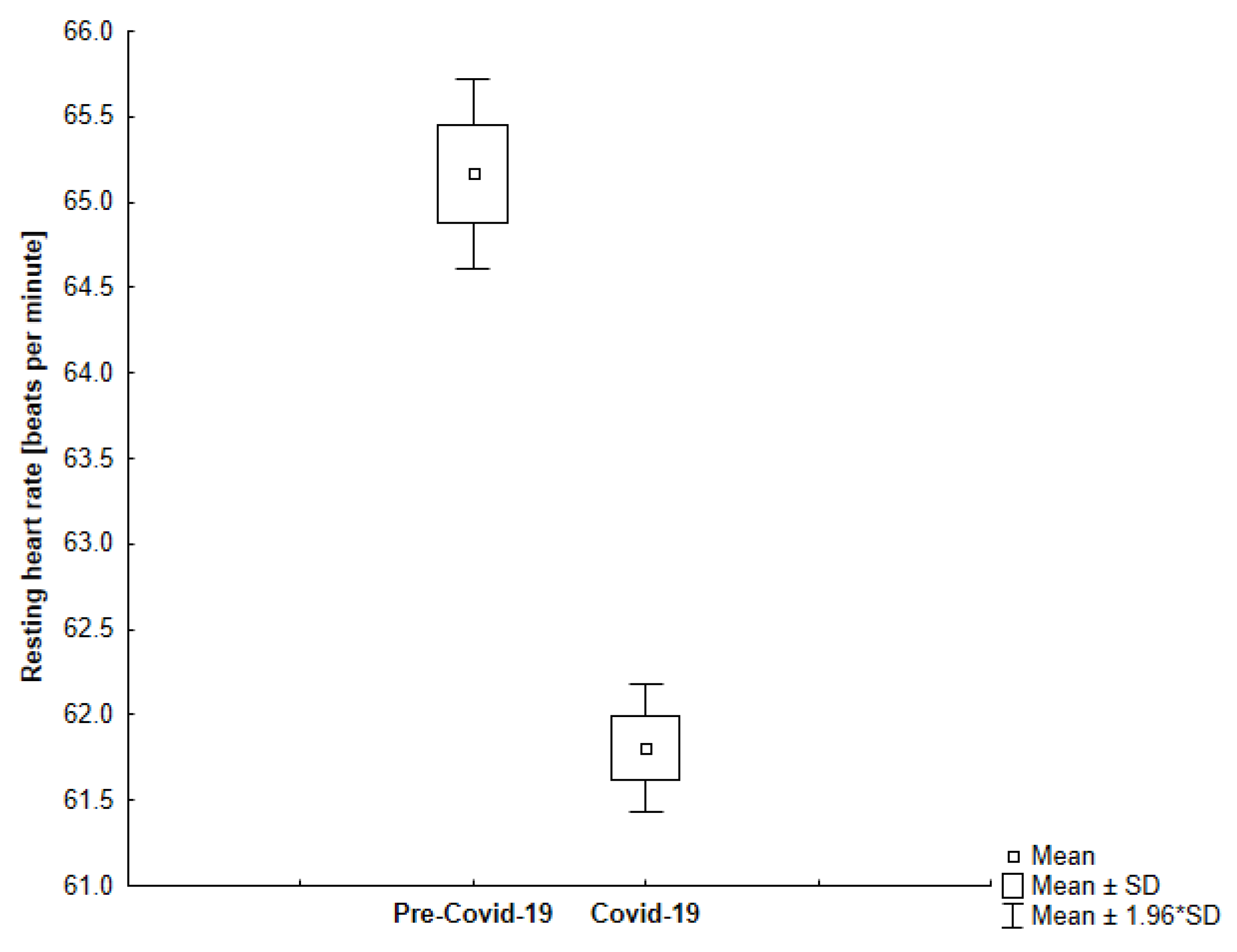
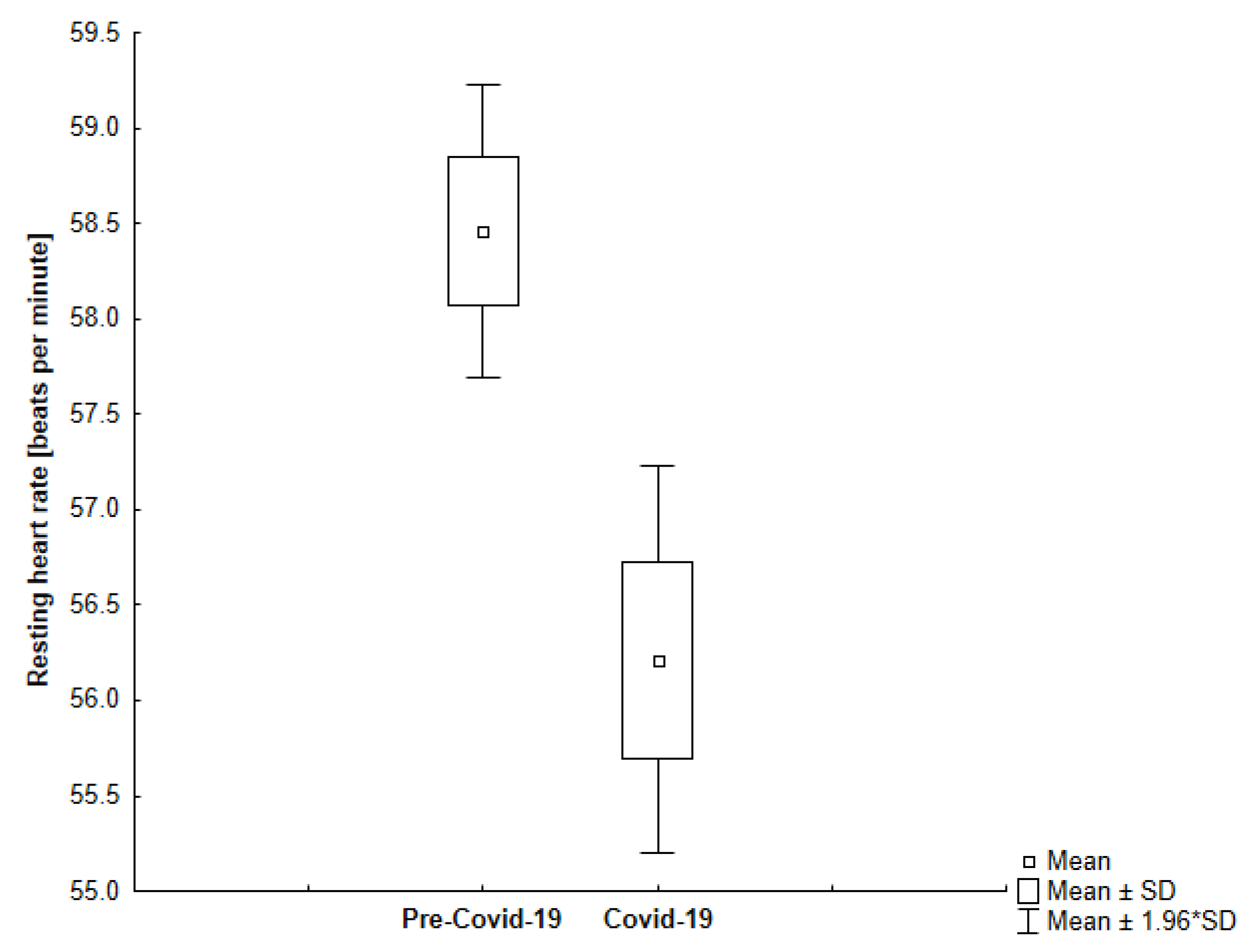
4.2. Resting Heart Rate
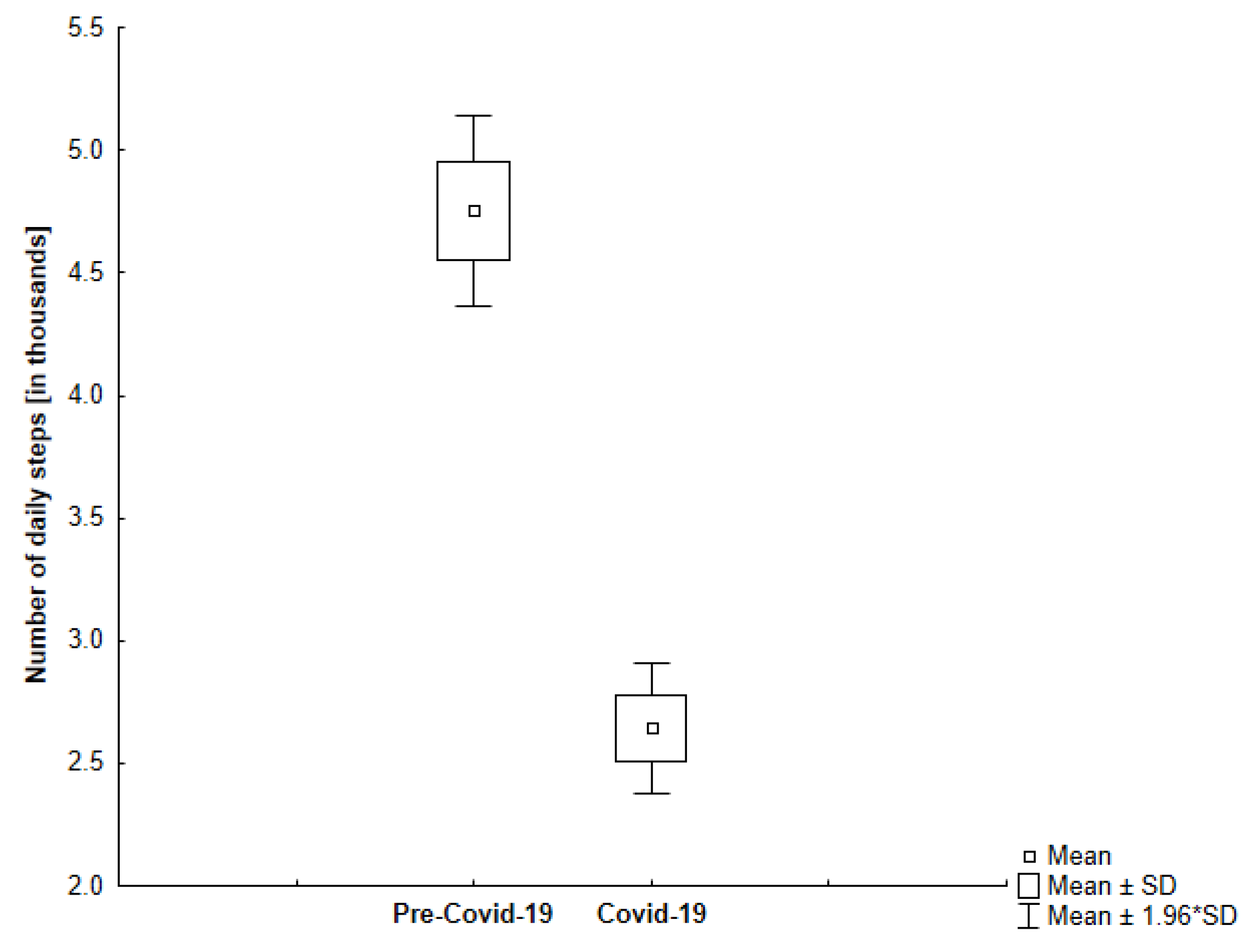
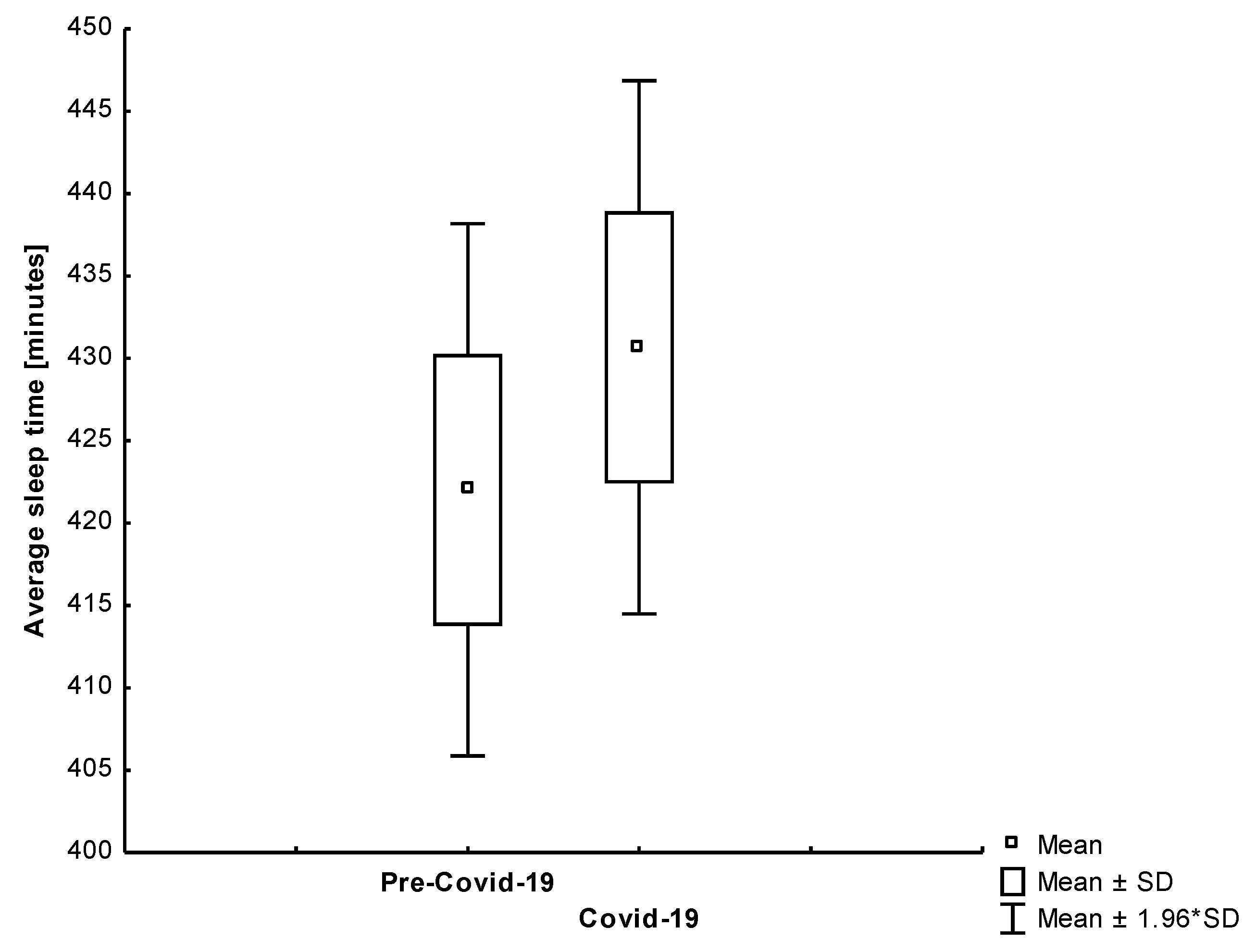
4.3. Sleep Duration and Quality
4.4. Cardiovascular and Pre-Frailty Risk Assessment Scores
5. Discussion
5.1. Smartwatch
5.2. Telemedical Solutions during the Covid-19
5.3. The Post-Covid-19 Face of Telemedical Services
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keesara, S.; Jonas, A.; Schulman, K. Covid-19 and Health Care’s Digital Revolution. N. Engl. J. Med. 2020, 382, e82. [Google Scholar] [CrossRef]
- Sim, I. Mobile Devices and Health. N. Engl. J. Med. 2019, 381, 956–968. [Google Scholar] [CrossRef]
- Kańtoch, E.; Kańtoch, A. What Features and Functions Are Desired in Telemedical Services Targeted at Polish Older Adults Delivered by Wearable Medical Devices?—Pre-COVID-19 Flashback. Sensors 2020, 20, 5181. [Google Scholar] [CrossRef]
- Semaan, S.; Dewland, T.A.; Tison, G.H.; Nah, G.; Vittinghoff, E.; Pletcher, M.J.; Olgin, J.E.; Marcus, G.M. Physical activity and atrial fibrillation: Data from wearable fitness trackers. Heart Rhythm 2020, 17, 842–846. [Google Scholar] [CrossRef] [PubMed]
- Kańtoch, A.; Gryglewska, B.; Wizner, B.; Parnicka, A.; Grodzicki, T. Assumptions of the European FRAILTOOLS project and description of the recruitment process for this study in Poland. Folia Med. Cracov. 2020, 60, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Turner, G.; Clegg, A. Best practice guidelines for the management of frailty: A British Geriatrics Society, Age UK and Royal College of General Practitioners report. Age Ageing 2014, 43, 744–747. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Bandeen-Roche, K.; Seplaki, C.L.; Huang, J.; Buta, B.; Kalyani, R.R.; Varadhan, R.; Xue, Q.L.; Walston, J.D.; Kasper, J.D. Frailty in Older Adults: A Nationally Representative Profile in the United States. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1427–1434. [Google Scholar] [CrossRef]
- Garcia-Garcia, F.J.; Gutierrez Avila, G.; Alfaro-Acha, A.; Amor Andres, M.S.; De Los Angeles De La Torre Lanza, M.; Escribano Aparicio, M.V.; Humanes Aparicio, S.; Larrion Zugasti, J.L.; Gomez-Serranillo Reus, M.; Rodriguez-Artalejo, F.; et al. The prevalence of frailty syndrome in an older population from Spain. The Toledo Study for Healthy Aging. J. Nutr. Health Aging 2011, 15, 852–856. [Google Scholar] [CrossRef]
- Gjaka, M.; Feka, K.; Bianco, A.; Tishukaj, F.; Giustino, V.; Parroco, A.M.; Palma, A.; Battaglia, G. The Effect of COVID-19 Lockdown Measures on Physical Activity Levels and Sedentary Behaviour in a Relatively Young Population Living in Kosovo. J. Clin. Med. 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Kehler, D.S.; Hay, J.L.; Stammers, A.N.; Hamm, N.C.; Kimber, D.E.; Schultz, A.S.; Szwajcer, A.; Arora, R.C.; Tangri, N.; Duhamel, T.A. A systematic review of the association between sedentary behaviors with frailty. Exp. Gerontol. 2018, 114, 1–12. [Google Scholar] [CrossRef]
- ESC Clinical Practice Guidelines: CVD Prevention in Clinical Practice (European Guidelines on) Guidelines. Available online: https://www.escardio.org/Guidelines/Clinical-Practice-Guidelines/CVD-Prevention-in-clinical-practice-European-Guidelines-on (accessed on 1 August 2020).
- Kańtoch, A.; Gryglewska, B.; Wójkowska-Mach, J.; Heczko, P.; Grodzicki, T. Treatment of Cardiovascular Diseases among Elderly Residents of Long-term Care Facilities. J. Am. Med. Dir. Assoc. 2018, 19, 428–432. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef]
- Yuki, A.; Otsuka, R.; Tange, C.; Nishita, Y.; Tomida, M.; Ando, F.; Shimokata, H.; Arai, H. Daily Physical Activity Predicts Frailty Development among Community-Dwelling Older Japanese Adults. J. Am. Med. Dir. Assoc. 2019, 20, 1032–1036. [Google Scholar] [CrossRef]
- Trost, S.G.; Owen, N.; Bauman, A.E.; Sallis, J.F.; Brown, W. Correlates of adults’ participation in physical activity: Review and update. Med. Sci. Sports Exerc. 2002, 34, 1996–2001. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.; Deterding, S.; Kuhn, K.A.; Staneva, A.; Stoyanov, S.; Hides, L. Gamification for health and wellbeing: A systematic review of the literature. Internet Interv. 2016, 6, 89–106. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.; Kerse, N.; Klenk, J.; Borotkanics, R.; Maddison, R. Exergames to Improve the Mobility of Long-Term Care Residents: A Cluster Randomized Controlled Trial. Games Health J. 2018, 7, 37–42. [Google Scholar] [CrossRef]
- Bonnechere, B.; Van Vooren, M.; Jansen, B.; Van Sint, J.S.; Rahmoun, M.; Fourtassi, M. Patients’ Acceptance of the Use of Serious Games in Physical Rehabilitation in Morocco. Games Health J. 2017, 6, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Gopinathannair, R.; Olshansky, B. Management of tachycardia. F1000Prime Rep. 2015, 7, 60. [Google Scholar] [CrossRef]
- Saxena, A.; Minton, D.; Lee, D.C.; Sui, X.; Fayad, R.; Lavie, C.J.; Blair, S.N. Protective role of resting heart rate on all-cause and cardiovascular disease mortality. Mayo Clin. Proc. 2013, 88, 1420–1426. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Shen, X.; Qi, X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. CMAJ 2016, 188, E53–E63. [Google Scholar] [CrossRef]
- Arnold, J.M.; Fitchett, D.H.; Howlett, J.G.; Lonn, E.M.; Tardif, J.C. Resting heart rate: A modifiable prognostic indicator of cardiovascular risk and outcomes? Can. J. Cardiol. 2008, 24 (Suppl. A), 3A–8A. [Google Scholar] [CrossRef]
- Aune, D.; Sen, A.; o’Hartaigh, B.; Janszky, I.; Romundstad, P.R.; Tonstad, S.; Vatten, L.J. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 504–517. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Bin, Y.S. Is Sleep Quality More Important Than Sleep Duration for Public Health? Sleep 2016, 39, 1629–1630. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J.P.; Dutil, C.; Sampasa-Kanyinga, H. Sleeping hours: What is the ideal number and how does age impact this? Nat. Sci. Sleep 2018, 10, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, G.G.; Ayas, N.T. The impact of daily sleep duration on health: A review of the literature. Prog. Cardiovasc. Nurs. 2004, 19, 56–59. [Google Scholar] [CrossRef]
- Cappuccio, F.P.; D’Elia, L.; Strazzullo, P.; Miller, M.A. Sleep duration and all-cause mortality: A systematic review and meta-analysis of prospective studies. Sleep 2010, 33, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, M.; Khormali, M.; Akbarpour, S.; Sadeghniiat-Hagighi, K.; Shamsipour, M. Sleep quality and its association with psychological distress and sleep hygiene: A cross-sectional study among pre-clinical medical students. Sleep Sci. 2018, 11, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.G.; Stinson, K.; Whitaker, K.L.; Moskovitz, D.; Virk, H. The subjective meaning of sleep quality: A comparison of individuals with and without insomnia. Sleep 2008, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Hsu, N.W.; Chou, P. Subgrouping Poor Sleep Quality in Community-Dwelling Older Adults with Latent Class Analysis—The Yilan Study, Taiwan. Sci. Rep. 2020, 10, 5432. [Google Scholar] [CrossRef] [PubMed]
- Le Hello, C.; Trombert, B.; Morel, A.; Chieh, A.; Brouard, B.; Boissier, C. Performance analysis of walking of 10,000 regular users of a connected activity tracker. J. Med. Vasc. 2018, 43, 231–237. [Google Scholar] [CrossRef]
- Saint-Maurice, P.F.; Troiano, R.P.; Bassett, D.R., Jr.; Graubard, B.I.; Carlson, S.A.; Shiroma, E.J.; Fulton, J.E.; Matthews, C.E. Association of Daily Step Count and Step Intensity with Mortality among US Adults. JAMA 2020, 323, 1151–1160. [Google Scholar] [CrossRef]
- King, C.E.; Sarrafzadeh, M. A Survey of Smartwatches in Remote Health Monitoring. J. Health Inf. Res. 2018, 2, 1–24. [Google Scholar] [CrossRef]
- Guk, K.; Han, G.; Lim, J.; Jeong, K.; Kang, T.; Lim, E.K.; Jung, J. Evolution of Wearable Devices with Real-Time Disease Monitoring for Personalized Healthcare. Nanomaterials 2019, 9, 813. [Google Scholar] [CrossRef]
- Kańtoch, E. Recognition of Sedentary Behavior by Machine Learning Analysis of Wearable Sensors during Activities of Daily Living for Telemedical Assessment of Cardiovascular Risk. Sensors 2018, 18, 3219. [Google Scholar] [CrossRef] [PubMed]
- Tudor-Locke, C.; Bassett, D.R., Jr. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004, 34, 1–8. [Google Scholar] [CrossRef]
- Bassett, D.R., Jr.; Toth, L.P.; LaMunion, S.R.; Crouter, S.E. Step Counting: A Review of Measurement Considerations and Health-Related Applications. Sports Med. 2017, 47, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Kado, D.M.; Lui, L.Y.; Cummings, S.R.; Study of Osteoporotic Fractures Research Group. Rapid resting heart rate: A simple and powerful predictor of osteoporotic fractures and mortality in older women. J. Am. Geriatr. Soc. 2002, 50, 455–460. [Google Scholar] [CrossRef]
- World Health Organization. Men, Ageing and Health: Achieving Health Across the Life Span; World Health Organization: Geneva, Switzerland, 2001; pp. 1–63. Available online: https://apps.who.int/iris/bitstream/handle/10665/66941/WHO_NMH_NPH_01.2.pdf;jsessioni (accessed on 22 March 2021).
- Henriksen, A.; Haugen Mikalsen, M.; Woldaregay, A.Z.; Muzny, M.; Hartvigsen, G.; Hopstock, L.A.; Grimsgaard, S. Using Fitness Trackers and Smartwatches to Measure Physical Activity in Research: Analysis of Consumer Wrist-Worn Wearables. J. Med. Internet. Res. 2018, 20, e110. [Google Scholar] [CrossRef]
- Sergi, G.; Veronese, N.; Fontana, L.; De Rui, M.; Bolzetta, F.; Zambon, S.; Corti, M.C.; Baggio, G.; Toffanello, E.D.; Crepaldi, G.; et al. Pre-frailty and risk of cardiovascular disease in elderly men and women: The Pro.V.A. study. J. Am. Coll. Cardiol. 2015, 65, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Mander, B.A.; Winer, J.R.; Walker, M.P. Sleep and Human Aging. Neuron 2017, 94, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Watson, P.L.; Ceriana, P.; Fanfulla, F. Delirium: Is sleep important? Best practice & research. Clin. Anaesthesiol. 2012, 26, 355–366. [Google Scholar] [CrossRef]
- Kukreja, D.; Günther, U.; Popp, J. Delirium in the elderly: Current problems with increasing geriatric age. Indian J. Med. Res. 2015, 142, 655–662. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.S.; Fong, T.G.; Hshieh, T.T.; Inouye, S.K. Delirium in Older Persons: Advances in Diagnosis and Treatment. JAMA 2017, 318, 1161–1174. [Google Scholar] [CrossRef]
- Toosizadeh, N.; Ehsani, H.; Parthasarathy, S.; Carpenter, B.; Ruberto, K.; Mohler, J.; Parvaneh, S. Frailty and heart response to physical activity. Arch. Gerontol. Geriatr. 2021, 93, 104323. [Google Scholar] [CrossRef] [PubMed]
- The Impact of Coronavirus on Global Activity. Available online: https://blog.fitbit.com/covid-19-global-activity/ (accessed on 1 August 2020).
- Are People Actually Sheltering-in-Place? Fitbit Looks at Steps to Find Out. Available online: https://blog.fitbit.com/social-distancing-mobility/ (accessed on 1 August 2020).
- McPhee, J.S.; French, D.P.; Jackson, D.; Nazroo, J.; Pendleton, N.; Degens, H. Physical activity in older age: Perspectives for healthy ageing and frailty. Biogerontology 2016, 17, 567–580. [Google Scholar] [CrossRef] [PubMed]
- The Impact of COVID-19 on Global Sleep Patterns. Available online: https://blog.fitbit.com/covid-19-sleep-patterns/?utm_source=ET&utm_medium=EM&utm_campaign=20200412_WB2_WW_FM_E_EI_FT_00_NA&utm_content=covidsleep+ (accessed on 1 August 2020).
- Omboni, S. Telemedicine during the COVID-19 in Italy: A Missed Opportunity? Telemed. J. e-Health 2020. [Google Scholar] [CrossRef]
- Hamilton, T.; Johnson, L.; Quinn, B.T.; Coppola, J.; Sachs, D.; Migliaccio, J.; Phipps, C.; Schwartz, J.; Capasso, M.; Carpenter, M.; et al. Telehealth Intervention Programs for Seniors: An Observational Study of a Community-Embedded Health Monitoring Initiative. Telemed. J. e-Health 2020, 26, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Zhai, Y.; Song, X.; Wang, Z.; Dou, D.; Li, Y. The Application of Mobile Telehealth System to Facilitate Patient Information Presentation and Case Discussion. Telemed. J. e-Health 2020, 26, 725–733. [Google Scholar] [CrossRef]
- Direito, A.; Tooley, M.; Hinbarji, M.; Albatal, R.; Jiang, Y.; Whittaker, R.; Maddison, R. Tailored Daily Activity: An Adaptive Physical Activity Smartphone Intervention. Telemed. J. e-Health 2020, 26, 426–437. [Google Scholar] [CrossRef]
- Zhou, X.; Snoswell, C.L.; Harding, L.E.; Bambling, M.; Edirippulige, S.; Bai, X.; Smith, A.C. The Role of Telehealth in Reducing the Mental Health Burden from COVID-19. Telemed. J. e-Health 2020, 26, 377–379. [Google Scholar] [CrossRef]
- Omron Health Guide Watch. Available online: https://omronhealthcare.com/products/heartguide-wearable-blood-pressure-monitor-bp8000m/ (accessed on 1 August 2020).

| Biomarker | Scores | Assumptions and References | ||||
|---|---|---|---|---|---|---|
| 0 | 0.5 | 1.0 | 1.5 | 2.0 | ||
| Daily Steps | >12,500 | 10,000–12,500 | 7500–9999 | 5000–7499 | <5000 | [38,39] |
| Resting Heart Rate | <60 bpm | 60–67 bpm | 68–74 bpm | 75–80 bpm | >80 bpm | [21,22,23,40] |
| Sleep Duration | 7–8 h/day | - | - | - | <7 h/day or >8 h/day | [27,28,29] |
| Age | <70 | 70–74 | 75–79 | 80–84 | 85+ | [41] |
| Variable | Pre-Covid-19 | Covid-19 | p-Value | |||
|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | |||
| Resting Heart Rate [bpm] | All | 63.28 | 4.36 | 60.23 | 3.69 | <0.001 |
| Group 1 | 65.17 | 3.28 | 61.80 | 2.18 | <0.001 | |
| Group 2 | 58.46 | 2.81 | 56.21 | 3.73 | <0.001 | |
| Steps Count [results in thousands] | All | 7550 | 4430 | 3230 | 1910 | <0.001 |
| Group 1 | 9800 | 4570 | 3700 | 2160 | <0.001 | |
| Group 2 | 4750 | 2010 | 2640 | 1360 | <0.001 | |
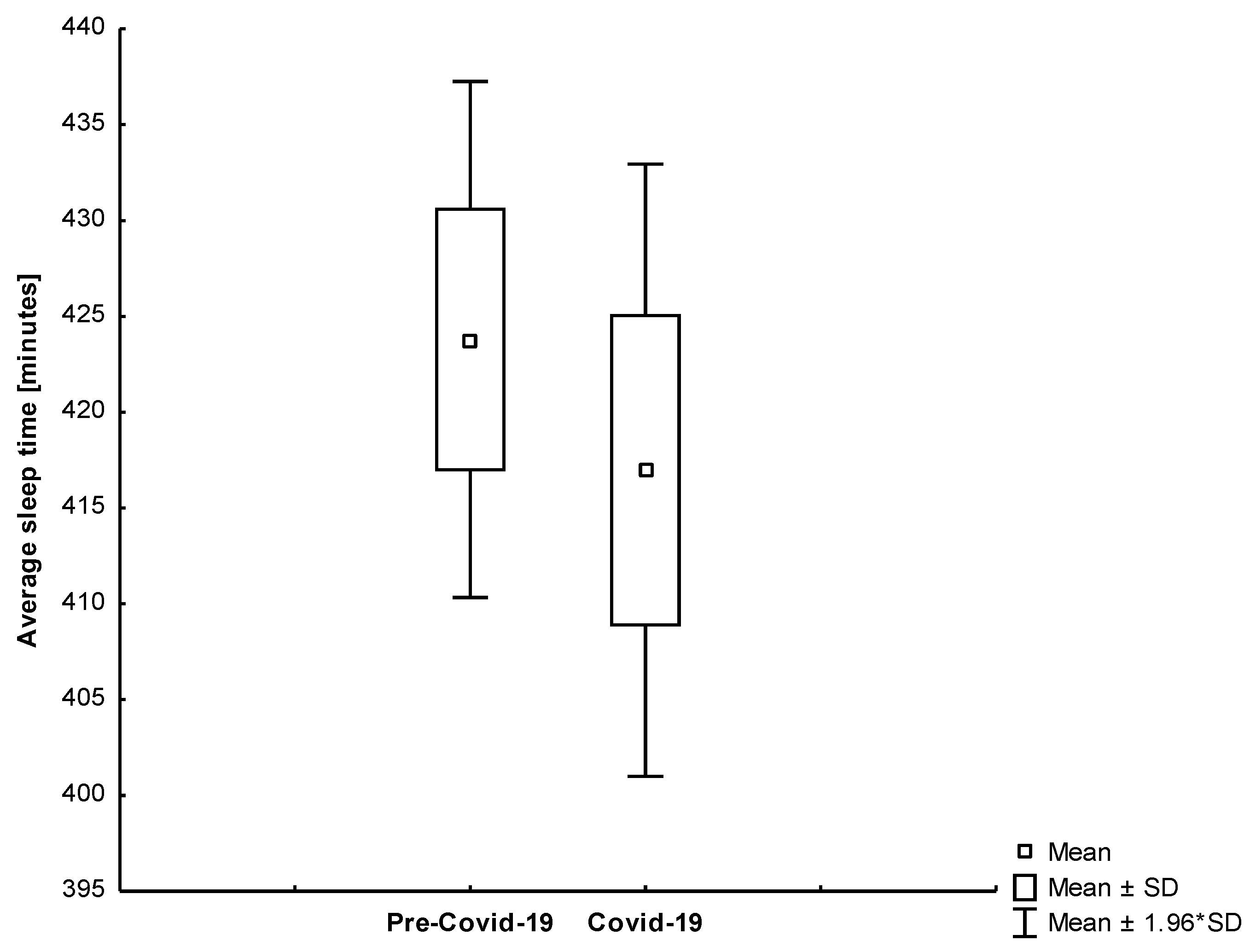
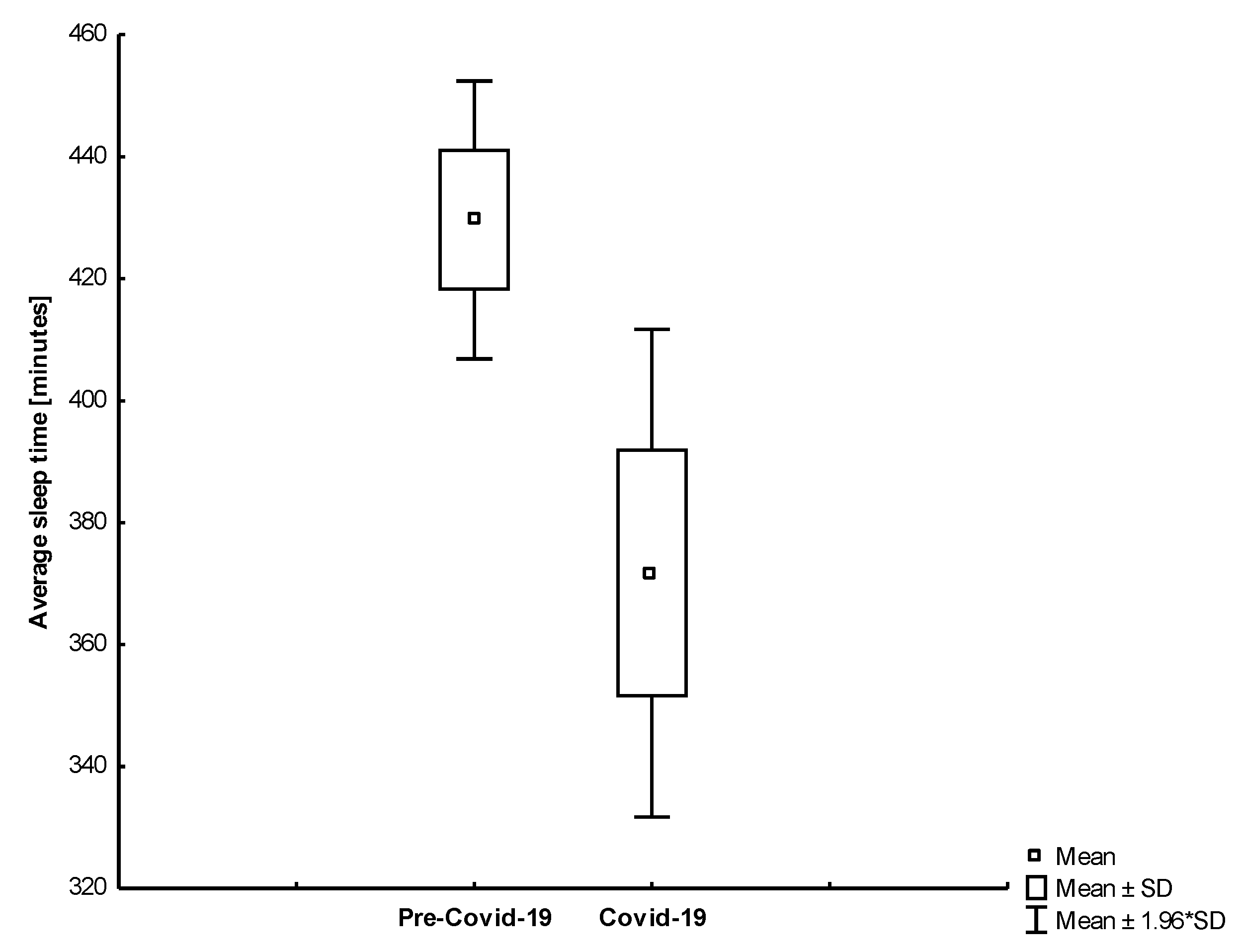
| Sleep Duration [min.] | All | 423.79 | 81.84 | 416.97 | 97.12 | 0.499 |
| Group 1 | 422.02 | 86.07 | 430.67 | 86.21 | 0.441 | |
| Group 2 | 429.64 | 66.80 | 371.73 | 117.19 | 0.008 | |
| Characteristic | Subject ID 1 | Subject ID 2 | Subject ID 3 | Subject ID 4 | Subject ID 5 |
|---|---|---|---|---|---|
| Age | 32 | 82 | 84 | 34 | 57 |
| Gender | Female | Female | Male | Male | Female |
| Race | White | White | White | White | White |
| Marital Status | Single | Married | Married | Single | Divorced |
| Occupational Status | Employment | Pension | Pension | Employment | Employment |
| Number of Household Members | 1 | 3 | 3 | 1 | 3 |
| Current Smoker | No | No | No | No | No |
| Number of Diagnosed Chronic Diseases | 0 | 8 | 9 | 1 | 1 |
| Number of Chronic Medication Used | 0 | 10 | 11 | 1 | 2 |
| History of Cardiovascular Diseases: | |||||
| Hypertension | - | + | + | - | - |
| Chronic Heart Failure | - | + | + | - | - |
| Coronary Heart Disease | - | + | + | - | - |
| Atrial Fibrillation | - | - | + | - | - |
| Dyslipidemia | - | + | + | - | - |
| Type 2 Diabetes | - | + | - | - | - |
| Thyroid Disease | - | + | - | + | - |
| Functional Status: | |||||
| Uses Assistive Device for Ambulation | - | - | - | - | - |
| Able to Ambulate without Assistive Device | + | + | + | + | + |
| Biomarker | Subject ID 1 | Subject ID 2 | Subject ID 3 | Subject ID 4 | Subject ID 5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Reference | Self-Isolation | Reference | Self-Isolation | Reference | Self-Isolation | Reference | Self-Isolation | Reference | Self-Isolation | |
| Daily steps | 0.5 | 2 | 2 | 2 | 2 | 2 | 0.5 | 2 | 0.5 | 2 |
| Resting heart rate | 1 | 0.5 | 0 | 0 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Sleep duration | 0 | 0 | 2 | 2 | 0 | 2 | 0 | 2 | 0 | 0 |
| Age | 0 | 0 | 1.50 | 1.50 | 2 | 2 | 0 | 0 | 0 | 0 |
| Cardiovascular and Pre-Frailty Risk score | 1.5 | 2.5 | 5.5 | 5.5 | 4.5 | 6.5 | 1 | 4.5 | 1 | 2.5 |
| Clinical validation–Cardiovascular and Pre-Frailty Risk | N | N | Y | Y | N | Y | N | N | N | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kańtoch, E.; Kańtoch, A. Cardiovascular and Pre-Frailty Risk Assessment during Shelter-In-Place Measures Based on Multimodal Biomarkers Collected from Smart Telemedical Wearables. J. Clin. Med. 2021, 10, 1997. https://doi.org/10.3390/jcm10091997
Kańtoch E, Kańtoch A. Cardiovascular and Pre-Frailty Risk Assessment during Shelter-In-Place Measures Based on Multimodal Biomarkers Collected from Smart Telemedical Wearables. Journal of Clinical Medicine. 2021; 10(9):1997. https://doi.org/10.3390/jcm10091997
Chicago/Turabian StyleKańtoch, Eliasz, and Anna Kańtoch. 2021. "Cardiovascular and Pre-Frailty Risk Assessment during Shelter-In-Place Measures Based on Multimodal Biomarkers Collected from Smart Telemedical Wearables" Journal of Clinical Medicine 10, no. 9: 1997. https://doi.org/10.3390/jcm10091997
APA StyleKańtoch, E., & Kańtoch, A. (2021). Cardiovascular and Pre-Frailty Risk Assessment during Shelter-In-Place Measures Based on Multimodal Biomarkers Collected from Smart Telemedical Wearables. Journal of Clinical Medicine, 10(9), 1997. https://doi.org/10.3390/jcm10091997






