Abstract
Currently, a bidirectional relationship between the gut microbiota and the nervous system, which is considered as microbiota-gut-brain axis, is being actively studied. This axis is believed to be a key mechanism in the formation of somatovisceral functions in the human body. The gut microbiota determines the level of activation of the hypothalamic–pituitary system. In particular, the intestinal microbiota is an important source of neuroimmune mediators in the pathogenesis of cardiovascular disease. This review reflects the current state of publications in PubMed and Scopus databases until December 2020 on the mechanisms of formation and participation of neuroimmune mediators associated with gut microbiota in the development of cardiovascular disease.
1. Introduction
Cardiovascular disease (CVD) is still the leading cause of death and disability in developed countries all over the world. Approximately one in three deaths in the United States and one in four deaths in European countries are due to CVD [1]. Moreover, the widespread cardiovascular risk factors such as metabolic syndrome, diabetes mellitus, obesity, and sex steroid hormones metabolism disorders define the necessity of searching for strategies that are more effective in the prevention of cardiometabolic disorders [2,3,4]. Recently, remarkable interest has been focused on the role of the human gut microbiota in the pathogenesis of CVD, and modern knowledge allows developing new personalized approaches to the prevention and treatment of CVD.
Atherosclerosis is one of the key factors in the development of CVD. Bacterial DNA presented in the intestinal microbiota was isolated from atherosclerotic plaques [5]. Moreover, another research demonstrates that Akkermansia muciniphila rebuilds the barrier function of the intestines and provides antiatherogenic effect [6]. Changes in the composition of gut microbiota and its metabolic status have also been associated with the risk of CVD development. In particular, trimethylamine N-oxide (TMAO) is considered to be a potential risk factor for atherosclerosis and cardiometabolic diseases [7,8,9]. Some research showed that gastrointestinal microbiota is connected with blood pressure regulation [10,11]. The increase of butyrate-producing bacteria from the genus Odorbacter count is associated with decrease of blood pressure among women with obesity [12,13]. More scientific evidence confirms the role of intestinal microbiota in the pathogenesis of heart failure. It was found that patients with heart failure and peripheral edema had higher concentrations of endotoxins and inflammatory cytokines in blood plasma than patients without edema [14,15].
Animal researches support the connection between obesity and the increase of gastrointestinal bacteria from phyla Firmicutes and Bacteroidetes. The decrease in short-chain fatty acids (SCFA) producing bacteria indicates a disorder of glucose homeostasis [16,17]. Animal studies also confirm that gastrointestinal bacteria regulate blood lipids level in human organisms. Bile acids synthesized by gastrointestinal bacteria, move from the intestine into bloodstream and modulate the hepatic and system metabolism of lipids and glucose [18,19].
Nowadays, the bidirectional relationship between the gastrointestinal microbiota and the nervous system, which is considered as microbiota–gut–brain axis, is being actively studied [20,21,22,23]. In this review, we have reflected the current state of publications in the Pubmed and Scopus databases until December 2020 on the formation mechanisms of neuroimmune mediators of intestinal microbiome origin and their role in the pathogenesis of CVD.
2. Human Intestinal Microbiome Variability
The gut microbiome develops and matures during growing up. The child’s first contact with microbes supposedly should happen after the rupture of the sterile amniotic sac. However, it was determined that the placenta and the infant’s first stool contained a full set of microbes [24,25,26] and it was shown that the traced strain of Enterococcus faecium could enter through the umbilical cord in mice [27]. The way of parturition is one of the key factors which defines the child’s gastrointestinal microbiota composition [28,29,30,31]. However, some authors conclude that during the first 6–12 weeks of life, an infant’s microbiota undergoes a considerable reorganization, which is mainly defined by the child’s living conditions but not by the way of parturition [32,33].
The intestine microbiota of an adult consists of the common bacterial core which includes two basic phyla—Firmicutes and Bacteriodetes, while the other part of the gastrointestinal microbiota is quite diverse. This variety often includes less common samples such as Proteobacteria, Verrumicrobia, Actinobacteria, Fusobacteria, Cyanobacteria, and Archaea [34]. The group number changes through the gastrointestinal tract under the influence of different factors and accessibility of nutrients [35].
Therefore, the difficulty in predicting the pathogenic role of the particular gastrointestinal bacteria is due to many factors, namely, different phases of gastrointestinal microbiota development, widely varied medicament concentrations in the organism, including antibiotics, the level of medicine development, living conditions, co-morbidities, and many others [36,37,38,39,40]. Consequently, any attempts to differentiate any bacteria that are responsible for the development of the disease are associated with a large number of objective difficulties.
3. The Anatomy–Functional Connection between the Autonomic Nervous System and CVD
The functioning of the cardiovascular system (CVS) is under constant and dynamic control of the autonomic nervous system (ANS). ANS adapts CVS to the effects of external and internal environmental factors. The ANS control over the CVS is carried out using cardiovascular neuroaxis which is presented at multiple levels of integrative centers. In particular, the intrinsic cardiac nervous system (ICNS), represented by ganglionated plexi, functions at the level of heart [41]. ICNS is connected with the sympathetic paravertebral ganglia, the extrathoracic cardiac ganglia, and central nervous system as well as with parasympathetic system and provides coordinated response of the heart to different stimuli [42]. Moreover, the mechanism of CVS afferent control by the ANS includes baroreceptors, chemoreceptors, and mechanoreceptors, which are located in the wall of large vessels, primarily in the aorto–carotid zone [43]. The parasympathetic nervous system interacts with ICNS via preganglionic parasympathetic fibers from the cervical vagus nerve, providing a coordinated response in the heart [42,44]. That is, the response of the CVS to external and internal factors is determined by the work and functional status of the ANS. The balance of afferent and efferent reactions of the ANS is carried out to ensure the necessary functional status of the CVS under conditions of rest, physical activity or stress.
Stress is the universal and obligatory component of CVD pathogenesis. Stress accompanies the CVD during the whole period of disease independently from the particular CVD nosology. In different periods, stress can act as an acute attacking factor, which leads to irreversible changes in the organism, organs, and tissues, or it can be a factor, which compensates the organism’s dysfunction during CVD [45,46]. Stress-induced cardiovascular reactions are the result of dynamic regulation of the efferent and afferent pathways of the ANS and, at the same time, the work of the hypothalamus–pituitary–adrenal axis. Cardiovascular changes due to autonomic and neuroendocrine regulation are necessary for the organism to respond to expected or current needs [47]. At the same time, in the course of chronic stress, cardiovascular reactions initiate pathophysiological changes in the CVS, such as the progression of atherosclerosis, the development of hypertension, myocardial infarction, heart remodeling, and others [48,49].
Thus, stress development should be considered as a mechanism which performs the filigree adjustment of an organism’s homeostasis towards the development of irreversible changes or the formation of compensatory and adaptive mechanisms during CVD. Consequently, stress control is a logical and reasonable task in CVD patient treatment.
4. Pathophysiological Mechanisms of Intestinal Barrier Insufficiency during CVD-Associated Stress
The stress associated with CVD affects the whole organism, including the gastrointestinal tract, by activating the sympathetic division of the ANS. Under the influence of ANS, the decreased blood supply to the intestines with the inhabited microbiota reduces the activity of the digestive glands, and intestinal peristalsis in the gastrointestinal tract slows down [50]. The above-mentioned mechanisms determine further disturbance of the intestinal epithelium due to CVD-associated stress (Figure 1).
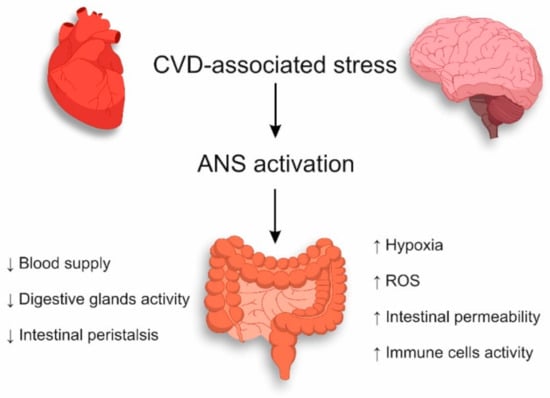
Figure 1.
Mechanisms of intestinal epithelium damage during CVD-associated stress.
The intestinal wall is innervated by adrenergic sympathetic nerve fibers which increase water and natrium absorption during the stimulation [51,52] that accompanies an increase of the intestinal permeability. At the same time, the mucus production by intestinal epithelial goblet cells decreases under the influence of the vagus nerve in the large intestine [53]. It is noteworthy that, on the one hand, mucus forms a protective shield for the intestinal epithelium from commensals and their metabolites and, on the other hand, the mucus blocks an untimely activation of immune cells. Consequently, the reduction of the mucus layer and the increase of the intestinal wall permeability can lead to the disorder of intestinal bacteria and spatial segregation of the intestinal epithelial cells [54,55]. It was shown in Wistar rats that limited nesting stress at early post-natal period leads to hypercorticosteronemia, increased intestinal permeability, and decrease of fecal microbial diversity with disbalance of gut microbiota composition [56]. The decrease of intestinal blood supply is not only due to the effect of the sympathetic division of the ANS but also due to the pathogenetic influence of CVD. It was shown in several studies that blood-supply failure in the intestine accompanies many types of CVD: myocardial infarction, severe atherosclerosis, chronic heart failure, diabetes mellitus, and obesity [57,58,59]. Thus, blood-supply failure in the intestine during CVD is determined simultaneously by several mechanisms. The decrease of intestinal blood supply is accompanied by tissue hypoxia. The intestinal mucus membrane is mostly sensitive to hypoxia [60]. It is an anatomical structure which supports the mucus layer as well as spatial segregation of microbiota from subepithelial tissue. During hypoxia, glucose transformation in aerobic and anaerobic catabolism cycles impairs biological synthesis of energy at intermediate stages. This results in the release of reactive oxygen species (ROS) [61,62]. Reperfusion increases damaging effects of ischemic injury due to accumulation of activated immune cells and generation of ROS [63]. ROS have a high reactivity towards proteins, lipids, carbohydrates, and nucleic acids, that leads to intestinal epithelium integrity damage [64,65]. As for the relationship between gut microbiota and ischemic intestinal injury, it was shown in a rat model that intestinal ischemia–reperfusion injury caused significant changes in the gut microbiome with an increase of the number of Escherichia coli and Prevotella oralis, followed by the enhancing of Lactobacilli in the healing stage [66]. At the same time, it was demonstrated in a model of rats with acute myocardial infarction that gut microbiota alterations cause the development of intestinal inflammation and apoptosis, that is, not only intestinal ischemia leads to imbalance of gut microbiome but vice versa—changes in the microbiome lead to gut damage [67].
In addition to the mucus layer, the intestinal epithelial layer plays an important role in providing functions of the intestinal barrier. Intestinal epithelial layer consists of epithelial cells connected with tight junction proteins, in particular, claudins, occludins, cadherins, and adhesion molecules [68,69]. Tight junction proteins are important as an element in the intestinal barrier in the structure of the gut–brain axis. It was shown that ghrelin, a brain–gut peptide, attenuates intestinal barrier dysfunction activating tight junction proteins zonula occludens-1 and claudin-5 after intracerebral hemorrhage in animal model [70]. Some studies demonstrate the relationship of gut microbiome changes with intestinal barrier injury through alteration of tight junction proteins. For example, Lactobacillus plantarum enhances the epithelial barrier stimulating expression of genes involved in the signaling pathway of tight junctions zonula occludens-1, zonula occludens-2, and occludin [71]. The same effects were demonstrated in the other study in a mice model, where treatment with a mixture of Lactobacillus, Bifidobacterium, and Streptococcus increased the expression of tight junctions zonula occludens-1 and claudins [72]. Alterations of tight junction integrity can lead to increased influx of bacteria or bacterial metabolites associated with an impaired metabolic host status, manifested in cardiometabolic disease [73].
5. Intestinal Microbiota in Neuroimmune Network Formation
The intestinal epithelium and mucus barrier is located between the intestinal environment, intestinal bacteria, and the immune system. It is known that the intestinal epithelial layer includes different types of cells: enterocytes, goblet cells, enteroendocrine cells, Paneth cells, tuft cells, and M-cells [74]. Multiple professional immune cells, such as lymphocytes, dendritic cells, and macrophages, are located in immediate proximity to the surface of the intestinal mucus membrane. Intraepithelial lymphocytes (IELs), the first immune cells responding to pathogenic factors, invade the epithelium and spread dendrites to detect luminal antigens [75]. Other cells are located in organized lymphoid structures, such as Peyer’s patches and cryptopatches, or are dispersed inside the lamina propria [76,77,78,79].
Similarly to professional immune cells, such as macrophages and dendritic cells, intestinal epithelial cells express innate immune receptors like pattern recognition receptors, including Toll-like receptors (TLR) and nucleotide-binding proteins, containing oligomerization domain (NOD). Synthesis of antimicrobial molecules by Paneth cells is regulated by TLR4/MyD88 and NOD2 signal transmissions, which are controlled by intestinal microorganisms [80,81,82]. TLR plays a fundamental role in the innate immune system by activating pro-inflammatory signaling pathways in response to microbial antigens.
Intestinal immune cells support the barrier function of the intestinal mucus membrane through cytokines or with direct cell junctions. So, IL-17 and IL-22 produced by Th17 cells or Type 3 innate lymphoid cells (ILC3), increase AMP and Reg3 family protein secretion by intestinal epithelial cells [83]. Moreover, IL-6 produced by intraepithelial lymphocytes, enhances intestinal epithelial cell proliferation and promotes the repair of the mucus membrane after injury [84]. However, other pro-inflammatory cytokines, such as TNF-α and IFN-γ, inhibit epithelial cell proliferation by the suppression of β-catenin/T cell factor (TCF) signal transmission [85,86].
Intestinal epithelial cells also modulate the host immune response by the secretion of cytokines and chemokines. During the stimulation of the intestinal endothelium with flagellin proteins of the gram-negative bacteria Escherichia and Proteus, TLR5 / MyD88 signaling promotes the production of IL-8, which recruits neutrophils into the lamina propria [87,88]. Cholecystokinin, glucagon-like peptide (GLP), and serotonin are secreted by intestinal endocrine cells and influence the activity of the immune system in the gut [89]. Cholecystokinin regulates the differentiation and production of cytokines by CD4+-cells and B-cells [90,91]. It is notable that the decrease of the activity of digestive glands regulated by the sympathetic nervous system indirectly influences the activity of the immune cells.
It is interesting that the microbiota’s influence on the epithelial intestinal barrier is determined not only by the immune component but also by other effects. In particular, short-chain fatty acids (SCFA) synthesized by gut microbiota are used as a source of energy for the epithelium and strengthen the epithelial barrier indirectly [92,93]. The microbial metabolite indole has a defensive barrier effect through the activation of the pregnane-X receptor and increases the secretion of glucagon-like peptide-1 [94].
The disability to save complex anatomical and functional characteristics of the intestinal epithelium decreases the antimicrobial, immunoregulatory, and regenerative capability of the epithelial barrier. The destruction of the mucous membrane leads to the translocation of the commensal bacteria and their metabolites from the intestine lumina into the subepithelial tissue, leading to the secretion of pro-inflammatory cytokines [95,96]. In turn, this causes the dysfunction of the organ and is accompanied by the inflammation of the intestinal mucosa [97,98].
Today, there are more and more proofs that metabolites of the intestinal bacteria reach the circulation through the destroyed intestinal barrier during inflammation [98,99,100,101]. Thaiss and co-authors highlight three factors affecting microbiome-mediated diseases at the same time [102]. Firstly, metabolites of the intestinal bacteria are permanent activators of the chronic immune reactions, which cause persistent inflammation in the intestine as well as in the whole organism. Secondly, dysbiotic disturbance of intestinal microecology during the maturation period of the innate immune system leads to impaired immunological tolerance, which subsequently manifests in autoimmune and auto-inflammatory disorders. Thirdly, the microbiome can influence immunological factors that control tissue-specific immunity in the distance from intestine. Therefore, the authors conclude that the intestinal microbiome provides pathophysiological mechanisms in normal and pathology. The barrier function of the intestinal epithelium is impaired during CVD, along with a disturbance of the spatial separation of the gut microbiota, synthesis of inflammatory mediators by immune cells, and their further penetration into all tissues of the organism.
Considering the role of the gut microbiota in the formation of the neuroimmune network, it is worth mentioning that the mechanisms underlying the formation of the microbiota–gut–brain axis are being actively studied. Brain-derived neurotrophic factor (BDNF) is a stress protein, a member of the neurotrophin family, which increases the resistance of neurons in the brain to dysfunction and provides the plasticity of the nervous system. A wide spectrum of processes are controlled by BDNF, including the involvement of microbiota–gut–brain axis in the pathogenesis of cardiometabolic disease [103]. It was demonstrated that BDNF signaling may mediate effects of intermittent fasting on glucose regulation and cardiovascular function [104]. Moreover, it was shown that treatment with high doses of probiotics can modulate behavior in Zebrafish, causing significant changes in the expression of some brain-relevant genes, such as BDNF [105]. Thus, BDNF may represent a molecular mechanism underlying the microbiota–gut–brain axis.
6. The Neuroimmune Axis: Microbiota–Intestine–Brain-CVD
Hypoxic injury of the intestinal mucus membrane, microbiota’s displacement to the subepithelial tissue, destruction of the intestinal epithelium’s barrier function, intestinal bacteria metabolites, and inflammation cytokines’ synthesis make the intestine the greatest endotoxin source. Inflammatory mediators reach centers of the nervous system through systemic blood and lymph circulation [67,106,107].
The blood–brain barrier (BBB) forms during gestation and serves as a selective filter between the brain and the blood circulatory system. The importance of the intestinal microbiota and microbial metabolites in the BBB formation was confirmed on gnotobiotic mice. In the absence of the intestinal microorganisms, the mice’s BBB becomes permeable compared with the BBB of normal animals [108].
It was discovered that the lymphatic system of the brain drains to the cerebrospinal fluid, goes to the subarachnoid space, and further travels to deep cervical lymph nodes. The lipids’ solubility, the proteins’ tertiary structure, the concentration, the molecular mass, and the charge of compounds determine the passage of mediators from the peripheral blood supply and the lymphatic system to the brain [109]. Cytokines presented in the peripheral blood are mainly hydrophilic and can modulate immunological functions in the nervous system [109,110]. It was also shown that the intravenous injection of indol, which is similar to the product of bacterial metabolism of tryptophan, allows overcoming the BBB [111]. Neuro-inflammatory effects of LPS act through TLR activation in peripheral tissues, causing secondary effects in the nervous system by BBB-positive pro-inflammatory cytokines [112,113,114].
The BBB and the lymphatic vascular system are considered as the entry for the signals going to the brain. For example, circulating immune cells and inflammation mediators (including hormones and neurotransmitters of both the host and bacteria) along with the vagus nerve stimulation represent mechanisms which contribute to direct or indirect microbial signals’ transmission from the intestine to the brain [115,116,117].
Inflammatory cytokines are also an important factor activating the central nervous system as the response to various stimuli, including pro-inflammatory cytokines which activate the hypothalamic–pituitary–adrenal axis during intestinal pathology. Cortisol and the proinflammatory cytokines interleukin (IL)-6 and IL-8 was significantly increased in patients with irritable bowel syndrome [118]. IL-1α cytokine stimulates the whole glucose metabolism in the organism on the central nervous system level [119]; IL-6, IL-1, TNF-α, and IFN cytokines stimulate the hypothalamic–pituitary–adrenal axis (HPA) independently of each other [120,121,122]. Besides inflammatory cytokines, prostaglandins synthesized in the cyclooxygenase system during the inflammation take part in the HPA axis activation [122]. Multiple researches studied the role of inflammatory cytokines (TNF-α, IL-1, and IL-6) in the HPA axis activation. The injection of any inflammatory cytokines stimulates the HPA axis and leads to an increase of the circulating corticosterone level [120,123]. It is noteworthy that the blockade of any cytokines does not block the HPA axis activation after the penetration of LPS, that is, if the intestinal epithelium barrier function brakes and LPS enters the bloodstream, then the duplicating effect of activation of HPA axis by cytokines is implemented [124,125]. Consequently, all inflammatory mediators promote HPA axis activation, while the blockade of any single cytokine cannot decrease the HPA axis stimulation because of the duplicating effects of each other [122,126].
Thus, the activation of the hypothalamic–pituitary–adrenal axis is one of the fundamental brain-mediated responses to disease. The HPA axis is considered as a basis of the neuroendocrine system that regulates the homeostasis in the organism under the influence of psychological and physical stress, including infections, promoting adequate reaction to the stress [127,128,129].
The considered mechanisms are of great importance in chronic stress. Since the threshold of emotional arousal is insufficient for the formation of stress during CVD, a full value stress response forms in the nervous system with the subsequent activation of the sympathetic division of the ANS through persistent activation of the HPA with inflammatory mediators from the intestine [130]. It is noteworthy that the whole complex of pathological changes in the organism develops by the acute stress pathway, while the emotional component (emotional stimulus) corresponds with the chronic stress threshold or is absent completely. This question requires further study. Therefore, reviewed mechanisms have an activating influence on nervous system centers, including ANS centers, which in turn innervate internal organs, including the intestine inhabited by microbiota.
It was shown that bacterial components of the gut microbiota in patients with attention deficit/hyperactivity disorder (ADHD) are associated with changes in the brain’s structure and functions as well as with behavior reactions [131,132]. The study results were based on intestinal microbiota transplantation.
Taking into account modern knowledge about intestinal microbiota and its connection with the disorder of the nervous system development through the gut–brain axis [133,134,135], it can be concluded that during CVD, mediators from the intestine enter the brain with the bloodstream and lymph and activate hypothalamus nuclei. Then, as far as the hypothalamus is a suprasegmental integral center of the ANS, the sympathetic division of the ANS is activated [136,137]. Thus, mediators from the intestine reach suprasegmentals centers of the ANS and activate the work of sympathetic and parasympathetic divisions, thereby closing the pathological circle of the intestinal microbiota participation in the CVD pathogenesis. Numerous publications demonstrate that an increase in microbiota-mediated inflammatory mediators aggravates the course and prognosis of disease in CVD [138,139,140,141]. It is also found that the correction of the intestinal microbiota in patients with CVD improves the prognosis of the disease. Prescribing in the complex therapy preparations that increase the number of bacteria from genus Akkermansia, Bifidobacteria, Lactobacillus, Bacteroides, and Prevotella ameliorates the course of CVD [142,143,144]. It is known that bacteria from Bifidobacteria and Lactobacillus genus provide a local anti-inflammatory effect on the intestinal wall. The recovery of the barrier function of intestinal epithelium occurs because of the decrease of inflammation in the intestinal wall, meaning that the level of inflammatory mediators decreases in systemic circulation and consequently their activating effect on the nervous system reduces [145,146,147]. In particular, hypertension is associated with disturbance of gut microbiome and dysregulation of gut–brain axis. It was demonstrated in a model of hypertensive rats that long-term kefir treatment reduced IL-6 and TNF-α protein density and abolished the microglial activation observed in the hypothalamic paraventricular nucleus and rostral ventrolateral medulla defending cardioregulatory nuclei from gut-mediated inflammation that provides the hypotensive effect of kefir [148]. Some studies in mice model of ischemic stroke or cerebral ischemia demonstrate that ischemic stroke brain injury promotes the development of gut dysbiosis with increase pro-inflammatory responses and infiltration of brain structures with cytokines, chemokines and immune cells that is associated with poor prognosis [149].
The mechanisms considered in the present review form a pathologic vicious circle where intestinal microbiota is involved in the pathogenesis of CVD and determines the inflammatory activation of HPA axis (Figure 2).
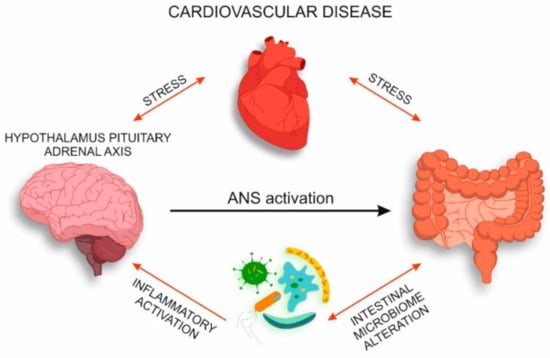
Figure 2.
Interaction of intestinal microbiota and nervous system in CVD.
Some studies investigated that microbiome-targeted preparations ameliorate the course of CVD, decrease the progression of atherosclerosis and risk of major CVD complications [150,151]. In the context of this review, we can suppose that beneficial cardioprotective mechanisms of microbiome-based treatment are due to its influence on the microbiome–gut–brain axis. It was shown in rats with induced myocardial infarction by occluding the left anterior coronary artery, administration of probiotics based on combination of Lactobacillus helveticus and Bifidobacterium longum reduces Bax/Bcl-2 (pro-apoptotic/anti-apoptotic) ratio and caspase-3 (pro-apoptotic) activity in the amygdala and dentate gyrus in comparison with the placebo group decreasing the predisposition of apoptosis in different cerebral regions associated with myocardial infarction [152]. Another study in mice demonstrates that antibiotic administration modulating gut microbiota reduces LPS levels and neuroinflammation in the ischemic brain after experimental stroke [153]. A study in patients with coronary artery disease found that probiotic Lactobacillus Rhamnosus in complex with prebiotic inulin provides beneficial effects on depression, anxiety, and inflammatory biomarkers [154].
7. Conclusions
The literature review elucidates the potential neuroimmune role of the intestinal microbiome in the pathogenesis of CVD. At the initial stage of CVD the role of the intestinal microbiota in its pathogenesis is of secondary significance, meaning that the qualitative and quantitative changes in bacteria are not as important as at the following stages. However, later, when the intestinal microbiota determines the level of inflammatory activation of the hypothalamus–pituitary–adrenal axis, changes in the intestinal microbiota become significant for CVD development. During CVD progression intestinal bacteria are in close interaction with the pathologic processes developing in the intestinal wall and become one of the key elements in CVD pathogenesis. In this regard, attempts to determine the intestinal bacteria most involved in the process of CVD progression can be an important step for the development of new relevant methods for the diagnosis, prevention, and therapy of CVD.
Author Contributions
Conceptualization and design the review, A.Y.P. and A.V.S.; bibliographic research, A.V.S., E.C., M.D.S. and I.S.S.; writing—original draft preparation, A.V.S., T.V.K., E.C. and I.S.S.; figure design, T.V.K. and V.A.K.; review final version approval, M.D.S. and A.V.S.; supervision, T.V.K., A.Y.P. and V.A.K.; funding acquisition, A.Y.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by RUSSIAN SCIENCE FOUNDATION, grant number 20-45-08002.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sanchis-Gomar, F.; Perez-Quilis, C.; Leischik, R.; Lucia, A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann. Transl. Med. 2016, 4, 256. [Google Scholar] [CrossRef]
- Tang, W.H.; Kitai, T.; Hazen, S.L. Gut Microbiota in Cardiovascular Health and Disease. Circ. Res. 2017, 120, 1183–1196. [Google Scholar] [CrossRef]
- Gerdts, E.; Regitz-Zagrosek, V. Sex differences in cardiometabolic disorders. Nat. Med. 2019, 25, 1657–1666. [Google Scholar] [CrossRef]
- Ambatiello, L.G.; Chazova, I.E. Cardiovascular and chronic obstructive pulmonary diseases: Pathophysiological processes and treatment tactics. Ter. Arkhiv 2020, 92, 78–83. [Google Scholar] [CrossRef]
- Koren, O.; Spor, A.; Felin, J.; Fåk, F.; Stombaugh, J.; Tremaroli, V.; Behre, C.J.; Knight, R.; Fagerberg, B.; Ley, R.E.; et al. Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 1), 4592–4598. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Li, H. The Role of Gut Microbiota in Atherosclerosis and Hypertension. Front. Pharmacol. 2018, 9, 1082. [Google Scholar] [CrossRef]
- Brown, J.M.; Hazen, S.L. The gut microbial endocrine organ: Bacterially derived signals driving cardiometabolic diseases. Annu. Rev. Med. 2015, 66, 343–359. [Google Scholar] [CrossRef] [PubMed]
- Senthong, V.; Wang, Z.; Fan, Y.; Wu, Y.; Hazen, S.L.; Tang, W.H. Trimethylamine N-Oxide and Mortality Risk in Patients with Peripheral Artery Disease. J. Am. Heart Assoc. 2016, 5, e004237. [Google Scholar] [CrossRef] [PubMed]
- Kirichenko, T.V.; Markina, Y.V.; Sukhorukov, V.N.; Khotina, V.A.; Wu, W.-K.; Orekhov, A.N. A Novel Insight at Atherogenesis: The Role of Microbiome. Front. Cell Dev. Biol. 2020, 8, 586189. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Santisteban, M.M.; Rodriguez, V.; Li, E.; Ahmari, N.; Carvajal, J.M.; Zadeh, M.; Gong, M.; Qi, Y.; Zubcevic, J.; et al. Gut dysbiosis is linked to hypertension. Hypertension 2015, 65, 1331–1340. [Google Scholar] [CrossRef]
- Louca, P.; Menni, C.; Padmanabhan, S. Genomic Determinants of Hypertension with a Focus on Metabolomics and the Gut Microbiome. Am. J. Hypertens. 2020, 33, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Arango, L.F.; Barrett, H.L.; McIntyre, H.D.; Callaway, L.K.; Morrison, M.; Dekker Nitert, M.; SPRING Trial Group. Increased Systolic and Diastolic Blood Pressure Is Associated with Altered Gut Microbiota Composition and Butyrate Production in Early Pregnancy. Hypertension 2016, 68, 974–981. [Google Scholar] [CrossRef]
- Chi, C.; Li, C.; Wu, D.; Buys, N.; Wang, W.; Fan, H.; Sun, J. Effects of Probiotics on Patients with Hypertension: A Systematic Review and Meta-analysis. Curr. Hypertens. Rep. 2020, 22, 33. [Google Scholar] [CrossRef]
- Niebauer, J.; Volk, H.D.; Kemp, M.; Dominguez, M.; Schumann, R.R.; Rauchhaus, M.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. Endotoxin and immune activation in chronic heart failure: A prospective cohort study. Lancet 1999, 353, 1838–1842. [Google Scholar] [CrossRef]
- Ameri, P.; Schiattarella, G.G.; Crotti, L.; Torchio, M.; Bertero, E.; Rodolico, D.; Forte, M.; Di Mauro, V.; Paolillo, R.; Chimenti, C.; et al. Novel Basic Science Insights to Improve the Management of Heart Failure: Review of the Working Group on Cellular and Molecular Biology of the Heart of the Italian Society of Cardiology. Int. J. Mol. Sci. 2020, 21, 1192. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial ecology: Human gut microbes associated with obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Samuel, B.S.; Shaito, A.; Motoike, T.; Rey, F.E.; Backhed, F.; Manchester, J.K.; Hammer, R.E.; Williams, S.C.; Crowley, J.; Yanagisawa, M.; et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl. Acad. Sci. USA 2008, 105, 16767–16772. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Houten, S.M.; Mataki, C.; Christoffolete, M.A.; Kim, B.W.; Sato, H.; Messaddeq, N.; Harney, J.W.; Ezaki, O.; Kodama, T.; et al. Bile acids induce energy expenditure by promoting intracellular thyroid hormone activation. Nature 2006, 439, 484–489. [Google Scholar] [CrossRef]
- Chait, A.; den Hartigh, L.J. Adipose Tissue Distribution, Inflammation and Its Metabolic Consequences, Including Diabetes and Cardiovascular Disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Gudasheva, T.A.; Povarnina, P.Y.; Volkova, A.A.; Kruglov, S.V.; Antipova, T.A.; Seredenin, S.B. A Nerve Growth Factor Dipeptide Mimetic Stimulates Neurogenesis and Synaptogenesis in the Hippocampus and Striatum of Adult Rats with Focal Cerebral Ischemia. Acta Nat. 2019, 11, 31–37. [Google Scholar] [CrossRef]
- Labus, J.S.; Osadchiy, V.; Hsiao, E.Y.; Tap, J.; Derrien, M.; Gupta, A.; Tillisch, K.; Le Nevé, B.; Grinsvall, C.; Ljungberg, M.; et al. Evidence for an association of gut microbial Clostridia with brain functional connectivity and gastrointestinal sensorimotor function in patients with irritable bowel syndrome, based on tripartite network analysis. Microbiome 2019, 7, 45. [Google Scholar] [CrossRef] [PubMed]
- Obrenovich, M.; Tabrez, S.; Siddiqui, B.; McCloskey, B.; Perry, G. The Microbiota-Gut-Brain Axis-Heart Shunt Part II: Prosaic Foods and the Brain-Heart Connection in Alzheimer Disease. Microorganisms 2020, 8, 493. [Google Scholar] [CrossRef]
- Obrenovich, M.; Flückiger, R.; Sykes, L.; Donskey, C. The Co-Metabolism within the Gut-Brain Metabolic Interaction: Potential Targets for Drug Treatment and Design. CNS Neurol. Disord. Drug Targets 2016, 15, 127–134. [Google Scholar] [CrossRef]
- Aagaard, K.; Ma, J.; Antony, K.M.; Ganu, R.; Petrosino, J.; Versalovic, J. The placenta harbors a unique microbiome. Sci. Transl. Med. 2014, 6, 237ra65. [Google Scholar] [CrossRef]
- Dobbler, P.; Mai, V.; Procianoy, R.S.; Silveira, R.C.; Corso, A.L.; Roesch, L.F.W. The vaginal microbial communities of healthy expectant Brazilian mothers and its correlation with the newborn’s gut colonization. World J. Microbiol. Biotechnol. 2019, 35, 159. [Google Scholar] [CrossRef]
- DiGiulio, D.B.; Romero, R.; Amogan, H.P.; Kusanovic, J.P.; Bik, E.M.; Gotsch, F.; Kim, C.J.; Erez, O.; Edwin, S.; Relman, D.A. Microbial prevalence, diversity and abundance in amniotic fluid during preterm labor: A molecular and culture-based investigation. PLoS ONE 2008, 3, e3056. [Google Scholar] [CrossRef]
- Jiménez, E.; Fernández, L.; Marín, M.L.; Martín, R.; Odriozola, J.M.; Nueno-Palop, C.; Narbad, A.; Olivares, M.; Xaus, J.; Rodríguez, J.M. Isolation of commensal bacteria from umbilical cord blood of healthy neonates born by cesarean section. Curr. Microbiol. 2005, 51, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Bello, M.G.; Costello, E.K.; Contreras, M.; Magris, M.; Hidalgo, G.; Fierer, N.; Knight, R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc. Natl. Acad. Sci. USA 2010, 107, 11971–11975. [Google Scholar] [CrossRef] [PubMed]
- Rutayisire, E.; Huang, K.; Liu, Y.; Tao, F. The mode of delivery affects the diversity and colonization pattern of the gut microbiota during the first year of infants’ life: A systematic review. BMC Gastroenterol. 2016, 16, 86. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.M.; Ma, J.; Prince, A.L.; Antony, K.M.; Seferovic, M.D.; Aagaard, K.M. Maturation of the infant microbiome community structure and function across multiple body sites and in relation to mode of delivery. Nat. Med. 2017, 23, 314–326. [Google Scholar] [CrossRef]
- Roduit, C.; Scholtens, S.; de Jongste, J.C.; Wijga, A.H.; Gerritsen, J.; Postma, D.S.; Brunekreef, B.; Hoekstra, M.O.; Aalberse, R.; Smit, H.A. Asthma at 8 years of age in children born by caesarean section. Thorax 2009, 64, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Feldman, A.S.; Rosas-Salazar, C.; James, K.; Escobar, G.; Gebretsadik, T.; Li, S.X.; Carroll, K.N.; Walsh, E.; Mitchel, E.; et al. Relative Importance and Additive Effects of Maternal and Infant Risk Factors on Childhood Asthma. PLoS ONE 2016, 11, e0151705, Erratum in 2016, 11, e0156473. [Google Scholar] [CrossRef] [PubMed]
- Tanoey, J.; Gulati, A.; Patterson, C.; Becher, H. Risk of Type 1 Diabetes in the Offspring Born through Elective or Non-elective Caesarean Section in Comparison to Vaginal Delivery: A Meta-Analysis of Observational Studies. Curr. Diabetes Rep. 2019, 19, 124. [Google Scholar] [CrossRef]
- Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature 2012, 486, 207–214. [Google Scholar] [CrossRef]
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Sekirov, I.; Tam, N.M.; Jogova, M.; Robertson, M.L.; Li, Y.; Lupp, C.; Finlay, B.B. Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect. Immun. 2008, 76, 4726–4736. [Google Scholar] [CrossRef] [PubMed]
- Azad, M.B.; Bridgman, S.L.; Becker, A.B.; Kozyrskyj, A.L. Infant antibiotic exposure and the development of childhood overweight and central adiposity. Int. J. Obes. (Lond.) 2014, 38, 1290–1298. [Google Scholar] [CrossRef]
- Gough, E.K.; Moodie, E.E.; Prendergast, A.J.; Johnson, S.M.; Humphrey, J.H.; Stoltzfus, R.J.; Walker, A.S.; Trehan, I.; Gibb, D.M.; Goto, R.; et al. The impact of antibiotics on growth in children in low and middle income countries: Systematic review and meta-analysis of randomised controlled trials. BMJ 2014, 348, g2267. [Google Scholar] [CrossRef] [PubMed]
- Becattini, S.; Taur, Y.; Pamer, E.G. Antibiotic-Induced Changes in the Intestinal Microbiota and Disease. Trends Mol. Med. 2016, 22, 458–478. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A.; Murphy, D.A.; Yuan, B.X.; Macdonald, S.; Hopkins, D.A. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat. Rec. 1997, 247, 289–298. [Google Scholar] [CrossRef]
- Ardell, J.L.; Rajendran, P.S.; Nier, H.A.; KenKnight, B.H.; Armour, J.A. Central-peripheral neural network interactions evoked by vagus nerve stimulation: Functional consequences on control of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1740–H1752. [Google Scholar] [CrossRef] [PubMed]
- Kember, G.C.; Armour, J.A.; Zamir, M. Mechanism of smart baroreception in the aortic arch. Phys. Rev. E Stat. Nonlinear Soft Matter Phys. 2006, 74 Pt 1, 031914. [Google Scholar] [CrossRef]
- Yamakawa, K.; Rajendran, P.S.; Takamiya, T.; Yagishita, D.; So, E.L.; Mahajan, A.; Shivkumar, K.; Vaseghi, M. Vagal nerve stimulation activates vagal afferent fibers that reduce cardiac efferent parasympathetic effects. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1579–H1590. [Google Scholar] [CrossRef]
- Sher, L.D.; Geddie, H.; Olivier, L.; Cairns, M.; Truter, N.; Beselaar, L.; Essop, M.F. Chronic stress and endothelial dysfunction: Mechanisms, experimental challenges, and the way ahead. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H488–H506. [Google Scholar] [CrossRef] [PubMed]
- Sazonova, M.A.; Sinyov, V.V.; Ryzhkova, A.I.; Sazonova, M.D.; Kirichenko, T.V.; Khotina, V.A.; Khasanova, Z.B.; Doroschuk, N.A.; Karagodin, V.P.; Orekhov, A.N.; et al. Some Molecular and Cellular Stress Mechanisms Associated with Neurodegenerative Diseases and Atherosclerosis. Int. J. Mol. Sci. 2021, 22, 699. [Google Scholar] [CrossRef] [PubMed]
- Gianaros, P.J.; Wager, T.D. Brain-Body Pathways Linking Psychological Stress and Physical Health. Curr. Dir. Psychol. Sci. 2015, 24, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Jennings, J.R.; Kamarck, T.W.; Everson-Rose, S.A.; Kaplan, G.A.; Manuck, S.B.; Salonen, J.T. Exaggerated blood pressure responses during mental stress are prospectively related to enhanced carotid atherosclerosis in middle-aged Finnish men. Circulation 2004, 110, 2198–2203. [Google Scholar] [CrossRef]
- Al’Absi, M.; Devereux, R.B.; Rao, D.C.; Kitzman, D.; Oberman, A.; Hopkins, P.; Arnett, D.K. Blood pressure stress reactivity and left ventricular mass in a random community sample of African-American and caucasian men and women. Am. J. Cardiol. 2006, 97, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Browning, K.N.; Travagli, R.A. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr. Physiol. 2014, 4, 1339–1368. [Google Scholar] [CrossRef] [PubMed]
- Brunsson, I.; Eklund, S.; Jodal, M.; Lundgren, O.; Sjövall, H. The effect of vasodilatation and sympathetic nerve activation on net water absorption in the cat’s small intestine. Acta Physiol. Scand. 1979, 106, 61–68. [Google Scholar] [CrossRef]
- Brierley, S.M.; Hibberd, T.J.; Spencer, N.J. Spinal Afferent Innervation of the Colon and Rectum. Front. Cell. Neurosci. 2018, 12, 467. [Google Scholar] [CrossRef]
- Metz, C.N.; Pavlov, V.A. Vagus nerve cholinergic circuitry to the liver and the gastrointestinal tract in the neuroimmune communicatome. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G651–G658. [Google Scholar] [CrossRef] [PubMed]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef] [PubMed]
- Viggiano, D.; Ianiro, G.; Vanella, G.; Bibbò, S.; Bruno, G.; Simeone, G.; Mele, G. Gut barrier in health and disease: Focus on childhood. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1077–1085. [Google Scholar]
- Moussaoui, N.; Jacobs, J.P.; Larauche, M.; Biraud, M.; Million, M.; Mayer, E.; Taché, Y. Chronic Early-life Stress in Rat Pups Alters Basal Corticosterone, Intestinal Permeability, and Fecal Microbiota at Weaning: Influence of Sex. J. Neurogastroenterol. Motil. 2017, 23, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, H.; Mizoguchi, K.; Oe, M.; Maeta, H. Intestinal ischemia induces late preconditioning against myocardial infarction: A role for inducible nitric oxide synthase. Cardiovasc. Res. 2001, 49, 391–398. [Google Scholar] [CrossRef][Green Version]
- Paterno, F.; Longo, W.E. The etiology and pathogenesis of vascular disorders of the intestine. Radiol. Clin. N. Am. 2008, 46, 877–885. [Google Scholar] [CrossRef]
- Haas, A.V.; McDonnell, M.E. Pathogenesis of Cardiovascular Disease in Diabetes. Endocrinol. Metab. Clin. N. Am. 2018, 47, 51–63. [Google Scholar] [CrossRef]
- Rosenberger, P.; Schwab, J.M.; Mirakaj, V.; Masekowsky, E.; Mager, A.; Morote-Garcia, J.C.; Unertl, K.; Eltzschig, H.K. Hypoxia-inducible factor-dependent induction of netrin-1 dampens inflammation caused by hypoxia. Nat. Immunol. 2009, 10, 195–202, Erratum in Nat. Immunol. 2015, 16, 544. [Google Scholar] [CrossRef]
- Poyton, R.O.; Castello, P.R.; Ball, K.A.; Woo, D.K.; Pan, N. Mitochondria and hypoxic signaling: A new view. Ann. N. Y. Acad. Sci. 2009, 1177, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Matsuno, T.; Omata, K.; Satoh, T. Relationship between hyposalivation and oxidative stress in aging mice. J. Clin. Biochem. Nutr. 2017, 61, 40–46. [Google Scholar] [CrossRef]
- Mallick, I.H.; Yang, W.; Winslet, M.C.; Seifalian, A.M. Ischemia-reperfusion injury of the intestine and protective strategies against injury. Dig. Dis. Sci. 2004, 49, 1359–1377. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: An essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Nadatani, Y.; Watanabe, T.; Shimada, S.; Otani, K.; Tanigawa, T.; Fujiwara, Y. Microbiome and intestinal ischemia/reperfusion injury. J. Clin. Biochem. Nutr. 2018, 63, 26–32. [Google Scholar] [CrossRef]
- Wang, F.; Li, Q.; Wang, C.; Tang, C.; Li, J. Dynamic alteration of the colonic microbiota in intestinal ischemia-reperfusion injury. PLoS ONE 2012, 7, e42027. [Google Scholar] [CrossRef]
- Sun, L.; Jia, H.; Li, J.; Yu, M.; Yang, Y.; Tian, D.; Zhang, H.; Zou, Z. Cecal Gut Microbiota and Metabolites Might Contribute to the Severity of Acute Myocardial Ischemia by Impacting the Intestinal Permeability, Oxidative Stress, and Energy Metabolism. Front. Microbiol. 2019, 10, 1745. [Google Scholar] [CrossRef] [PubMed]
- Groschwitz, K.R.; Hogan, S.P. Intestinal barrier function: Molecular regulation and disease pathogenesis. J. Allergy Clin. Immunol. 2009, 124, 3–20. [Google Scholar] [CrossRef]
- Bhattarai, Y. Microbiota-gut-brain axis: Interaction of gut microbes and their metabolites with host epithelial barriers. Neurogastroenterol. Motil. 2018, 30, e13366. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Wei, Y.; Yang, W.; Cai, Y.; Chen, B.; Yang, G.; Shang, H.; Zhao, W. Ghrelin Attenuates Intestinal Barrier Dysfunction Following Intracerebral Hemorrhage in Mice. Int. J. Mol. Sci. 2016, 17, 2032. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C.; Cookson, A.L.; McNabb, W.C.; Park, Z.; McCann, M.J.; Kelly, W.J.; Roy, N.C. Lactobacillus plantarum MB452 enhances the function of the intestinal barrier by increasing the expression levels of genes involved in tight junction formation. BMC Microbiol. 2010, 10, 316. [Google Scholar] [CrossRef]
- Mennigen, R.; Nolte, K.; Rijcken, E.; Utech, M.; Loeffler, B.; Senninger, N.; Bruewer, M. Probiotic mixture VSL#3 protects the epithelial barrier by maintaining tight junction protein expression and preventing apoptosis in a murine model of colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 296, G1140–G1149. [Google Scholar] [CrossRef]
- Massier, L.; Blüher, M.; Kovacs, P.; Chakaroun, R.M. Impaired Intestinal Barrier and Tissue Bacteria: Pathomechanisms for Metabolic Diseases. Front. Endocrinol. (Lausanne) 2021, 12, 616506. [Google Scholar] [CrossRef] [PubMed]
- Okumura, R.; Takeda, K. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp. Mol. Med. 2017, 49, e338. [Google Scholar] [CrossRef]
- Hu, M.D.; Jia, L.; Edelblum, K.L. Policing the Intestinal Epithelial Barrier: Innate Immune Functions of Intraepithelial Lymphocytes. Curr. Pathobiol. Rep. 2018, 6, 35–46. [Google Scholar] [CrossRef]
- McLaughlin, T.; Ackerman, S.E.; Shen, L.; Engleman, E. Role of innate and adaptive immunity in obesity-associated metabolic disease. J. Clin. Investig. 2017, 127, 5–13. [Google Scholar] [CrossRef]
- Shimotoyodome, A.; Meguro, S.; Hase, T.; Tokimitsu, I.; Sakata, T. Short chain fatty acids but not lactate or succinate stimulate mucus release in the rat colon. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2000, 125, 525–531. [Google Scholar] [CrossRef]
- Cash, H.L.; Whitham, C.V.; Behrendt, C.L.; Hooper, L.V. Symbiotic bacteria direct expression of an intestinal bactericidal lectin. Science 2006, 313, 1126–1130. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; MacDonald, T.T.; Hermoso, M.A. Editorial: Intestinal Homeostasis and Disease: A Complex Partnership between Immune Cells, Non-Immune Cells, and the Microbiome. Front. Immunol. 2019, 10, 2775. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, T.; Satchell, D.P.; Wilson, C.L.; Parks, W.C.; Selsted, M.E.; Ouellette, A.J. Secretion of microbicidal alpha-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 2000, 1, 113–118. [Google Scholar] [CrossRef]
- Vaishnava, S.; Yamamoto, M.; Severson, K.M.; Ruhn, K.A.; Yu, X.; Koren, O.; Ley, R.; Wakeland, E.K.; Hooper, L.V. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science 2011, 334, 255–258. [Google Scholar] [CrossRef]
- Kobayashi, K.S.; Chamaillard, M.; Ogura, Y.; Henegariu, O.; Inohara, N.; Nuñez, G.; Flavell, R.A. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 2005, 307, 731–734. [Google Scholar] [CrossRef]
- Liang, S.C.; Tan, X.Y.; Luxenberg, D.P.; Karim, R.; Dunussi-Joannopoulos, K.; Collins, M.; Fouser, L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006, 203, 2271–2279. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Manieri, N.A.; Liu, T.C.; Stappenbeck, T.S. IL-6 stimulates intestinal epithelial proliferation and repair after injury. PLoS ONE 2014, 9, e114195. [Google Scholar] [CrossRef]
- Capaldo, C.T.; Beeman, N.; Hilgarth, R.S.; Nava, P.; Louis, N.A.; Naschberger, E.; Stürzl, M.; Parkos, C.A.; Nusrat, A. IFN-γ and TNF-α-induced GBP-1 inhibits epithelial cell proliferation through suppression of β-catenin/TCF signaling. Mucosal Immunol. 2012, 5, 681–690. [Google Scholar] [CrossRef]
- Pull, S.L.; Doherty, J.M.; Mills, J.C.; Gordon, J.I.; Stappenbeck, T.S. Activated macrophages are an adaptive element of the colonic epithelial progenitor niche necessary for regenerative responses to injury. Proc. Natl. Acad. Sci. USA 2005, 102, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Zeng, H.; Lyons, S.; Carlson, A.; Merlin, D.; Neish, A.S.; Gewirtz, A.T. TLR5-mediated activation of p38 MAPK regulates epithelial IL-8 expression via posttranscriptional mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G282–G290. [Google Scholar] [CrossRef]
- Chervy, M.; Barnich, N.; Denizot, J. Adherent-Invasive, E. coli: Update on the Lifestyle of a Troublemaker in Crohn’s Disease. Int. J. Mol. Sci. 2020, 21, 3734. [Google Scholar] [CrossRef]
- Worthington, J.J. The intestinal immunoendocrine axis: Novel cross-talk between enteroendocrine cells and the immune system during infection and inflammatory disease. Biochem. Soc. Trans. 2015, 43, 727–733. [Google Scholar] [CrossRef]
- Saia, R.S.; Mestriner, F.L.; Bertozi, G.; Cunha, F.Q.; Cárnio, E.C. Cholecystokinin inhibits inducible nitric oxide synthase expression by lipopolysaccharide-stimulated peritoneal macrophages. Mediat. Inflamm. 2014, 2014, 896029. [Google Scholar] [CrossRef]
- Zhang, J.G.; Cong, B.; Li, Q.X.; Chen, H.Y.; Qin, J.; Fu, L.H. Cholecystokinin octapeptide regulates lipopolysaccharide-activated B cells co-stimulatory molecule expression and cytokines production in vitro. Immunopharmacol. Immunotoxicol. 2011, 33, 157–163. [Google Scholar] [CrossRef]
- Kelly, C.J.; Zheng, L.; Campbell, E.L.; Saeedi, B.; Scholz, C.C.; Bayless, A.J.; Wilson, K.E.; Glover, L.E.; Kominsky, D.J.; Magnuson, A.; et al. Crosstalk between Microbiota-Derived Short-Chain Fatty Acids and Intestinal Epithelial HIF Augments Tissue Barrier Function. Cell Host Microbe 2015, 17, 662–671. [Google Scholar] [CrossRef]
- Zhgun, E.S.; Kislun, Y.V.; Kalachniuk, T.N.; Veselovsky, V.A.; Urban, A.S.; Tikhonova, P.O.; Pavlenko, A.V.; Ilchenko, G.N.; Ilina, E.N. Evaluation of metabolites levels in feces of patients with inflammatory bowel diseases. Biomed. Khim. 2020, 66, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Chimerel, C.; Emery, E.; Summers, D.K.; Keyser, U.; Gribble, F.M.; Reimann, F. Bacterial metabolite indole modulates incretin secretion from intestinal enteroendocrine L cells. Cell Rep. 2014, 9, 1202–1208. [Google Scholar] [CrossRef]
- Jostins, L.; Ripke, S.; Weersma, R.K.; Duerr, R.H.; McGovern, D.P.; Hui, K.Y.; Lee, J.C.; Schumm, L.P.; Sharma, Y.; Anderson, C.A.; et al. Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012, 491, 119–124. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Hotamisligil, G.S. Inflammation, metaflammation and immunometabolic disorders. Nature 2017, 542, 177–185. [Google Scholar] [CrossRef]
- Tilg, H.; Zmora, N.; Adolph, T.E.; Elinav, E. The intestinal microbiota fuelling metabolic inflammation. Nat. Rev. Immunol. 2020, 20, 40–54. [Google Scholar] [CrossRef] [PubMed]
- Ogden, H.B.; Fallowfield, J.L.; Child, R.B.; Davison, G.; Fleming, S.C.; Edinburgh, R.M.; Delves, S.K.; Millyard, A.; Westwood, C.S.; Layden, J.D. Reliability of gastrointestinal barrier integrity and microbial translocation biomarkers at rest and following exertional heat stress. Physiol. Rep. 2020, 8, e14374. [Google Scholar] [CrossRef]
- Genser, L.; Poitou, C.; Brot-Laroche, É.; Rousset, M.; Vaillant, J.C.; Clément, K.; Thenet, S.; Leturque, A. Alteration of intestinal permeability: The missing link between gut microbiota modifications and inflammation in obesity? Med. Sci. (Paris) 2016, 32, 461–469. [Google Scholar] [CrossRef][Green Version]
- de Punder, K.; Pruimboom, L. Stress induces endotoxemia and low-grade inflammation by increasing barrier permeability. Front. Immunol. 2015, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Thaiss, C.A.; Zmora, N.; Levy, M.; Elinav, E. The microbiome and innate immunity. Nature 2016, 535, 65–74. [Google Scholar] [CrossRef]
- Kowiański, P.; Lietzau, G.; Czuba, E.; Waśkow, M.; Steliga, A.; Moryś, J. BDNF: A Key Factor with Multipotent Impact on Brain Signaling and Synaptic Plasticity. Cell. Mol. Neurobiol. 2018, 38, 579–593. [Google Scholar] [CrossRef]
- Mattson, M.P. Energy intake, meal frequency, and health: A neurobiological perspective. Annu. Rev. Nutr. 2005, 25, 237–260. [Google Scholar] [CrossRef]
- Cuomo, M.; Borrelli, L.; Della Monica, R.; Coretti, L.; De Riso, G.; D’Angelo Lancellotti di Durazzo, L.; Fioretti, A.; Lembo, F.; Dinan, T.G.; Cryan, J.F.; et al. DNA Methylation Profiles of Tph1A and BDNF in Gut and Brain of L. Rhamnosus-Treated Zebrafish. Biomolecules 2021, 11, 142. [Google Scholar] [CrossRef]
- Dopkins, N.; Nagarkatti, P.S.; Nagarkatti, M. The role of gut microbiome and associated metabolome in the regulation of neuroinflammation in multiple sclerosis and its implications in attenuating chronic inflammation in other inflammatory and autoimmune disorders. Immunology 2018, 154, 178–185. [Google Scholar] [CrossRef]
- Pan, W.; Stone, K.P.; Hsuchou, H.; Manda, V.K.; Zhang, Y.; Kastin, A.J. Cytokine signaling modulates blood-brain barrier function. Curr. Pharm. Des. 2011, 17, 3729–3740. [Google Scholar] [CrossRef]
- Braniste, V.; Al-Asmakh, M.; Kowal, C.; Anuar, F.; Abbaspour, A.; Tóth, M.; Korecka, A.; Bakocevic, N.; Ng, L.G.; Kundu, P.; et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014, 6, 263ra158. [Google Scholar] [CrossRef]
- Banks, W.A. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009, 9 (Suppl. 1), S3. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, E.G.; Banks, W.A.; Kastin, A.J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 1993, 47, 169–176. [Google Scholar] [CrossRef]
- Lexchin, J.L.; Cude-Simpson, K.D.; Stancer, H.C. Brain and blood indole metabolites after peripheral administration of (14)C-5-HT in rat. Neurochem. Res. 1977, 2, 39–50. [Google Scholar] [CrossRef]
- Kannan, R.; Kuhlenkamp, J.F.; Jeandidier, E.; Trinh, H.; Ookhtens, M.; Kaplowitz, N. Evidence for carrier-mediated transport of glutathione across the blood-brain barrier in the rat. J. Clin. Investig. 1990, 85, 2009–2013. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Robinson, S.M. Minimal penetration of lipopolysaccharide across the murine blood-brain barrier. Brain Behav. Immun. 2010, 24, 102–109. [Google Scholar] [CrossRef]
- Ugalde-Muñiz, P.; Fetter-Pruneda, I.; Navarro, L.; García, E.; Chavarría, A. Chronic Systemic Inflammation Exacerbates Neurotoxicity in a Parkinson’s Disease Model. Oxid. Med. Cell. Longev. 2020, 2020, 4807179. [Google Scholar] [CrossRef] [PubMed]
- Sharon, G.; Sampson, T.R.; Geschwind, D.H.; Mazmanian, S.K. The Central Nervous System and the Gut Microbiome. Cell 2016, 167, 915–932. [Google Scholar] [CrossRef] [PubMed]
- Aspelund, A.; Antila, S.; Proulx, S.T.; Karlsen, T.V.; Karaman, S.; Detmar, M.; Wiig, H.; Alitalo, K. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J. Exp. Med. 2015, 212, 991–999. [Google Scholar] [CrossRef] [PubMed]
- Louveau, A.; Smirnov, I.; Keyes, T.J.; Eccles, J.D.; Rouhani, S.J.; Peske, J.D.; Derecki, N.C.; Castle, D.; Mandell, J.W.; Lee, K.S.; et al. Structural and functional features of central nervous system lymphatic vessels. Nature 2015, 523, 337–341. [Google Scholar] [CrossRef]
- Dinan, T.G.; Quigley, E.M.; Ahmed, S.M.; Scully, P.; O’Brien, S.; O’Mahony, L.; O’Mahony, S.; Shanahan, F.; Keeling, P.W. Hypothalamic-pituitary-gut axis dysregulation in irritable bowel syndrome: Plasma cytokines as a potential biomarker? Gastroenterology 2006, 130, 304–311. [Google Scholar] [CrossRef]
- Lang, C.H.; Molina, P.E.; Yousef, K.A.; Tepper, P.G.; Abumrad, N.N. Role of IL-1 alpha in central nervous system immunomodulation of glucoregulation. Brain Res. 1993, 624, 53–60. [Google Scholar] [CrossRef]
- Dunn, A.J. Cytokine activation of the HPA axis. Ann. N. Y. Acad. Sci. 2000, 917, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Goebel, M.U.; Baase, J.; Pithan, V.; Exton, M.; Saller, B.; Schedlowski, M.; Limmroth, V. Acute interferon beta-1b administration alters hypothalamic-pituitary-adrenal axis activity, plasma cytokines and leukocyte distribution in healthy subjects. Psychoneuroendocrinology 2002, 27, 881–892. [Google Scholar] [CrossRef]
- Zimomra, Z.R.; Porterfield, V.M.; Camp, R.M.; Johnson, J.D. Time-dependent mediators of HPA axis activation following live Escherichia coli. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R1648–R1657. [Google Scholar] [CrossRef]
- Turnbull, A.V.; Rivier, C.L. Regulation of the hypothalamic-pituitary-adrenal axis by cytokines: Actions and mechanisms of action. Physiol. Rev. 1999, 79, 1–71. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.J. Effects of the IL-1 receptor antagonist on the IL-1- and endotoxin-induced activation of the HPA axis and cerebral biogenic amines in mice. Neuroimmunomodulation 2000, 7, 36–45. [Google Scholar] [CrossRef]
- Wieczorek, M.; Swiergiel, A.H.; Pournajafi-Nazarloo, H.; Dunn, A.J. Physiological and behavioral responses to interleukin-1beta and LPS in vagotomized mice. Physiol. Behav. 2005, 85, 500–511. [Google Scholar] [CrossRef] [PubMed]
- de Sousa Rodrigues, M.E.; Bekhbat, M.; Houser, M.C.; Chang, J.; Walker, D.I.; Jones, D.P.; Oller do Nascimento, C.M.P.; Barnum, C.J.; Tansey, M.G. Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain Behav. Immun. 2017, 59, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Chapman, C.R.; Tuckett, R.P.; Song, C.W. Pain and stress in a systems perspective: Reciprocal neural, endocrine, and immune interactions. J. Pain 2008, 9, 122–145. [Google Scholar] [CrossRef] [PubMed]
- Prevot, V. Plasticity of neuroendocrine systems. Eur. J. Neurosci. 2010, 32, 1987–1988. [Google Scholar] [CrossRef]
- Kudryashov, N.V.; Kalinina, T.S.; Shimshirt, A.A.; Volkova, A.V.; Narkevich, V.B.; Naplekova, P.L.; Kasabov, K.A.; Kudrin, V.S.; Voronina, T.A.; Fisenko, V.P. The Behavioral and Neurochemical Aspects of the Interaction between Antidepressants and Unpredictable Chronic Mild Stress. Acta Nat. 2020, 12, 63–72. [Google Scholar] [CrossRef]
- Xu, C.; Lee, S.K.; Zhang, D.; Frenette, P.S. The Gut Microbiome Regulates Psychological-Stress-Induced Inflammation. Immunity 2020, 53, 417–428. [Google Scholar] [CrossRef]
- Tengeler, A.C.; Dam, S.A.; Wiesmann, M.; Naaijen, J.; van Bodegom, M.; Belzer, C.; Dederen, P.J.; Verweij, V.; Franke, B.; Kozicz, T.; et al. Gut microbiota from persons with attention-deficit/hyperactivity disorder affects the brain in mice. Microbiome 2020, 8, 44. [Google Scholar] [CrossRef]
- Bundgaard-Nielsen, C.; Knudsen, J.; Leutscher, P.D.C.; Lauritsen, M.B.; Nyegaard, M.; Hagstrøm, S.; Sørensen, S. Gut microbiota profiles of autism spectrum disorder and attention deficit/hyperactivity disorder: A systematic literature review. Gut Microbes 2020, 11, 1172–1187. [Google Scholar] [CrossRef] [PubMed]
- Fond, G.; Boukouaci, W.; Chevalier, G.; Regnault, A.; Eberl, G.; Hamdani, N.; Dickerson, F.; Macgregor, A.; Boyer, L.; Dargel, A.; et al. The “psychomicrobiotic”: Targeting microbiota in major psychiatric disorders: A systematic review. Pathol. Biol. (Paris) 2015, 63, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Li, J.; Wu, F.; Zheng, H.; Peng, Q.; Zhou, H. Altered composition and function of intestinal microbiota in autism spectrum disorders: A systematic review. Transl. Psychiatry 2019, 9, 43. [Google Scholar] [CrossRef]
- Cenit, M.C.; Nuevo, I.C.; Codoñer-Franch, P.; Dinan, T.G.; Sanz, Y. Gut microbiota and attention deficit hyperactivity disorder: New perspectives for a challenging condition. Eur. Child Adolesc. Psychiatry 2017, 26, 1081–1092. [Google Scholar] [CrossRef]
- Sudo, N. Role of microbiome in regulating the HPA axis and its relevance to allergy. Chem. Immunol. Allergy 2012, 98, 163–175. [Google Scholar] [CrossRef]
- Farzi, A.; Fröhlich, E.E.; Holzer, P. Gut Microbiota and the Neuroendocrine System. Neurotherapeutics 2018, 15, 5–22. [Google Scholar] [CrossRef]
- Maev, I.V.; Kazulin, A.N.; Andreev, D.N. The cardiovascular system in patients with functional and inflammatory bowel diseases. Ter. Arkhiv 2018, 90, 59–64. [Google Scholar] [CrossRef]
- Jia, Q.; Li, H.; Zhou, H.; Zhang, X.; Zhang, A.; Xie, Y.; Li, Y.; Lv, S.; Zhang, J. Role and Effective Therapeutic Target of Gut Microbiota in Heart Failure. Cardiovasc. Ther. 2019, 2019, 5164298. [Google Scholar] [CrossRef] [PubMed]
- Pieczynska, M.D.; Yang, Y.; Petrykowski, S.; Horbanczuk, O.K.; Atanasov, A.G.; Horbanczuk, J.O. Gut Microbiota and Its Metabolites in Atherosclerosis Development. Molecules 2020, 25, 594. [Google Scholar] [CrossRef]
- Chakaroun, R.M.; Massier, L.; Kovacs, P. Gut Microbiome, Intestinal Permeability, and Tissue Bacteria in Metabolic Disease: Perpetrators or Bystanders? Nutrients 2020, 12, 1082. [Google Scholar] [CrossRef]
- Lyu, M.; Wang, Y.F.; Fan, G.W.; Wang, X.Y.; Xu, S.Y.; Zhu, Y. Balancing Herbal Medicine and Functional Food for Prevention and Treatment of Cardiometabolic Diseases through Modulating Gut Microbiota. Front. Microbiol. 2017, 8, 2146. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, W.; Zuo, L.; Zhu, W.; Wang, B.; Li, Q.; Li, J. Bifidobacteria may be beneficial to intestinal microbiota and reduction of bacterial translocation in mice following ischaemia and reperfusion injury. Br. J. Nutr. 2013, 109, 1990–1998. [Google Scholar] [CrossRef]
- Drapkina, O.M.; Korneeva, O.N. Gut microbiota and obesity: Pathogenetic relationships and ways to normalize the intestinal microflora. Ter. Arkhiv 2016, 88, 135–142. [Google Scholar] [CrossRef]
- Athari Nik Azm, S.; Djazayeri, A.; Safa, M.; Azami, K.; Ahmadvand, B.; Sabbaghziarani, F.; Sharifzadeh, M.; Vafa, M. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in β-amyloid (1-42) injected rats. Appl. Physiol. Nutr. Metab. 2018, 43, 718–726. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Ståhlman, M.; Arike, L.; Johansson, M.E.V.; Hansson, G.C.; Bäckhed, F. Bifidobacteria or Fiber Protects against Diet-Induced Microbiota-Mediated Colonic Mucus Deterioration. Cell Host Microbe 2018, 23, 27–40.e7. [Google Scholar] [CrossRef]
- Paiva, I.H.R.; Duarte-Silva, E.; Peixoto, C.A. The role of prebiotics in cognition, anxiety, and depression. Eur. Neuropsychopharmacol. 2020, 34, 1–18. [Google Scholar] [CrossRef]
- de Almeida Silva, M.; Mowry, F.E.; Peaden, S.C.; Andrade, T.U.; Biancardi, V.C. Kefir ameliorates hypertension via gut-brain mechanisms in spontaneously hypertensive rats. J. Nutr. Biochem. 2020, 77, 108318. [Google Scholar] [CrossRef]
- Chen, Z.; Venkat, P.; Seyfried, D.; Chopp, M.; Yan, T.; Chen, J. Brain-Heart Interaction: Cardiac Complications after Stroke. Circ. Res. 2017, 121, 451–468. [Google Scholar] [CrossRef]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of Gut Microbiota, Probiotics and Prebiotics in the Cardiovascular Diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef]
- Zhao, X.; Oduro, P.K.; Tong, W.; Wang, Y.; Gao, X.; Wang, Q. Therapeutic potential of natural products against atherosclerosis: Targeting on gut microbiota. Pharmacol. Res. 2021, 163, 105362. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.A.; Bah, T.M.; Kaloustian, S.; Lada-Moldovan, L.; Rondeau, I.; Tompkins, T.A.; Godbout, R.; Rousseau, G. Lactobacillus helveticus and Bifidobacterium longum taken in combination reduce the apoptosis propensity in the limbic system after myocardial infarction in a rat model. Br. J. Nutr. 2009, 102, 1420–1425. [Google Scholar] [CrossRef]
- Kurita, N.; Yamashiro, K.; Kuroki, T.; Tanaka, R.; Urabe, T.; Ueno, Y.; Miyamoto, N.; Takanashi, M.; Shimura, H.; Inaba, T.; et al. Metabolic endotoxemia promotes neuroinflammation after focal cerebral ischemia. J. Cereb. Blood Flow Metab. 2020, 40, 2505–2520. [Google Scholar] [CrossRef] [PubMed]
- Moludi, J.; Khedmatgozar, H.; Nachvak, S.M.; Abdollahzad, H.; Moradinazar, M.; Sadeghpour Tabaei, A. The effects of co-administration of probiotics and prebiotics on chronic inflammation, and depression symptoms in patients with coronary artery diseases: A randomized clinical trial. Nutr. Neurosci. 2021, 28, 1–10. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).