Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future
Abstract
1. Introduction
2. Standard and Advanced Echocardiography
2.1. Left Ventricular Morphology and Systolic Function
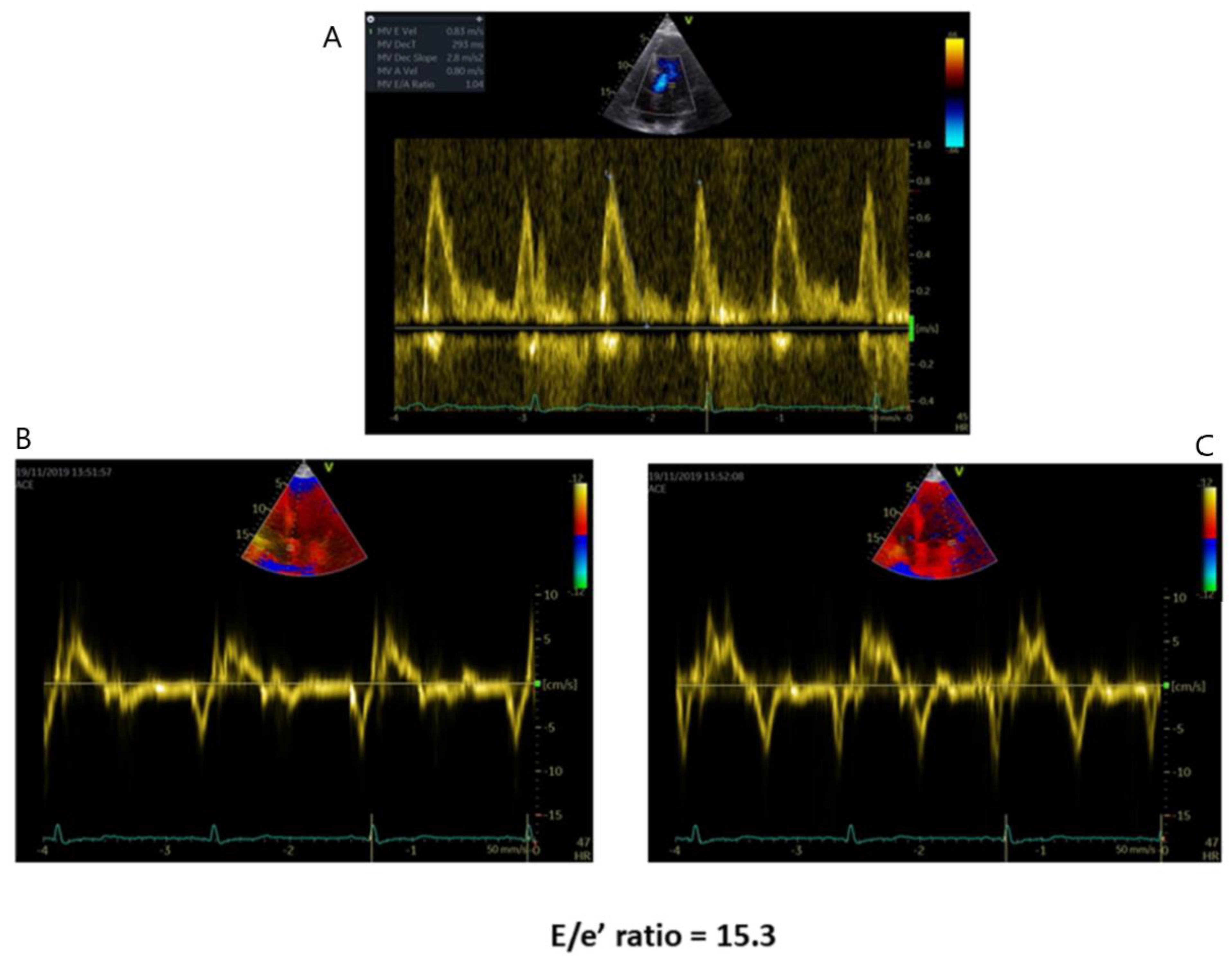
2.2. Left Ventricular Diastolic Function
2.3. Right Ventricle
2.4. Aorta
2.5. Valve Disease
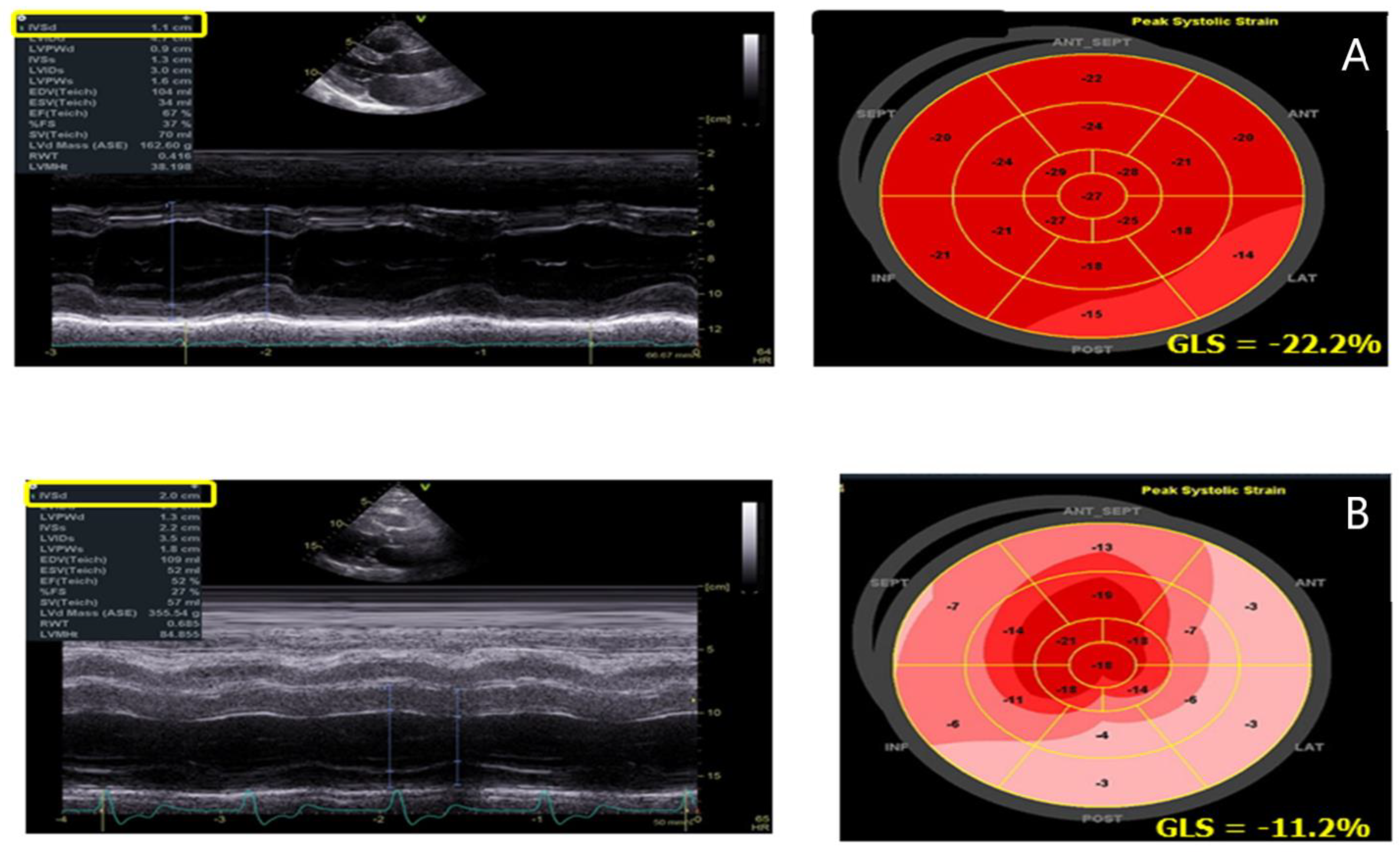
3. Speckle Tracking Echocardiography
4. Three-Dimensional Echocardiography
5. Cardiac Magnetic Resonance
6. Tissue Characterization
6.1. T1 and T2 Mapping
6.2. Late Gadolinium Enhancement
6.3. Speckle Tracking Analysis in CMR
6.4. Cardiovascular Magnetic Resonance Perfusion Mapping
7. Nuclear Scintigraphy and Positron Emission Tomography
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desnick, R.J.; Ioannou, Y.A.; Eng, C.M. α-Galactosidase A deficiency: Fabry disease. In The Metabolic and Molecular Bases of Inherited Disease, 8th ed.; Scriver, C.R., Beaudet, A.L., Sly, W.S., Valle, D., Eds.; McGraw Hill: New York, NY, USA, 2001; pp. 3733–3774. [Google Scholar]
- Pisani, A.; Visciano, B.; Imbriaco, M.; Di Nuzzi, A.; Mancini, A.; Marchetiello, C.; Riccio, E. The kidney in Fabry’s disease. Clin. Genet. 2014, 86, 301–309. [Google Scholar] [CrossRef]
- Weidemann, F.; Sanchez-Niño, M.D.; Politei, J.; Oliveira, J.P.; Wanner, C.; Warnock, D.G.; Ortiz, A. Fibrosis: A key feature of Fabry disease with potential therapeutic implications. Orphanet J. Rare Dis. 2013, 8, 116. [Google Scholar] [CrossRef] [PubMed]
- Mehta, A.; Clarke, J.T.; Giugliani, R.; Elliott, P.; Linhart, A.; Beck, M.; Sunder-Plassmann, G.; FOS Investigators. Natural course of Fabry disease: Changing pattern of causes of death in FOS—Fabry Outcome Survey. J. Med. Genet. 2009, 46, 548–552. [Google Scholar] [CrossRef]
- Spada, M.; Pagliardini, S.; Yasuda, M.; Tukel, T.; Thiagarajan, G.; Sakuraba, H.; Ponzone, A.; Desnick, R.J. High incidence of later-onset Fabry disease revealed by newborn screening. Am. J. Hum. Genet. 2006, 79, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.R.; Elliott, S.; Buroker, N.; Thomas, L.I.; Keutzer, J.; Glass, M.; Gelb, M.H.; Turecek, F. Identification of infants at risk for developing Fabry, Pompe, or mucopolysaccharidosis-I from newborn blood spots by tandem mass spectrometry. J. Pediatr. 2013, 163, 498–503. [Google Scholar] [CrossRef]
- Patel, V.; O’Mahony, C.; Hughes, D.; Rahman, M.S.; Coats, C.; Murphy, E.; Lachmann, R.; Mehta, A.; Elliott, P.M. Clinical and genetic predictors of major cardiac events in patients with Anderson-Fabry disease. Heart 2015, 101, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Rombach, S.M.; Smid, B.E.; Linthorst, G.E.; Dijkgraaf, M.G.; Hollak, C.E. Natural course of Fabry disease and the effectiveness of enzyme replacement therapy: A systematic review and meta-analysis: Effectiveness of ERT in different disease stages. J. Inherit. Metab. Dis. 2014, 37, 341–352. [Google Scholar] [CrossRef]
- Linhart, A.; Palecek, T.; Bultas, J.; Ferguson, J.J.; Hrudová, J.; Karetová, D.; Zeman, J.; Ledvinová, J.; Poupetová, H.; Elleder, M.; et al. New insights in cardiac structural changes in patients with Fabry’s disease. Am. Heart J. 2000, 139, 1101–1108. [Google Scholar] [CrossRef]
- Linhart, A.; Kampmann, C.; Zamorano, J.L.; Sunder-Plassmann, G.; Beck, M.; Mehta, A.; Elliott, P.M.; European FOS Investigators. Cardiac manifestations of Anderson-Fabry disease: Results from the international Fabry outcome survey. Eur. Heart J. 2007, 28, 1228–1235. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.C.; Sheppard, M.; Reed, E.; Lee, P.; Elliott, P.M.; Pennell, D.J. The histological basis of late gadolinium enhancement cardiovascular magnetic resonance in a patient with Anderson-Fabry disease. J. Cardiovasc. Magn. Reson. 2006, 8, 479–482. [Google Scholar] [CrossRef]
- Takenaka, T.; Teraguchi, H.; Yoshida, A.; Taguchi, S.; Ninomiya, K.; Umekita, Y.; Yoshida, H.; Horinouchi, M.; Tabata, K.; Yonezawa, S.; et al. Terminal stage cardiac findings in patients with cardiac Fabry disease: An electrocardiographic, echocardiographic, and autopsy study. J. Cardiol. 2008, 51, 50–59. [Google Scholar] [CrossRef]
- Imbriaco, M.; Messalli, G.; Avitabile, G.; Cuocolo, A.; Maurea, S.; Soscia, F.; Pisani, A. Cardiac magnetic resonance imaging illustrating Anderson-Fabry disease progression. Br. J. Radiol. 2010, 83, e249–e251. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, B.; Takenaka, T.; Teraguchi, H.; Tei, C.; Lee, P.; McKenna, W.J.; Elliott, P.M. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy. Circulation 2002, 105, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Pieroni, M.; Morgante, E.; Antuzzi, D.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation 2004, 110, 1047–1053. [Google Scholar] [CrossRef]
- Ramaswami, U. Update on role of agalsidase alfa in management of Fabry disease. Drug Des. Devel. Ther. 2011, 145, 155–173. [Google Scholar] [CrossRef] [PubMed][Green Version]
- El Dib, R.P.; Nascimento, P.; Pastores, G.M. Enzyme replacement therapy for Anderson-Fabry disease. Cochrane Database Syst. Rev. 2013, 282, CD006663. [Google Scholar] [CrossRef]
- Riccio, E.; Zanfardino, M.; Ferreri, L.; Santoro, C.; Cocozza, S.; Capuano, I.; Imbriaco, M.; Feriozzi, S.; Pisani, A.; AFFIINITY Group. Switch from enzyme replacement therapy to oral chaperone migalastat for treating fabry disease: Real-life data. Eur. J. Hum. Genet. 2020, 28, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Müntze, J.; Gensler, D.; Maniuc, O.; Liu, D.; Cairns, T.; Oder, D.; Hu, K.; Lorenz, K.; Frantz, S.; Wanner, C.; et al. Oral chaperone therapy migalastat for treating fabry disease: Enzymatic response and serum biomarker changes after 1 year. Clin. Pharmacol. Ther. 2019, 105, 1224–1233. [Google Scholar] [CrossRef]
- Weidemann, F.; Niemann, M.; Störk, S.; Breunig, F.; Beer, M.; Sommer, C.; Herrmann, S.; Ertl, G.; Wanner, C. Long-term outcome of enzyme-replacement therapy in advanced Fabry disease: Evidence for disease progression towards serious complications. J. Intern. Med. 2013, 274, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.E.; Cantor, R.; Schwartz, M.F.; Baker, M.; Desnick, R.J. Echocardiographic abnormalities and disease severity in Fabry’s disease. J. Am. Coll. Cardiol. 1986, 7, 1157–1161. [Google Scholar] [CrossRef]
- Jain, R.; Kalvin, L.; Johnson, B.; Muthukumar, L.; Khandheria, B.K.; Tajik, A.J. Many Facesof Fabry’s Cardiomyopathy. JACC Cardiovasc. Imaging 2018, 11, 644–647. [Google Scholar] [CrossRef] [PubMed]
- Kampmann, C.; Linhart, A.; Baehner, F.; Palecek, T.; Wiethoff, C.M.; Miebach, E.; Whybra, C.; Gal, A.; Bultas, J.; Beck, M. Onset and progression of the Anderson-Fabry disease related cardiomyopathy. Int. J. Cardiol. 2008, 130, 367–373. [Google Scholar] [CrossRef]
- Wu, J.C.; Ho, C.Y.; Skali, H.; Abichandani, R.; Wilcox, W.R.; Banikazemi, M.; Packman, S.; Sims, K.; Solomon, S.D. Cardiovascular manifestations of Fabry disease: Relationships between left ventricular hypertrophy, disease severity, and alpha-galactosidase Aactivity. Eur. Heart J. 2010, 31, 1088–1097. [Google Scholar] [CrossRef] [PubMed]
- Calcagnino, M.; O’Mahony, C.; Coats, C.; Cardona, M.; Garcia, A.; Janagarajan, K.; Mehta, A.; Hughes, D.; Murphy, E.; Lachmann, R.; et al. Exercise-induced left ventricular outflow tract obstruction in symptomatic patients with Anderson-Fabry disease. J. Am. Coll Cardiol. 2011, 58, 88–89. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Baig, S.; Abdel-Gadir, A.; Medina-Menacho, K.; Rosmini, S.; Captur, G.; Tchan, M.; Geberhiwot, T.; Murphy, E.; et al. Cardiac Phenotype of Prehypertrophic Fabry Disease. Circ. Cardiovasc. Imaging 2018, 11, e007168. [Google Scholar] [CrossRef]
- Pieroni, M.; Chimenti, C.; De Cobelli, F.; Morgante, E.; Del Maschio, A.; Gaudio, C.; Russo, M.A.; Frustaci, A. Fabry’s disease cardiomyopathy: Echocardiographicdetection of endomyocardial glycosphingolipid compartmentalization. J. Am. Coll. Cardiol. 2006, 47, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Kounas, S.; Demetrescu, C.; Pantazis, A.A.; Keren, A.; Lee, P.J.; Hughes, D.; Mehta, A.; Elliott, P.M. The binary endocardial appearance is a poor discriminator of Anderson-Fabry disease from familial hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2058–2061. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mundigler, G.; Gaggl, M.; Heinze, G.; Graf, S.; Zehetgruber, M.; Lajic, N.; Voigtländer, T.; Mannhalter, C.; Sunder-Plassmann, R.; Paschke, E.; et al. The endocardial binary appearance (‘binary sign’) is an unreliable marker for echocardiographic detection of Fabry disease in patients with left ventricularhypertrophy. Eur. J. Echocardiogr. 2011, 12, 744–749. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niemann, M.; Liu, D.; Hu, K.; Herrmann, S.; Breunig, F.; Strotmann, J.; Störk, S.; Voelker, W.; Ertl, G.; Wanner, C.; et al. Prominent papillary muscles in Fabry disease: Adiagnostic marker? Ultrasound Med. Biol. 2011, 37, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M. Echocardiographic assessment of fabry cardiomyopathy: Early diagnosis and follow-up. J. Am. Soc. Echocardiogr. 2011, 24, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Palecek, T.; Linhart, A.; Lubanda, J.C.; Magage, S.; Karetova, D.; Bultas, J.; Aschermann, M. Early diastolic mitral annular velocity and color M-mode flow propagation velocity in the evaluation of left ventricular diastolic function in patients with Fabry disease. Heart Vessels. 2006, 21, 13–19. [Google Scholar] [CrossRef]
- Pieroni, M.; Chimenti, C.; Ricci, R.; Sale, P.; Russo, M.A.; Frustaci, A. Early detection of Fabry cardiomyopathy by tissue Doppler imaging. Circulation 2003, 107, 1978–1984. [Google Scholar] [CrossRef] [PubMed]
- Pieroni, M.; Chimenti, C.; Russo, A.; Russo, M.A.; Maseri, A.; Frustaci, A. Tissue Doppler imaging in Fabry disease. Curr. Opin. Cardiol. 2004, 19, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Zamorano, J.; Serra, V.; Pérez de Isla, L.; Feltes, G.; Calli, A.; Barbado, F.J.; Torras, J.; Hernandez, S.; Herrera, J.; Herrero, J.A.; et al. Usefulness of tissue Doppler on early detection of cardiac disease in Fabry patients and potential role of enzyme replacement therapy (ERT) for avoiding progression of disease. Eur. J. Echocardiogr. 2011, 12, 671–677. [Google Scholar] [CrossRef]
- Linhart, A.; Lubanda, J.C.; Palecek, T.; Bultas, J.; Karetová, D.; Ledvinová, J.; Elleder, M.; Aschermann, M. Cardiac manifestations in Fabry disease. J. Inherit. Metab. Dis. 2001, 24 (Suppl. 2), 75–83; discussion 65. [Google Scholar] [CrossRef] [PubMed]
- Barbey, F.; Qanadli, S.D.; Juli, C.; Brakch, N.; Palacek, T.; Rizzo, E.; Jeanrenaud, X.; Eckhardt, B.; Linhart, A. Aortic remodelling in Fabry disease. Eur. Heart J. 2010, 31, 347–353. [Google Scholar] [CrossRef]
- Niemann, M.; Breunig, F.; Beer, M.; Hu, K.; Liu, D.; Emmert, A.; Herrmann, S.; Ertl, G.; Wanner, C.; Takenaka, T.; et al. Tei index in fabry disease. J. Am. Soc. Echocardiogr. 2011, 24, 1026–1032. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Lo, Q.; Devine, K.; Tchan, M.C.; Sillence, D.O.; Sadick, N.; Richards, D.A.; Thomas, L. Left atrial enlargement and reduced atrial compliance occurs early in Fabry cardiomyopathy. J. Am. Soc. Echocardiogr. 2013, 26, 1415–1423. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Russo, M.A.; Frustaci, A. Atrial biopsy evidence of Fabry disease causing lone atrial fibrillation. Heart 2010, 96, 1782–1783. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Oder, D.; Salinger, T.; Hu, K.; Müntze, J.; Weidemann, F.; Herrmann, S.; Ertl, G.; Wanner, C.; Frantz, S.; et al. Association and diagnostic utility of diastolic dysfunction and myocardial fibrosis in patients with Fabry disease. Open Heart 2018, 5, e000803. [Google Scholar] [CrossRef] [PubMed]
- Ferrans, V.J.; Hibbs, R.G.; Burda, C.D. The heart in Fabry’s disease. A histochemical and electron microscopic study. Am. J. Cardiol. 1969, 24, 95–110. [Google Scholar] [CrossRef]
- Krämer, J.; Niemann, M.; Liu, D.; Hu, K.; Machann, W.; Beer, M.; Wanner, C.; Ertl, G.; Weidemann, F. Two-dimensional speckle tracking as a non-invasive tool for identification of myocardial fibrosis in Fabry disease. Eur. Heart J. 2013, 34, 1587–1596. [Google Scholar] [CrossRef] [PubMed]
- Niemann, M.; Weidemann, F. Echocardiography in Fabry disease. Cardiogenetics 2013, 3, e3. [Google Scholar] [CrossRef]
- Palecek, T.; Dostalova, G.; Kuchynka, P.; Karetova, D.; Bultas, J.; Elleder, M.; Linhart, A. Right ventricular involvement in Fabry disease. J. Am. Soc. Echocardiogr. 2008, 21, 1265–1268. [Google Scholar] [CrossRef] [PubMed]
- Niemann, M.; Breunig, F.; Beer, M.; Herrmann, S.; Strotmann, J.; Hu, K.; Emmert, A.; Voelker, W.; Ertl, G.; Wanner, C.; et al. The right ventricle in Fabry disease: Natural history and impact of enzyme replacement therapy. Heart 2010, 96, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Graziani, F.; Laurito, M.; Pieroni, M.; Pennestrì, F.; Lanza, G.A.; Coluccia, V.; Camporeale, A.; Pedicino, D.; Verrecchia, E.; Manna, R.; et al. Right VentricularHypertrophy, SystolicFunction, and DiseaseSeverity in Anderson-FabryDisease: An EchocardiographicStudy. J. Am. Soc. Echocardiogr. 2017, 30, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Desnick, R.J.; Blieden, L.C.; Sharp, H.L.; Hofschire, P.J.; Moller, J.H. Cardiac valvular anomalies in Fabry disease. Clinical, morphologic, and biochemical studies. Circulation 1976, 54, 818–825. [Google Scholar] [CrossRef]
- Sheppard, M.N. The heart in Fabry’s disease. Cardiovasc. Pathol. 2011, 20, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Putko, B.N.; Wen, K.; Thompson, R.B.; Mullen, J.; Shanks, M.; Yogasundaram, H.; Sergi, C.; Oudit, G.Y. Anderson-Fabry cardiomyopathy: Prevalence, pathophysiology, diagnosis and treatment. Heart Fail. Rev. 2015, 20, 179–191. [Google Scholar] [CrossRef]
- D’Andrea, A.; Radmilovic, J.; Ballo, P.; Mele, D.; Agricola, E.; Cameli, M.; Rossi, A.; Esposito, R.; Novo, G.; Mondillo, S.; et al. Left ventricular hypertrophy or storage disease? the incremental value of speckle tracking strain bull’s-eye. Echocardiography 2017, 34, 746–759. [Google Scholar] [CrossRef] [PubMed]
- Shanks, M.; Thompson, R.B.; Paterson, I.D.; Putko, B.; Khan, A.; Chan, A.; Becher, H.; Oudit, G.Y. Systolic and diastolic function assessment in fabry disease patients using speckle-tracking imaging and comparison with conventional echocardiographic measurements. J. Am. Soc. Echocardiogr. 2013, 26, 1407–1414. [Google Scholar] [CrossRef] [PubMed]
- Esposito, R.; Galderisi, M.; Santoro, C.; Imbriaco, M.; Riccio, E.; Maria Pellegrino, A.; Sorrentino, R.; Lembo, M.; Citro, R.; Angela Losi, M.; et al. Prominent longitudinal strain reduction of left ventricular basal segments in treatment-naïve Anderson-Fabry disease patients. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Hu, K.; Nordbeck, P.; Ertl, G.; Störk, S.; Weidemann, F. Longitudinal strain bull’s eye plot patterns in patients with cardiomyopathy and concentric left ventricular hypertrophy. Eur. J. Med. Res. 2016, 10, 21. [Google Scholar] [CrossRef]
- Gruner, C.; Verocai, F.; Carasso, S.; Vannan, M.A.; Jamorski, M.; Clarke, J.T.; Care, M.; Iwanochko, R.M.; Rakowski, H. Systolic myocardial mechanics in patients with Anderson-Fabry disease with and without left ventricular hypertrophy and in comparison to nonobstructive hypertrophic cardiomyopathy. Echocardiography 2012, 29, 810–817. [Google Scholar] [CrossRef]
- Esposito, R.; Santoro, C.; Sorrentino, R.; Riccio, E.; Citro, R.; Buonauro, A.; Di Risi, T.; Imbriaco, M.; Trimarco, B.; Pisani, A.; et al. Layer-specific longitudinal strain in Anderson-Fabry disease at diagnosis: A speckle tracking echocardiography analysis. Echocardiography 2019, 36, 1273–1281. [Google Scholar] [CrossRef]
- Pichette, M.; Serri, K.; Pagè, M.; Zhao, L.; Bichet, D.G.; Poulin, F. Impaired left atrial function in Fabry disease: A longitudinal Speckle-Tracking Echocardiography study. J. Am. Soc. Echocardiogr. 2017, 30, 170–179. [Google Scholar] [CrossRef]
- Morris, D.A.; Blaschke, D.; Canaan-Kühl, S.; Krebs, A.; Knobloch, G.; Walter, T.C.; Haverkamp, W. Global cardiac alterations detected by speckle-tracking echocardiography in Fabry disease: Left ventricular, right ventricular, and left atrial dysfunction are common and linked to worse symptomatic status. Int. J. Cardiovasc. Imaging 2015, 31, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Cianciulli, T.F.; Saccheri, M.C.; Rísolo, M.A.; Lax, J.A.; Méndez, R.J.; Morita, L.A.; Beck, M.A.; Kazelián, L.R. Mechanical dispersion in Fabry disease assessed with speckle tracking echocardiography. Echocardiography 2020, 37, 293–301. [Google Scholar] [CrossRef]
- Chang, S.A.; Kim, H.K.; Lee, S.C.; Kim, E.Y.; Hahm, S.H.; Kwon, O.M.; Park, S.W.; Choe, Y.H.; Oh, J.K. Assessment of left ventricular mass in hypertrophic cardiomyopathy by real-time three-dimensional echocardiography using single-beat capture image. J. Am. Soc. Echocardiogr. 2013, 26, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.S.; Lee, P.; Hughes, D.; Thaman, R.; Sachdev, B.; Pellerin, D.; Mehta, A.; Elliott, P.M. The natural history of left ventricular systolic function in Anderson-Fabry disease. Heart 2005, 91, 533–534. [Google Scholar] [CrossRef] [PubMed]
- Alkema, M.; Spitzer, E.; Soliman, O.I.; Loewe, C. Multimodality Imaging for Left Ventricular Hypertrophy Severity Grading: A Methodological Review. J. Cardiovasc. Ultrasound 2016, 24, 257–267. [Google Scholar] [CrossRef]
- Rickers, C.; Wilke, N.M.; Jerosch-Herold, M.; Casey, S.A.; Panse, P.; Panse, N.; Weil, J.; Zenovich, A.G.; Maron, B.J. Utility of cardiac magnetic resonance imaging in the diagnosis of hypertrophic cardiomyopathy. Circulation 2005, 112, 855–861. [Google Scholar] [CrossRef]
- Hazari, H.; Belenkie, I.; Kryski, A.; White, J.A.; Oudit, G.Y.; Thompson, R.; Fung, T.; Dehar, N.; Khan, A. Comparison of Cardiac Magnetic Resonance Imaging and Echocardiography in Assessment of Left Ventricular Hypertrophy in Fabry Disease. Can. J. Cardiol. 2018, 34, 1041–1047. [Google Scholar] [CrossRef]
- Imbriaco, M.; Pisani, A.; Spinelli, L.; Cuocolo, A.; Messalli, G.; Capuano, E.; Marmo, M.; Liuzzi, R.; Visciano, B.; Cianciaruso, B.; et al. Effects of enzyme-replacement therapy in patients with Anderson-Fabry disease: A prospective long-term cardiac magnetic resonance imaging study. Heart 2009, 95, 1103–1107. [Google Scholar] [CrossRef]
- Kozor, R.; Callaghan, F.; Tchan, M.; Hamilton-Craig, C.; Figtree, G.A.; Grieve, S.M. A disproportionate contribution of papillary muscles and trabeculations to total left ventricular mass makes choice of cardiovascular magnetic resonance analysis technique critical in Fabry disease. J. Cardiovasc. Magn. Reson. 2015, 17, 22. [Google Scholar] [CrossRef]
- Kozor, R.; Nordin, S.; Treibel, T.A.; Rosmini, S.; Castelletti, S.; Fontana, M.; Captur, G.; Baig, S.; Steeds, R.P.; Hughes, D.; et al. Insight into hypertrophied hearts: A cardiovascular magnetic resonance study of papillary muscle mass and T1 mapping. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1034. [Google Scholar] [CrossRef] [PubMed]
- Deva, D.P.; Hanneman, K.; Li, Q.; Ng, M.Y.; Wasim, S.; Morel, C.; Iwanochko, R.M.; Thavendiranathan, P.; Crean, A.M. Cardiovascular magnetic resonance demonstration of the spectrum of morphological phenotypes and patterns of myocardial scarring in Anderson-fabry disease. J. Cardiovasc. Magn. Reson. 2016, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Spirito, P.; Bellone, P.; Harris, K.M.; Bernabo, P.; Bruzzi, P.; Maron, B.J. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N. Engl. J. Med. 2000, 342, 1778–1785. [Google Scholar] [CrossRef] [PubMed]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 19, 75. [Google Scholar] [CrossRef]
- Thompson, R.B.; Chow, K.; Khan, A.; Chan, A.; Shanks, M.; Paterson, I.; Oudit, G.Y. T₁ mapping with cardiovascular MRI is highly sensitive for Fabry disease independent of hypertrophy and sex. Circ. Cardiovasc. Imaging 2013, 6, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Sado, D.M.; White, S.K.; Piechnik, S.K.; Banypersad, S.M.; Treibel, T.; Captur, G.; Fontana, M.; Maestrini, V.; Flett, A.S.; Robson, M.D.; et al. Identification and assessment of Anderson-Fabry disease by cardiovascular magnetic resonance noncontrast myocardial T1 mapping. Circ. Cardiovasc. Imaging 2013, 6, 392–398. [Google Scholar] [CrossRef]
- Deborde, E.; Dubourg, B.; Bejar, S.; Brehin, A.C.; Normant, S.; Michelin, P.; Dacher, J.N. Differentiation between Fabry disease and hypertrophic cardiomyopathy with cardiac T1 mapping. Diagn. Interv. Imaging 2020, 101, 59–67. [Google Scholar] [CrossRef]
- Nordin, S.; Kozor, R.; Vijapurapu, R.; Augusto, J.B.; Knott, K.D.; Captur, G.; Treibel, T.A.; Ramaswami, U.; Tchan, M.; Geberhiwot, T.; et al. Myocardial Storage, Inflammation, and Cardiac Phenotype in Fabry Disease After One Year of Enzyme Replacement Therapy. Circ. Cardiovasc. Imaging 2019, 12, e009430. [Google Scholar] [CrossRef] [PubMed]
- Nordin, S.; Kozor, R.; Bulluck, H.; Castelletti, S.; Rosmini, S.; Abdel-Gadir, A.; Baig, S.; Mehta, A.; Hughes, D.; Moon, J.C. Cardiac Fabry disease with late gadolinium enhancement is a chronic inflammatory cardiomyopathy. J. Am. Coll. Cardiol. 2016, 68, 1707–1708. [Google Scholar] [CrossRef]
- Perry, R.; Shah, R.; Saiedi, M.; Patil, S.; Ganesan, A.; Linhart, A.; Selvanayagam, J.B. The Role of Cardiac Imaging in the Diagnosis and Management of Anderson-Fabry Disease. JACC Cardiovasc. Imaging 2019, 12, 1230–1242. [Google Scholar] [CrossRef]
- Koeppe, S.; Neubauer, H.; Breunig, F.; Weidemann, F.; Wanner, C.; Sandstede, J.; Machann, W.; Hahn, D.; Köstler, H.; Beer, M. MR-based analysis of regional cardiac function in relation to cellular integrity in Fabry disease. Int. J. Cardiol. 2012, 160, 53–58. [Google Scholar] [CrossRef]
- Strotmann, J.; Beer, M.; Machann, W.; Voelker, W.; Ertl, G.; Wanner, C.; Weidemann, F. Differences in fabry cardiomyopathy between female and male patients: Consequences for diagnostic assessment. JACC Cardiovasc. Imaging 2011, 4, 592–601. [Google Scholar] [CrossRef]
- Hsu, T.R.; Chang, F.P.; Chu, T.H.; Sung, S.H.; Bizjajeva, S.; Yu, W.C.; Niu, D.M. Correlations between endomyocardial biopsies and cardiac manifestations in taiwanese patients with the Chinese hotspot IVS4+919G>A mutation: Data from the fabry outcome survey. Int. J. Mol. Sci. 2017, 18, 119. [Google Scholar] [CrossRef] [PubMed]
- Weidemann, F.; Beer, M.; Kralewski, M.; Siwy, J.; Kampmann, C. Early detection of organ involvement in fabry disease by biomarker assessment in conjunction with LGE cardiac MRI: Results from the SOPHIA study. Mol. Genet. Metab. 2019, 126, 169–182. [Google Scholar] [CrossRef] [PubMed]
- Smid, B.E.; van der Tol, L.; Cecchi, F.; Elliott, P.M.; Hughes, D.A.; Linthorst, G.E.; Timmermans, J.; Weidemann, F.; West, M.L.; Biegstraaten, M.; et al. Uncertain diagnosis of Fabry disease: Consensus recommendation on diagnosis in adults with left ventricular hypertrophy and genetic variants of unknown significance. Int. J. Cardiol. 2014, 177, 400–408. [Google Scholar] [CrossRef]
- De Cobelli, F.; Esposito, A.; Belloni, E.; Pieroni, M.; Perseghin, G.; Chimenti, C.; Frustaci, A.; Del Maschio, A. Delayed-enhanced cardiac MRI for differentiation of Fabry’s disease from symmetric hypertrophic cardiomy-opathy. AJR Am. J. Roentgenol. 2009, 192, W97–W102. [Google Scholar] [CrossRef]
- Hanneman, K.; Karur, G.R.; Wasim, S.; Wald, R.M.; Iwanochko, R.M.; Morel, C.F. Left Ventricular Hypertrophy and Late Gadolinium Enhancement at Cardiac MRI Are Associated with Adverse Cardiac Events in Fabry Disease. Radiology 2019, 00, 1–9. [Google Scholar] [CrossRef]
- Augustine, D.; Lewandowski, A.J.; Lazdam, M.; Rai, A.; Francis, J.; Myerson, S.; Noble, A.; Becher, H.; Neubauer, S.; Petersen, S.E.; et al. Global and regional left ventricular myocardial deformation measures by magnetic resonance feature tracking in healthy volunteers: Comparison with tagging and relevance of gender. J. Cardiovasc. Magn. Reson. 2013, 15, 8. [Google Scholar] [CrossRef] [PubMed]
- Mathur, S.; Dreisbach, J.G.; Karur, G.R.; Iwanochko, R.M.; Morel, C.F.; Wasim, S.; Nguyen, E.T.; Wintersperger, B.J.; Hanneman, K. Loss of base-to-apex circumferential strain gradient assessed by cardiovascular magnetic resonance in Fabry disease: Relationship to T1 mapping, late gadolinium enhancement and hypertrophy. J. Cardiovasc. Magn. Reson. 2019, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.C.; Ambach, S.; Madueme, P.C.; Khoury, P.R.; Hopkin, R.J.; Jefferies, J.L. Comparison of Native T1, Strain, and Traditional Measures of Cardiovascular Structure and Function by Cardiac Magnetic Resonance Imaging in Patients With Anderson-Fabry Disease. Am. J. Cardiol. 2018, 122, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Augusto, J.B.; Johner, N.; Shah, D.; Nordin, S.; Knott, K.D.; Rosmini, S.; Lau, C.; Alfarih, M.; Hughes, R.; Seraphim, A.; et al. The myocardial phenotype of Fabry disease pre-hypertrophy and pre-detectable storage. Eur. Heart J. Cardiovasc. Imaging 2020, jeaa101. [Google Scholar] [CrossRef]
- Knott, K.D.; Augusto, J.B.; Nordin, S.; Kozor, R.; Camaioni, C.; Xue, H.; Hughes, R.K.; Manisty, C.; Brown, L.A.E.; Kellman, P.; et al. Quantitative Myocardial Perfusion in Fabry Disease. Circ. Cardiovasc. Imaging 2019, 12, e008872. [Google Scholar] [CrossRef] [PubMed]
- Chimenti, C.; Morgante, E.; Tanzilli, G.; Mangieri, E.; Critelli, G.; Gaudio, C.; Russo, M.A.; Frustaci, A. Angina in fabrydiseasereflects coro- nary small vessel disease. Circ. Heart Fail. 2008, 1, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Tomberli, B.; Cecchi, F.; Sciagrà, R.; Berti, V.; Lisi, F.; Torricelli, F.; Morrone, A.; Castelli, G.; Yacoub, M.H.; Olivotto, I. Coronary microvascular dysfunction is an early feature of cardiac involvement in patients with Anderson-fabry disease. Eur. J. Heart Fail. 2013, 15, 1363–1373. [Google Scholar] [CrossRef]
- Elliott, P.M.; Kindler, H.; Shah, J.S.; Sachdev, B.; Rimoldi, O.E.; Thaman, R.; Tome, M.T.; McKenna, W.J.; Lee, P.; Camici, P.G. Coronary microvascular dysfunction in male patients with Anderson-fabry disease and the effect of treatment with alpha galactosidase A. Heart 2006, 92, 357–360. [Google Scholar] [CrossRef]
- Spinelli, L.; Imbriaco, M.; Nappi, C.; Nicolai, E.; Giugliano, G.; Ponsiglione, A.; Diomiaiuti, T.C.; Riccio, E.; Duro, G.; Pisani, A.; et al. Early Cardiac Involvement Affects Left Ventricular Longitudinal Function in Females Carrying α-Galactosidase A Mutation: Role of Hybrid Positron Emission Tomography and Magnetic Resonance Imaging and Speckle-Tracking Echocardiography. Circ. Cardiovasc. Imaging 2018, 11, e007019. [Google Scholar] [CrossRef] [PubMed]
- Nappi, C.; Altiero, M.; Imbriaco, M.; Nicolai, E.; Giudice, C.A.; Aiello, M.; Diomiaiuti, C.T.; Pisani, A.; Spinelli, L.; Cuocolo, A. First experience of simultane- ous PET/MRI for the early detection of cardiac involvement in patients with Anderson-fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1025–1031. [Google Scholar] [CrossRef] [PubMed]
- Imbriaco, M.; Nappi, C.; Ponsiglione, A.; Pisani, A.; Dell’Aversana, S.; Nicolai, E.; Spinelli, L.; Aiello, M.; Diomiaiuti, C.T.; Riccio, E.; et al. Hybrid positron emission tomography-magnetic resonance imaging for assessing different stages of cardiac impairment in patients with Anderson-Fabry disease: AFFINITY study group. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 1004–1011. [Google Scholar] [CrossRef]
- Alamartine, E.; Sury, A.; Roche, F.; Pichot, V.; Barthelemy, J. Autonomic nervous system activity in patients with Fabry disease. Open J. Intern. Med. 2012, 2, 116–122. [Google Scholar] [CrossRef]
- Spinelli, L.; Imbriaco, M.; Giugliano, G.; Nappi, C.; Gaudieri, V.; Riccio, E.; Pisani, A.; Trimarco, B.; Cuocolo, A. Focal reduction in left ventricular 123I-metaiodobenzylguanidine uptake and impairment in systolic function in patients with Anderson-Fabry disease. J. Nucl. Cardiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Imbriaco, M.; Pellegrino, T.; Piscopo, V.; Petretta, M.; Ponsiglione, A.; Nappi, C.; Puglia, M.; Dell’Aversana, S.; Riccio, E.; Spinelli, L.; et al. Cardiac sympathetic neuronal damage precedes myocardial fibrosis in patients with Anderson- fabry disease. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 2266–2273. [Google Scholar] [CrossRef]
- Yamamoto, S.; Suzuki, H.; Sugimura, K.; Tatebe, S.; Aoki, T.; Miura, M.; Yaoita, N.; Sato, H.; Kozu, K.; Ota, H.; et al. Focal Reduction in Cardiac 123I-Metaiodobenzylguanidine Uptake in Patients With Anderson-Fabry Disease. Circ. J. 2016, 80, 2550–2551. [Google Scholar] [CrossRef]
- Hoffmann, B.; Mayatepek, E. Fabry disease-often seen, rarely diagnosed. Dtsch. Arztebl. Int. 2009, 106, 440–447. [Google Scholar] [CrossRef]



| Standard Echofindings | Description | Features |
|---|---|---|
| LV Hypertrophy | -Usuallysymmetric with concentric geometry -Rarely asymmetric septal hypertrophy, apical hypertrophy or eccentric geometry | -Predominant manifestation of AFD cardiomyopathy -Occurs in the 4th decade of life in men, later in women |
| Binary Sign | -Hyperechogenic endocardial surface adjacent to a hypoechogenic subendocardial layer | -Once considered pathognomonic -Overall low sensitivity and specificity |
| Prominent Papillary Muscles | -Papillary muscle thickening and hyperechogenicity | -Late sign, not for screening purposes |
| Preserved LV EF | -LV EF is usually in the normal range | -LV systolic dysfunction is a marker of severe cardiac involvement related to a poor prognosis |
| Diastolic Dysfunction | -Mitral flow Doppler parameters alteration -E/e’ ratio increase -LA dilation | -In AFD patients with LV hypertrophy, diastolic dysfunction underlies the symptoms of heart failure |
| Right Ventricle Hypertrophy | -Usually RV systolic function is preserved -Often associated withRV diastolic dysfunction | -Its prevalence varies between studies -No sex differences |
| Advanced Echocardiography | Description | Features |
|---|---|---|
| GLS | -Reduction in LV GLS with a prevalent involvement of the infero-lateral wall of the LV | -Correlates with LGE at CMR |
| GCS | -Reduction in the normal base-to-apex CS gradient | -Differential diagnosis with HCM where GCS increases with a preserved base-to-apex gradient |
| RVLS | -Reduction in the RV Longitudinal strain | -Early sign of RV dysfunction |
| CMR Sequences | Description | Features |
|---|---|---|
| Cine-sequences | -Measurement of LV mass, ventricular volumes, LV and RV EF, wall motion assessment | -Better quantification of LV papillary muscle mass |
| LGE | -Fibrosis usually localized at mid-wall in the basal infero-lateral area of LV -Very extensive and diffuse in advanced AFD | -Suggestive of AFD when in the typical localization -Additionally present in patients without LVH -Strongly correlated with more CV events |
| T1 mapping | -Lower native T1 times | -Early sign of cardiac involvement -Pathognomonic of AFD -Pseudo-normalization of T1 times correlates with the presence of LGE |
| T2 mapping | -Elevation of T2 times in inferolateral wall or LGE areas | -Suggestive of myocardial inflammation -T2 times elevation correlates with troponin elevation -No pathognomonic |
| ECV | Normal values except in LGE areas | -No pathognomonic -Reflects interstitial fibrosis |
| Speckle Tracking Analysis | -GLS shows no significant differences -GCS has a significant increase in LVH patients with loss of base-to-apex GCS gradient | -GCS may be an early marker of cardiac involvement -Speckle tracking analysis is not commonly used in CMR, further investigations are required |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Esposito, R.; Santoro, C.; Mandoli, G.E.; Cuomo, V.; Sorrentino, R.; La Mura, L.; Pastore, M.C.; Bandera, F.; D’Ascenzi, F.; Malagoli, A.; et al. Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future. J. Clin. Med. 2021, 10, 1994. https://doi.org/10.3390/jcm10091994
Esposito R, Santoro C, Mandoli GE, Cuomo V, Sorrentino R, La Mura L, Pastore MC, Bandera F, D’Ascenzi F, Malagoli A, et al. Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future. Journal of Clinical Medicine. 2021; 10(9):1994. https://doi.org/10.3390/jcm10091994
Chicago/Turabian StyleEsposito, Roberta, Ciro Santoro, Giulia Elena Mandoli, Vittoria Cuomo, Regina Sorrentino, Lucia La Mura, Maria Concetta Pastore, Francesco Bandera, Flavio D’Ascenzi, Alessandro Malagoli, and et al. 2021. "Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future" Journal of Clinical Medicine 10, no. 9: 1994. https://doi.org/10.3390/jcm10091994
APA StyleEsposito, R., Santoro, C., Mandoli, G. E., Cuomo, V., Sorrentino, R., La Mura, L., Pastore, M. C., Bandera, F., D’Ascenzi, F., Malagoli, A., Benfari, G., D’Andrea, A., & Cameli, M. (2021). Cardiac Imaging in Anderson-Fabry Disease: Past, Present and Future. Journal of Clinical Medicine, 10(9), 1994. https://doi.org/10.3390/jcm10091994










