The Influence of Multidrug-Resistant Bacteria on Clinical Outcomes of Diabetic Foot Ulcers: A Systematic Review
Abstract
1. Introduction
2. Methodology
2.1. Search Strategy
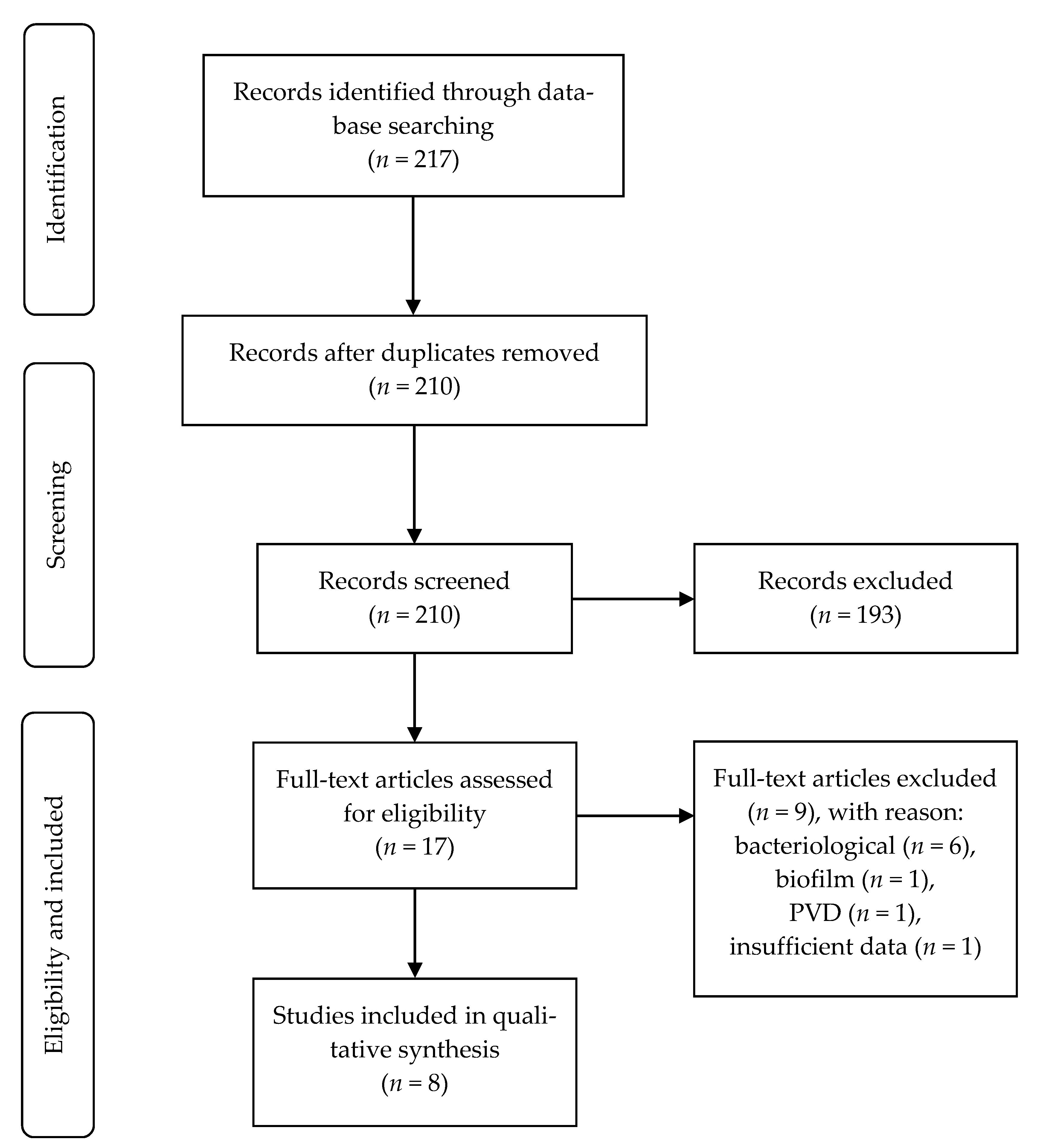
2.2. Selection of Studies
2.3. Data Collection
2.4. Quality Assessment
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Mohler, M.J.; Wendel, C.S.; Lipsky, B.A. Risk Factors for Foot Infections in Individuals With Diabetes. Diabetes Care 2006, 29, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Swerdlow, M.A.; Armstrong, A.A.; Conte, M.S.; Padula WV Bus, S.A. Five year mortality and direct costs of care for people with diabetic foot complications are comparable to cancer. J. Foot Ankle Res. 2020, 13, 16. [Google Scholar] [CrossRef]
- Lipsky, B.A. Bone of Contention: Diagnosing Diabetic Foot Osteomyelitis. Clin. Infect. Dis. 2008, 47, 528–530. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Nguyen, T.T. Strategy for Treatment of Infected Diabetic Foot Ulcers. Acc. Chem. Res. 2021, 54, 1080–1093. [Google Scholar] [CrossRef] [PubMed]
- van Baal, J.G. Surgical Treatment of the Infected Diabetic Foot. Clin. Infect. Dis. 2004, 39, S123–S128. [Google Scholar] [CrossRef]
- Lipsky, B.A. Aragón-Sánchez J, Diggle M, Embil J, Kono S, Lavery L; et al. IWGDF guidance on the diagnosis and management of foot infections in persons with diabetes. Diabetes Metab. Res. Rev. 2016, 32, 45–74. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Senneville, É.; Abbas, Z.G.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab. Res. Rev. 2020, 36, e3280. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Berendt, A.R.; Cornia, P.B.; Pile, J.C.; Peters, E.J.; Armstrong, D.G.; Deery, H.G.; Embil, J.M.; Joseph, W.S.; Karchmer, A.W.; et al. 2012 Infectious Diseases Society of America Clinical Practice Guideline for the Diagnosis and Treatment of Diabetic. Foot Infect. Clin. Infect. Dis. 2012, 54, e132–e173. [Google Scholar] [CrossRef]
- Aragón-Sánchez, J.; Lipsky, B.A. Modern management of diabetic foot osteomyelitis. The when, how and why of conservative approaches. Expert Rev. Anti Infect. Ther. 2018, 16, 35–50. [Google Scholar] [CrossRef]
- Kwon, K.T.; Armstrong, D.G. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infect. Chemother 2018, 50, 11. [Google Scholar] [CrossRef]
- Eleftheriadou, I.; Tentolouris, N.; Argiana, V.; Jude, E.; Boulton, A.J. Methicillin-Resistant Staphylococcus aureus in Diabetic Foot Infections. Drugs 2010, 70, 1785–1797. [Google Scholar] [CrossRef]
- Stacey, H.J.; Clements, C.S.; Welburn, S.C.; Jones, J.D. The prevalence of methicillin-resistant Staphylococcus aureus among diabetic patients: A meta-analysis. Acta Diabetol. 2019, 56, 907–921. [Google Scholar] [CrossRef]
- Saltoglu, N.; Ergonul, O.; Tulek, N.; Yemisen, M.; Kadanali, A.; Karagoz, G.; Batirel, A.; Ak, O.; Sonmezer, C.; Eraksoy, H.; et al. Influence of multidrug resistant organisms on the outcome of diabetic foot infection. Int. J. Infect. Dis. 2018, 70, 10–14. [Google Scholar] [CrossRef]
- Chen, Y.; Ding, H.; Wu, H.; Chen, H.-L. The Relationship Between Osteomyelitis Complication and Drug-Resistant Infection Risk in Diabetic Foot Ulcer: A Meta-analysis. Int. J. Low. Extrem. Wounds 2017, 16, 183–190. [Google Scholar] [CrossRef]
- Ji, X.; Jin, P.; Chu, Y.; Feng, S.; Wang, P. Clinical Characteristics and Risk Factors of Diabetic Foot Ulcer With Multidrug-Resistant Organism Infection. Int. J. Low. Extrem. Wounds 2014, 13, 64–71. [Google Scholar] [CrossRef]
- Dai, J.; Jiang, C.; Chen, H.; Chai, Y. Assessment of the Risk Factors of Multidrug-Resistant Organism Infection in Adults With Type 1 or Type 2 Diabetes and Diabetic Foot Ulcer. Can. J. Diabetes 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hartemann-Heurtier, A.; Robert, J.; Jacqueminet, S.; Ha Van, G.; Golmard, J.L.; Jarlier, V.; Grimaldi, A. Diabetic foot ulcer and multidrug-resistant organisms: Risk factors and impact. Diabet. Med. 2004, 21, 710–715. [Google Scholar] [CrossRef]
- Gadepalli, R.; Dhawan, B.; Sreenivas, V.; Kapil, A.; Ammini, A.C.; Chaudhry, R. A Clinico-microbiological Study of Diabetic Foot Ulcers in an Indian Tertiary Care Hospital. Diabetes Care 2006, 29, 1727–1732. [Google Scholar] [CrossRef] [PubMed]
- Kandemir, Ö.; Akbay, E.; Şahin, E.; Milcan, A.; Gen, R. Risk factors for infection of the diabetic foot with multi-antibiotic resistant microorganisms. J. Infect. 2007, 54, 439–445. [Google Scholar] [CrossRef]
- Zhang, J.; Chu, Y.; Wang, P.; Ji, X.; Li, X.; Wang, C.; Peng, Y. Clinical Outcomes of Multidrug Resistant Pseudomonas aeruginosa Infection and the Relationship With Type III Secretion System in Patients With Diabetic Foot. Int. J. Low. Extrem. Wounds 2014, 13, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Noor, S.; Borse, A.G.; Ozair, M.; Raghav, A.; Parwez, I.; Ahmad, J. Inflammatory markers as risk factors for infection with multidrug-resistant microbes in diabetic foot subjects. Foot 2017, 32, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Malik, A.; Ahmad, J. Clinico-microbiological study and antimicrobial drug resistance profile of diabetic foot infections in North India. Foot 2010, 21, 6–14. [Google Scholar] [CrossRef] [PubMed]
- Richard, J.L.; Sotto, A.; Jourdan, N.; Combescure, C.; Vannereau, D.; Rodier, M.; Lavigne, J.P.; Nîmes University Hospital Working Group on the Diabetic Foot. Risk factors and healing impact of multidrug-resistant bacteria in diabetic foot ulcers. Diabetes Metab. 2008, 34, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; The_PRISMA_Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann. Intern. Med. 2009, 151, 264. [Google Scholar] [CrossRef]
- Oxford Centre for Evidence-Based Medicine. Levels of Evidence (March 2009) 2009. Available online: http://www.cebm.net/oxford-centre-evidence-based-medicine-levels-evidence-march-2009/ (accessed on 30 May 2020).
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef]
- Gristina, A.G.; Costerton, J.W. Bacterial adherence and the glycocalyx and their role in musculoskeletal infection. Orthop. Clin. N. Am. 1984, 15, 517–535. [Google Scholar] [CrossRef]
- Uçkay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes. Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef]
- Saseedharan, S.; Sahu, M.; Chaddha, R.; Pathrose, E.; Bal, A.; Bhalekar, P.; Sekar, P.; Krishnan, P. Epidemiology of diabetic foot infections in a reference tertiary hospital in India. Braz. J. Microbiol. 2018, 49, 401–406. [Google Scholar] [CrossRef]
- Lázaro-Martínez, J.L.; Álvaro-Afonso, F.J.; Sevillano-Fernández, D.; Molines-Barroso, R.J.; García-Álvarez, Y.; García-Morales, E. Clinical and Antimicrobial Efficacy of a Silver Foam Dressing With Silicone Adhesive in Diabetic Foot Ulcers With Mild Infection. Int. J. Low. Extrem. Wounds 2019, 18, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.G.; Lavery, L.A.; Harkless, L.B. Validation of a Diabetic Wound Classification System: The contribution of depth, infection, and ischemia to risk of amputation. Diabetes Care 1998, 21, 855–859. [Google Scholar] [CrossRef]
- Lavery, L.A.; Armstrong, D.G.; Murdoch, D.P.; Peters, E.J.G.; Lipsky, B.A. Validation of the Infectious Diseases Society of America’s Diabetic Foot Infection Classification System. Clin. Infect. Dis. 2007, 44, 562–565. [Google Scholar] [CrossRef]
- Zhu, H.; Conibear, T.C.R.; Bandara, R.; Aliwarga, Y.; Stapleton, F.; Willcox, M.D.P. Type III Secretion System–Associated Toxins, Proteases, Serotypes, and Antibiotic Resistance of Pseudomonas aeruginosa Isolates Associated with Keratitis. Curr. Eye Res. 2006, 31, 297–306. [Google Scholar] [CrossRef]
- Schulert, G.S.; Feltman, H.; Rabin, S.D.; Martin, C.G.; Battle, S.E.; Rello, J.; Hauser, A.R. Secretion of the Toxin ExoU Is a Marker for Highly Virulent Pseudomonas aeruginosa Isolates Obtained from Patients with Hospital-Acquired Pneumonia. J. Infect. Dis. 2003, 188, 1695–1706. [Google Scholar] [CrossRef] [PubMed]
- Wukich, D.K.; Hobizal, K.B.; Raspovic, K.M.; Rosario, B.L. SIRS Is Valid in Discriminating Between Severe and Moderate Diabetic Foot Infections. Diabetes Care 2013, 36, 3706–3711. [Google Scholar] [CrossRef]
- Wukich, D.K.; Hobizal, K.B.; Brooks, M.M. Severity of Diabetic Foot Infection and Rate of Limb Salvage. Foot Ankle Int. 2013, 34, 351–358. [Google Scholar] [CrossRef]
- Abramson, M.A.; Sexton, D.J. Nosocomial Methicillin-Resistant and Methicillin-Susceptible Staphylococcus Aureus Primary Bacteremia: At What Costs? Infect. Control Hosp. Epidemiol. 1999, 20, 408–411. [Google Scholar] [CrossRef]
- Rello, J.; Torres, A.; Ricart, M.; Valles, J.; Gonzalez, J.; Artigas, A.; Rodriguez-Roisin, R. Ventilator-associated pneumonia by Staphylococcus aureus. Comparison of methicillin-resistant and methicillin-sensitive episodes. Am. J. Respir. Crit. Care Med. 1994, 150, 1545–1549. [Google Scholar] [CrossRef]
- Soriano, A.; Martinez, J.A.; Mensa, J.; Marco, F.; Almela, M.; Moreno-Martinez, A.; Sanchez, F.; Munoz, I.; Jimenez de Anta, M.T.; Soriano, E. Pathogenic Significance of Methicillin Resistance for Patients with Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2000, 30, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.T.; Carmeli, Y.; Falagas, M.T.; Giske, C.T.; Harbarth, S.; Hindler, J.T.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
| Study Design | Level of Evidence | Degree of Recommendation | |
|---|---|---|---|
| Ji et al. [15] | Retrospective cohort | 2b | B |
| Hartemann et al. [17] | Prospective cohort | 1b | A |
| Gadepalli et al. [18] | Retrospective cohort | 2b | B |
| Kandemir et al. [19] | Retrospective cohort | 2b | B |
| Zhang et al. [20] | Retrospective cohort | 2b | B |
| Noor et al. [21] | Retrospective cohort | 2b | B |
| Zubair et al. [22] | Retrospective cohort | 2b | B |
| Richard et al. [23] | Prospective cohort | 1b | A |
| Item Number–STROBE Guidelines | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | ||
| T | A | ||||||||||||||||||||||
| Ji et al. [15] | N | Y | Y | N | N | Y | Y | Y | Y | N | N | N | N | Y | Y | Y | Y | N | Y | Y | Y | N | Y |
| Hartemann et al. [17] | N | Y | Y | N | N | Y | Y | Y | Y | Y | N | Y | N | Y | Y | Y | N | Y | Y | N | Y | Y | N |
| Gadepalli et al. [18] | N | Y | N | N | N | Y | N | Y | Y | N | N | Y | N | N | Y | Y | Y | N | N | N | Y | N | Y |
| Kandemir et al. [19] | N | Y | Y | N | N | Y | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | N | Y | Y | N | Y | Y | N |
| Zhang et al. [20] | N | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y |
| Noor et al. [21] | N | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | N | N | Y | N | Y | Y | Y |
| Zubair et al. [22] | N | Y | Y | N | Y | Y | Y | Y | Y | N | N | Y | N | Y | Y | Y | Y | Y | N | N | Y | Y | Y |
| Richard et al. [23] | N | Y | N | N | Y | Y | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | Y | Y |
| Study/Year | Country | Participants Age N = MDRO+/MDRO− | Ulcer Duration MDRO+/MDRO− p-Value | Time to Ulcer HealingMDRO+/MDRO− p-Value | Event MDRO+/MDRO− p-Value | |
|---|---|---|---|---|---|---|
| Ji et al. [15]/2014 | China | 64.1 ± 10.5/64.4 ± 11 118 = 64/54 | 8.54 ± 8.43/5.96 ± 5.94 0.053 | No description | No description | |
| Hartemann et al. [17]/2004 | France | 65 ± 12 180 = 32/148 | 19.71 ± 36/28.71 ± 55.71 0.30 | Survival analysis No include mean ± SD 0.71 | No description | |
| Gadepalli et al. [18]/2006 | India | 53.9 ± 12.1 80 = 58/22 | 12.43 ± 9.04/15.86 ± 11.14 0.14 | No description | Duration of hospitalisation | 2.76 ± 0.88/2.65 ± 0.8 0.61 |
| Surgery (amputation included) | 47 (81)/10 (45.5) <0.01 * | |||||
| Death | 2 (3.4)/0 (0.0) 0.38 | |||||
| Kandemir et al. [19]/2006 | Turkey | 60 ± 11 73 = 36/37 | 25.71 ± 77.43/16.14 ± 28.43 0.32 | No description | Duration of hospitalisation | 5.29 ± 4.14/2.86 ± 2.71 0.00 ** |
| Zhang et al. [20]/2014 | China | 65 ± 12.3/64 ± 10.8 117 = 43/74 | 10.6 ± 8.8/9.4 ± 6.3 0.457 | Follow up 12 weeks 9 (20.9)/31 (41.9) 0.032 | Amputation | 14 (32.6)/12 (16.2) 0.032 |
| Noor et al. [21]/2017 | India | 53 ± 9 65 = 37/28 | 9 ± 6.86/6.86 ± 3.43 <0.05 ** | No description | Amputation | 19 (51.35)/7 (25) <0.05 ** |
| Zubair et al. [22]/2010 | India | 49.11 ± 12.46 102 = 46/56 | No description | No description | Duration of hospitalisation | 3.84 ± 1.03/2.56 ± 0.41 0.005 ** |
| Amputation | 19 (41.3)/4 (7.1) <0.001 ** | |||||
| Death | 4 (8.6)/1 (1.7) 0.002 ** | |||||
| Richard et al. [23]/2008 | France | 68.0 (no include SD) 188 = 45/143 | No description | Follow up 10 weeks 25 (51.1)/99 (69.2) 0.04 * Survival analysis 14/10 (Does not include SD) 0.036 * | Total amputation | 16 (35.6)/16 (11.2) <0.001 ** |
| Major amputation | 3 (18.8)/1 (6.3) 0.05 ** | |||||
| Minor amputation | 13 (81.2)/15 (93.7) 0.02 ** | |||||
| Death | 2 (4.4)/8 (5.6) NS | |||||
| Definition of MDRO | |
|---|---|
| Ji et al. [15] | Bacteria resistant to at least one agent in three or more antimicrobial categories. |
| Hartemann et al. [17] | MRSA; bacteria producing extended spectrum ESBL; Pseudomonas aeruginosa resistant to both ceftazidime and imipenem; Acinetobacter baumannii resistant to imipenem. |
| Gadepalli et al. [18] | MRSA; ESBL producing bacteria; methicillin-resistant coagulase-negative Staphylococci. |
| Kandemir et al. [19] | MRSA; methicillin-resistant Staphylococcus epidermidis; penicillin resistant Staphylococcus pneumoniae; Enterococcus spp.; ESBL producing bacteria and inducible beta-lactamase; P. aeruginosa resistant to both ceftazidime and imipenem; A. baumannii resistant to imipenem. |
| Zhang et al. [20] | Bacteria resistant to at least 1 agent in each of the 3 or more antipseudomonal agents. |
| Noor et al. [21] | No description. |
| Zubair et al. [22] | Bacteria resistant to two or more antimicrobial classes; MRSA; ESBL producing organisms. |
| Richard et al. [23] | MRSA, Enterobacteriaceae resistant to third-generation cephalosporins; P. aeruginosa resistant to two antibiotics from among ticarcillin, ciprofloxacin, ceftazidime and imipenem; Enterococcus spp. resistant to glycopeptides; A. baumannii resistant to ticarcillin; Stenotrophomonas maltophilia. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matta-Gutiérrez, G.; García-Morales, E.; García-Álvarez, Y.; Álvaro-Afonso, F.J.; Molines-Barroso, R.J.; Lázaro-Martínez, J.L. The Influence of Multidrug-Resistant Bacteria on Clinical Outcomes of Diabetic Foot Ulcers: A Systematic Review. J. Clin. Med. 2021, 10, 1948. https://doi.org/10.3390/jcm10091948
Matta-Gutiérrez G, García-Morales E, García-Álvarez Y, Álvaro-Afonso FJ, Molines-Barroso RJ, Lázaro-Martínez JL. The Influence of Multidrug-Resistant Bacteria on Clinical Outcomes of Diabetic Foot Ulcers: A Systematic Review. Journal of Clinical Medicine. 2021; 10(9):1948. https://doi.org/10.3390/jcm10091948
Chicago/Turabian StyleMatta-Gutiérrez, Gianmarco, Esther García-Morales, Yolanda García-Álvarez, Francisco Javier Álvaro-Afonso, Raúl Juan Molines-Barroso, and José Luis Lázaro-Martínez. 2021. "The Influence of Multidrug-Resistant Bacteria on Clinical Outcomes of Diabetic Foot Ulcers: A Systematic Review" Journal of Clinical Medicine 10, no. 9: 1948. https://doi.org/10.3390/jcm10091948
APA StyleMatta-Gutiérrez, G., García-Morales, E., García-Álvarez, Y., Álvaro-Afonso, F. J., Molines-Barroso, R. J., & Lázaro-Martínez, J. L. (2021). The Influence of Multidrug-Resistant Bacteria on Clinical Outcomes of Diabetic Foot Ulcers: A Systematic Review. Journal of Clinical Medicine, 10(9), 1948. https://doi.org/10.3390/jcm10091948











