Immunoglobulin-Storing Histiocytosis: A Case Based Systemic Review
Abstract
1. Introduction
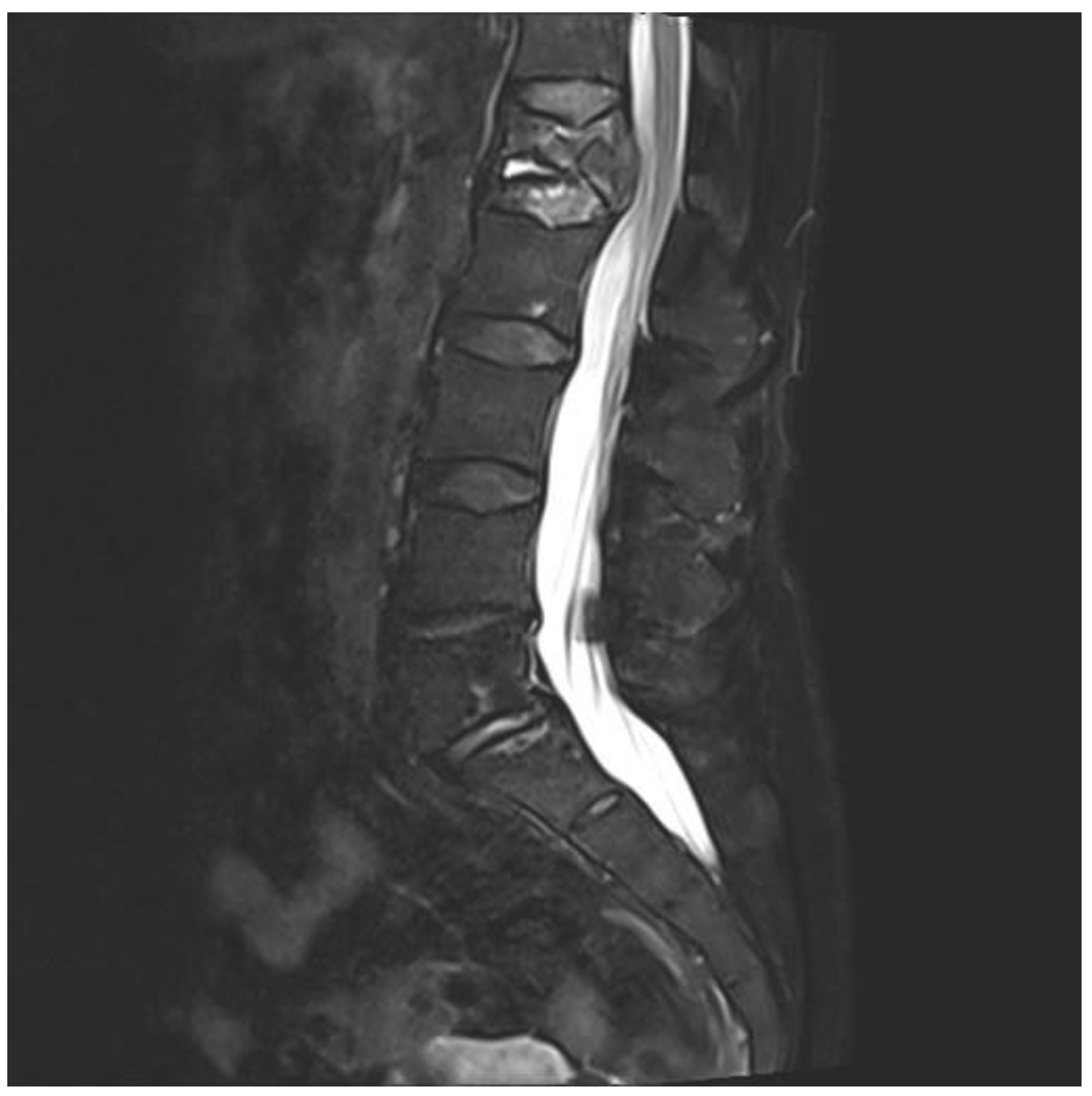
2. Case Report
3. Literature Review
3.1. Methods and Classification
3.2. Classification and Etiology
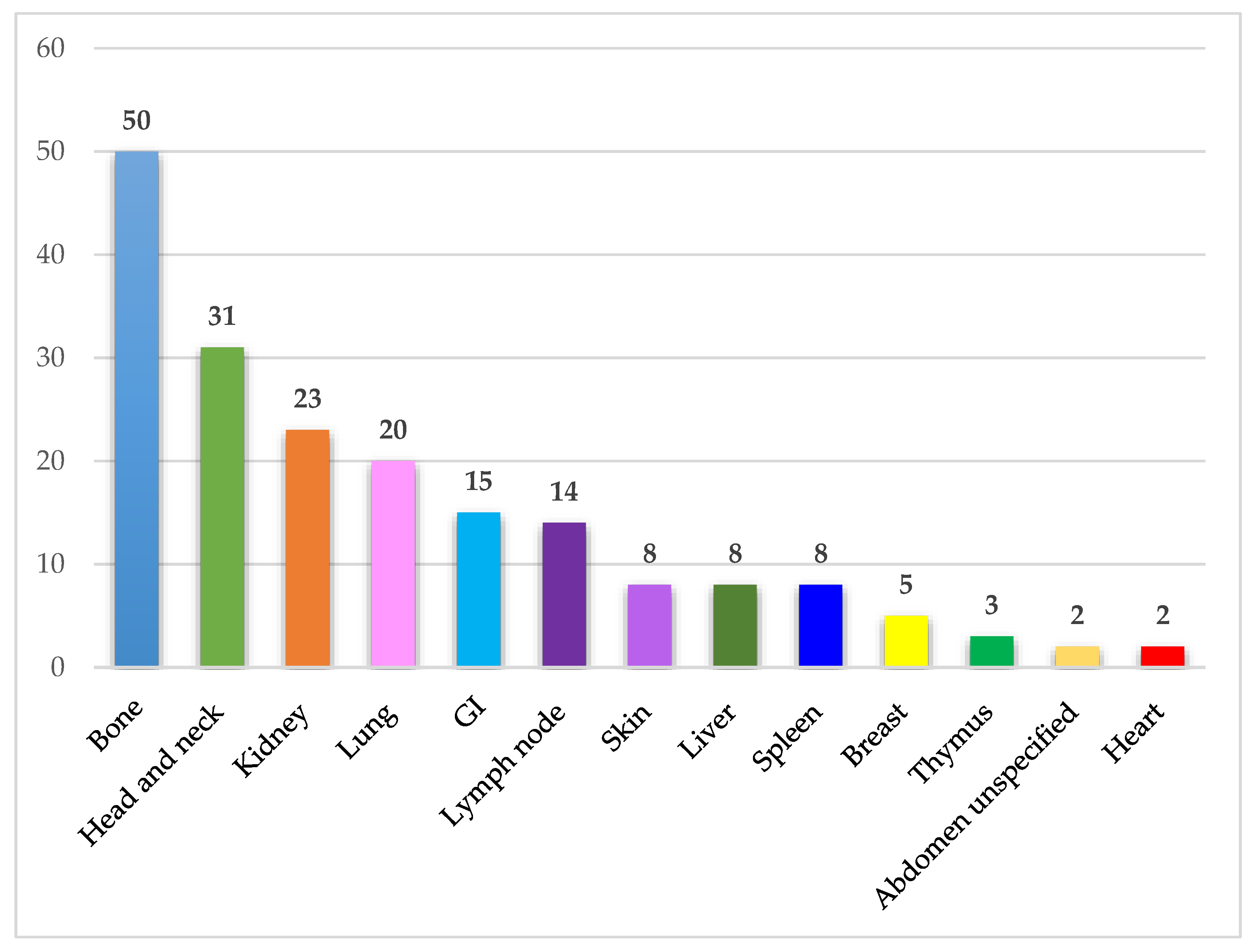
3.3. Organ Affection
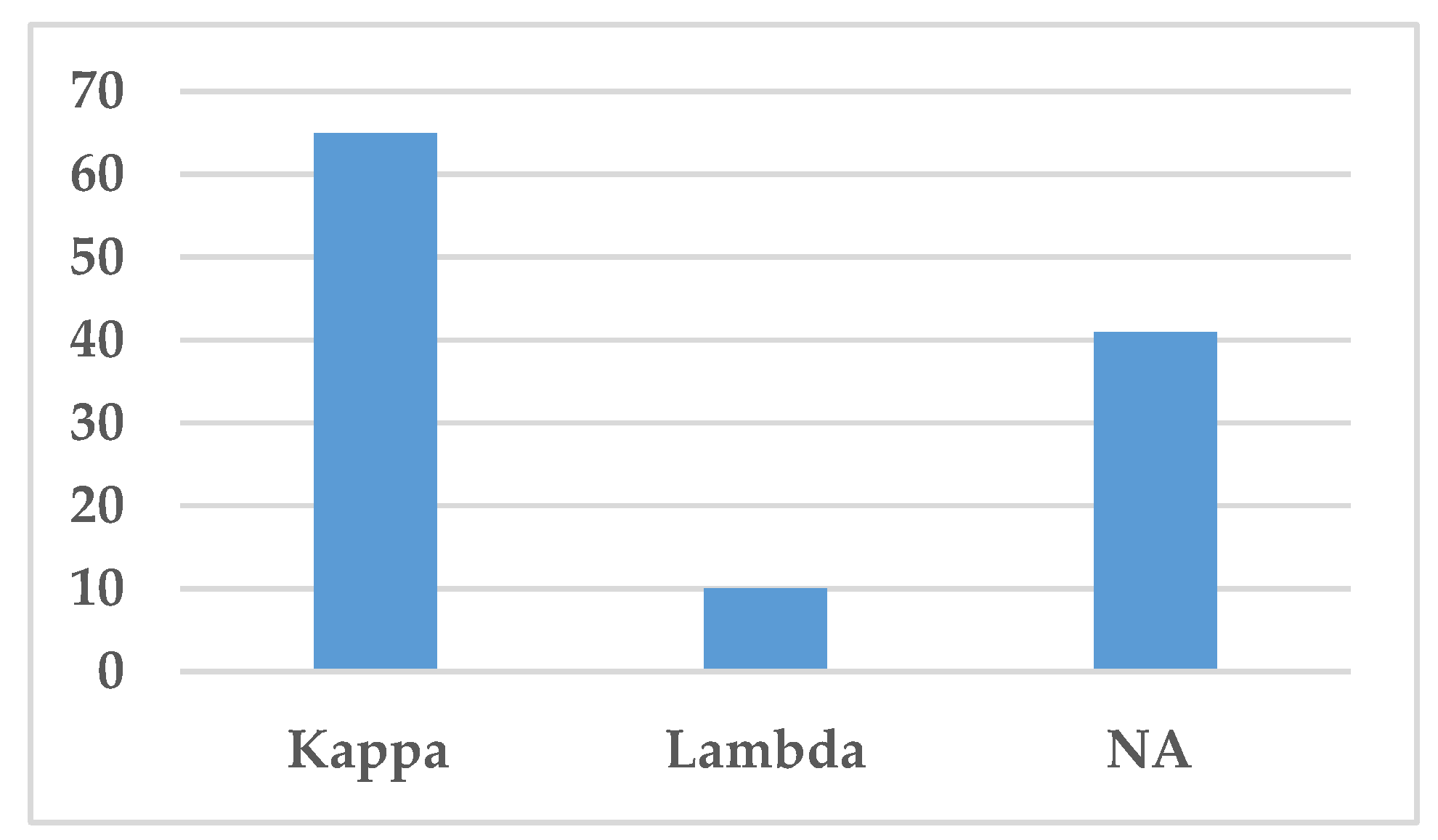
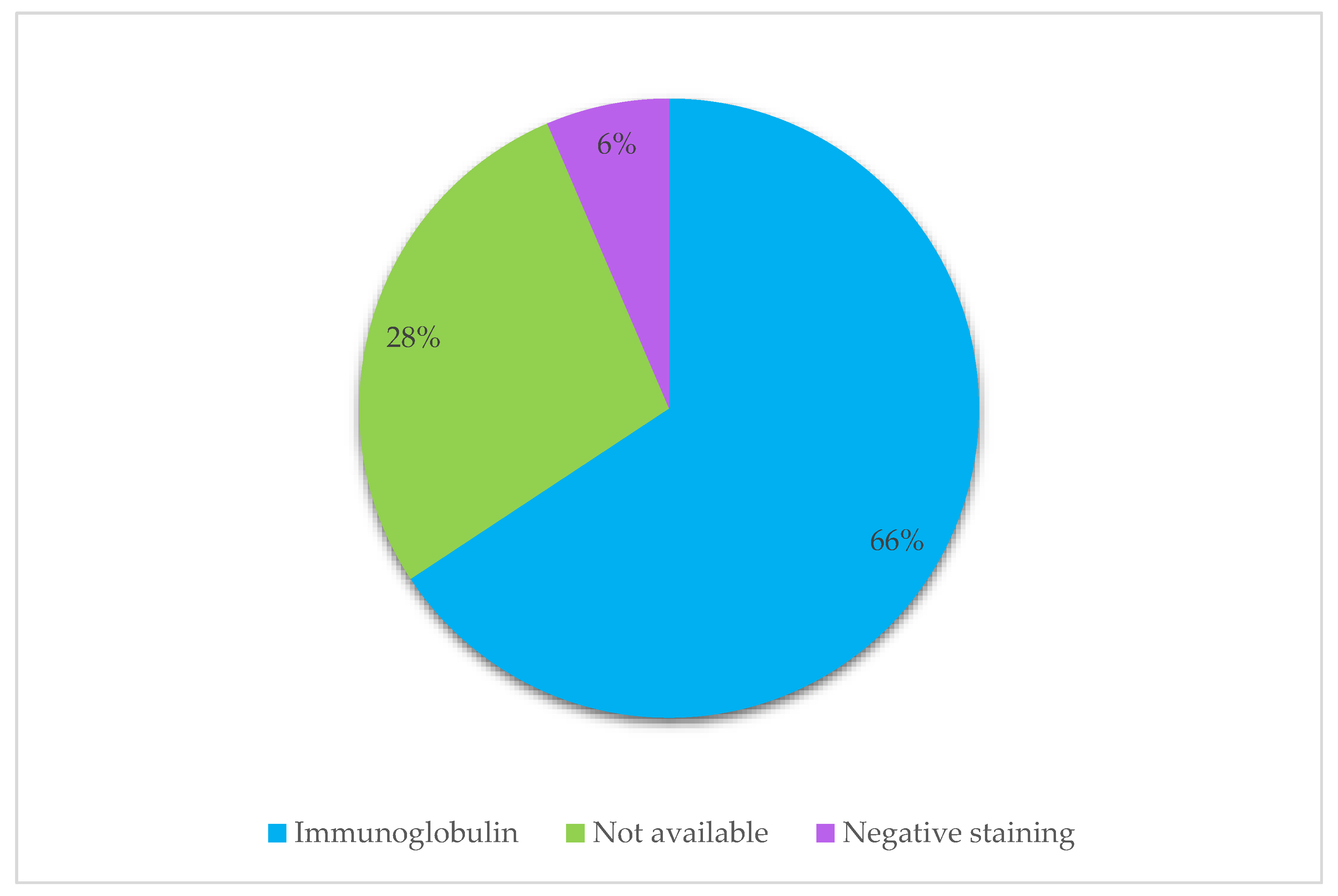
3.4. Immunoglobulin Classes and Immunoglobulin Restriction
3.5. Etiology of Material Deposited
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Flanagan, M.E.; Keene, C.D.; Louis, D.N.; Juric-Sekhar, G. Localized crystal-storing histiocytosis of the posterior fossa. Neuropathology 2018, 38, 529–534. [Google Scholar] [CrossRef]
- Fang, H.; Chiu, A.; Reichard, K.K. Crystal-Storing Histiocytosis in Bone Marrow: A Clinicopathologic Study of Eight Cases and Review of the Literature. Am. J. Clin. Pathol. 2018, 149, 148–163. [Google Scholar] [CrossRef]
- Lebeau, A.; Zeindl-Eberhart, E.; Muller, E.C.; Muller-Hocker, J.; Jungblut, P.R.; Emmerich, B.; Lohrs, U. Generalized crystal-storing histiocytosis associated with monoclonal gammopathy: Molecular analysis of a disorder with rapid clinical course and review of the literature. Blood 2002, 100, 1817–1827. [Google Scholar] [CrossRef]
- Chantranuwat, C. Noncrystallized form of immunoglobulin-storing histiocytosis as a cause of chronic lung infiltration in multiple myeloma. Ann. Diagn. Pathol 2007, 11, 220–222. [Google Scholar] [CrossRef]
- Kurabayashi, A.; Iguchi, M.; Matsumoto, M.; Hiroi, M.; Kume, M.; Furihata, M. Thymic mucosa-associated lymphoid tissue lymphoma with immunoglobulin-storing histiocytosis in Sjogren’s syndrome. Pathol. Int. 2010, 60, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Zizzo, M.; De Marco, L.; Zanelli, M.; Annessi, V.; Manenti, A.; Ascani, S.; Pedrazzoli, C. Localized Crystal-Storing Histiocytosis Involving Lower Rectum. Int. J. Surg. Pathol. 2020, 28, 415–416. [Google Scholar] [CrossRef]
- Reeders, J.; Arnold, C.; Chen, J.; Kirwan, P.; Lynnhtun, K. Crystal-storing histiocytosis leading to the identification of IgG-kappa secreting lymphoplasmacytic lymphoma with crystalline nephropathy. Pathology 2020, 52, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Kilic, I.; Picken, M.M.; Velankar, M.M.; Pambuccian, S.E. Bone marrow imprints of crystal-storing histiocytosis. Diagn. Cytopathol. 2020, 48, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Kim, N.H. Gastric crystal-storing histiocytosis with concomitant mucosa-associated lymphoid tissue lymphoma. J. Pathol. Transl. Med. 2020, 54, 332–335. [Google Scholar] [CrossRef] [PubMed]
- Contejean, A.; Larousserie, F.; Bouscary, D.; Dohan, A.; Deau-Fischer, B.; Szwebel, T.A.; Dhooge, M.; Terris, B.; Vignon, M. A colonic mass revealing a disseminated crystal storing histiocytosis secondary to indolent multiple myeloma: A case report with literature review. BMC Gastroenterol. 2020, 20, 239. [Google Scholar] [CrossRef]
- Vogel, A.N.; Casey, J.; Kaur, J.; Uppal, G. Two Cases of Crystal-storing Histiocytosis Diagnosed by Morphology, Immunohistochemistry, and Ultrastructural Examination. Appl. Immunohistochem. Mol. Morphol. 2019, 29, e1–e4. [Google Scholar] [CrossRef] [PubMed]
- Tomsula, J.; Meis, J.M.; Koy, R.D.; Monheit, J.; Zieske, A.; Ro, J.; Ayala, A. Crystal storing histiocytosis: Unusual clinical presentations in two patients. Ann. Diagn. Pathol. 2019, 40, 13–17. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, W.; Chen, Z.; Gao, L.; Yan, J.; Wang, M.; Xiang, C.; Liu, W. Generalized crystal-storing histiocytosis with diffuse large B-cell lymphoma and monoclonal gammopathy in a Chinese elderly woman: A case report. BMC Cancer 2019, 19, 514. [Google Scholar] [CrossRef]
- Rebecchini, C.; Trimeche, M.; Rosselet, A.; de Leval, L. Crystal-Storing Histiocytosis. Int. J. Surg. Pathol. 2019, 27, 399–400. [Google Scholar] [CrossRef] [PubMed]
- Michon, A.; Cohen Aubart, F.; Haroche, J.; Charlotte, F.; Maksud, P.; Amoura, Z. Long-bones involvement in generalized crystal-storing histiocytosis. Jt. Bone Spine 2019, 86, 652–653. [Google Scholar] [CrossRef]
- Jaitly, V.; Hu, Z.; Ayala, G.; Wahed, M.A.; Nguyen, N.D.; Brown, R.E. M2 Macrophages in Crystal Storing Histiocytosis Associated with Plasma Cell Myeloma. Ann. Clin. Lab. Sci. 2019, 49, 666–670. [Google Scholar]
- Gupta, R.K.; Rosenberg, A.Z.; Bagnasco, S.M.; Arend, L.J. Renal crystal-storing histiocytosis involving glomeruli—A comprehensive clinicopathologic analysis. Ann. Diagn. Pathol. 2019, 43, 151403. [Google Scholar] [CrossRef] [PubMed]
- Galeano-Valle, F.; Diaz-Crespo, F.J.; Melero-Martin, R.; Apaza-Chavez, J.E.; Del-Toro-Cervera, J.; Demelo-Rodriguez, P. Massive generalized crystal-storing histiocytosis associated with extracellular crystalline nephropathy: Clinical, immunohistochemical, and ultrastructural studies of a unique disorder and review of the literature. CEN Case Rep. 2019, 8, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.C.; Hafeez, S.; Shen, P. Crystal-Storing Histiocytosis with Plasma Cell Neoplasm in the Setting of Chronic Carbamazepine Exposure. J. Pathol. Transl. Med. 2019, 53, 142–144. [Google Scholar] [CrossRef]
- Boudhabhay, I.; Titah, C.; Talbot, A.; Harel, S.; Verine, J.; Touchard, G.; Kaaki, S.; Gabison, E.; Vasseur, V.; Mauget-Faysse, M.; et al. Multiple myeloma with crystal-storing histiocytosis, crystalline podocytopathy, and light chain proximal tubulopathy, revealed by retinal abnormalities: A case report. Medicine 2018, 97, e13638. [Google Scholar] [CrossRef]
- Wu, C.K.; Yang, A.H.; Lai, H.C.; Lin, B.S. Combined proximal tubulopathy, crystal-storing histiocytosis, and cast nephropathy in a patient with light chain multiple myeloma. BMC Nephrol. 2017, 18, 170. [Google Scholar] [CrossRef]
- Uthamalingam, P.; Mehta, S. Crystal-Storing Histiocytosis: Report of a Rare Case Presenting with Pathological Fracture of Femur. Is There More to the Entity? Int. J. Surg. Pathol. 2017, 25, 458–461. [Google Scholar] [CrossRef]
- Kokuho, N.; Terasaki, Y.; Kunugi, S.; Onda, N.; Urushiyama, H.; Terasaki, M.; Hino, M.; Gemma, A.; Hatori, T.; Shimizu, A. Localized pulmonary crystal-storing histiocytosis complicating pulmonary mucosa-associated lymphoid tissue lymphoma presenting with multiple mass lesions. Hum. Pathol. 2017, 65, 180–186. [Google Scholar] [CrossRef]
- Balakrishna, J.P.; Jaffe, E.S. Crystal-storing histiocytosis associated with thymic extranodal marginal zone lymphoma. Blood 2017, 130, 1683. [Google Scholar] [CrossRef]
- Shah, S.; Sethi, S.; Arend, L.; Geetha, D. Crystal-storing histiocytosis. Kidney Int. 2016, 89, 507. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kanagal-Shamanna, R.; Xu-Monette, Z.Y.; Miranda, R.N.; Dogan, A.; Zou, D.; Luthra, R.; Weber, D.M.; O’Malley, D.P.; Jorgensen, J.L.; Khoury, J.D.; et al. Crystal-storing histiocytosis: A clinicopathological study of 13 cases. Histopathology 2016, 68, 482–491. [Google Scholar] [CrossRef] [PubMed]
- Woehrer, A.; Kovacs, G.G. Clinical Neuropathology image 1-2015: Crystal-storing histiocytosis of the central nervous system. Clin. Neuropathol. 2015, 34, 4–5. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mittal, R.; Damato, B.; Coupland, S.E. Conjunctival extranodal marginal zone B-cell lymphoma with crystal-storing histiocytosis. Acta Ophthalmol. 2015, 93, e602–e603. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Liu, Y.; Li, X.; Yan, Q.; Wang, Z. Plasmacytoma with crystal-storing histiocytosis exhibiting FGFR3 and IgH translocation. Pathology 2015, 47, 82–85. [Google Scholar] [CrossRef]
- Loghavi, S.; Khoury, J.D. Unusual breast mass: Lymphoma with crystal-storing histiocytosis. Blood 2015, 125, 2445. [Google Scholar] [CrossRef]
- Li, J.J.; Henderson, C. Cutaneous crystal storing histiocytosis: A report of two cases. J. Cutan. Pathol. 2015, 42, 136–143. [Google Scholar] [CrossRef]
- Lee, J.S.; Im, K.; Park, S.N.; Park, H.S.; Kim, J.A.; Choi, Q.; Kim, S.Y.; Cha, C.H.; Oh, H.S.; Kim, I.H.; et al. A challenging diagnosis: Crystal-storing histiocytosis in plasma cell myeloma. Am. J. Clin. Pathol. 2015, 143, 300–304. [Google Scholar] [CrossRef] [PubMed]
- Baird, S.M.; Kenealy, M.K.; Hoy, R. Complete remission of Waldenstrom’s associated generalized crystal-storing histiocytosis of IgM lambda subtype with bortezomib-based combination chemotherapy. Leuk. Lymphoma 2015, 56, 3233–3235. [Google Scholar] [CrossRef]
- Aline-Fardin, A.; Bender, S.; Fabiani, B.; Buob, D.; Brahimi, S.; Verpont, M.C.; Mothy, M.; Ronco, P.; Boffa, J.J.; Aucouturier, P.; et al. Pseudo-Peritoneal Carcinomatosis Presentation of a Crystal-Storing Histiocytosis With an Unmutated Monoclonal kappa Light Chain. Medicine 2015, 94, e1247. [Google Scholar] [CrossRef] [PubMed]
- Vaid, A.; Caradine, K.D.; Lai, K.K.; Rego, R. Isolated gastric crystal-storing histiocytosis: A rare marker of occult lymphoproliferative disorders. J. Clin. Pathol. 2014, 67, 740–741. [Google Scholar] [CrossRef]
- Tsuji, T.; Yamasaki, H.; Hirano, T.; Toyozumi, Y.; Arima, N.; Tsuda, H. Crystal-storing histiocytosis complicating marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. Int. J. Hematol. 2014, 100, 519–520. [Google Scholar] [CrossRef]
- Thakral, B.; Courville, E. Crystal-storing histiocytosis with IgD kappa-associated plasma cell neoplasm. Blood 2014, 123, 3540. [Google Scholar] [CrossRef][Green Version]
- Tahara, K.; Miyajima, K.; Ono, M.; Sugio, Y.; Yamamoto, I.; Tamiya, S. Crystal-storing histiocytosis associated with marginal-zone lymphoma. Jpn. J. Radiol. 2014, 32, 296–301. [Google Scholar] [CrossRef]
- Saluja, K.; Thakral, B.; Eldibany, M.; Goldschmidt, R.A. Crystal storing histiocytosis associated with marginal zone B-cell lymphoma: A rare initial clinical presentation diagnosed by fine-needle aspiration. CytoJournal 2014, 11, 17. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Maneksha, V.; Adulkar, N. Crystal-storing histiocytosis masquerading ocular adnexal lymphoma: A case report and review of literature. Ophthalmic Plast. Reconstr. Surg. 2014, 30, e67–e69. [Google Scholar] [CrossRef] [PubMed]
- Orr, B.A.; Gallia, G.L.; Dogan, A.; Rodriguez, F.J. IgA/kappa-restricted crystal storing histiocytosis involving the central nervous system characterized by proteomic analysis. Clin. Neuropathol. 2014, 33, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, S.; Navarro, M.; Laser, J.; Berman, E.; Bhuiya, T. Localized crystal-storing histiocytosis presenting as a breast nodule: An unusual presentation of a rare entity. Breast J. 2014, 20, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Myers, J.L. Crystal-storing histiocytosis complicating primary pulmonary marginal zone lymphoma of mucosa-associated lymphoid tissue. Arch. Pathol. Lab. Med. 2013, 137, 1199–1204. [Google Scholar] [CrossRef]
- Zardawi, I.M.; Szabo, F. Monoclonal plasma cell proliferation associated with crystal-storing histiocytosis on a background of plasmacytoid dendritic cell tumour in a patient with stable chronic myelomonocytic leukaemia. Histopathology 2013, 62, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.C.; Yao, M.; Liao, S.L. Crystal-storing histiocytosis in a patient with ocular extranodal marginal zone lymphoma. Br. J. Haematol. 2013, 160, 419. [Google Scholar] [CrossRef] [PubMed]
- Yano, Y.; Nagahama, T.; Matsui, T.; Chuman, K.; Takeichi, M.; Hirai, F.; Yao, K.; Nishimata, N.; Haraoka, S.; Iwashita, A. Gastric crystal-storing histiocytosis detected with asymptomatic Sjogren’s syndrome: Report of a case and summary. Clin. J. Gastroenterol. 2013, 6, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.; De Rosa, N.; Cavazza, A.; Mengoli, M.C.; Della Casa, G.; Nannini, N.; Colby, T.V. Localized pleuropulmonary crystal-storing histiocytosis: 5 cases of a rare histiocytic disorder with variable clinicoradiologic features. Am. J. Surg. Pathol. 2013, 37, 906–912. [Google Scholar] [CrossRef]
- Rossi, G.; Morandi, U.; Nannini, N.; Fontana, G.; Pifferi, M.; Casali, C. Crystal-storing histiocytosis presenting with pleural disease. Histopathology 2010, 56, 403–405. [Google Scholar] [CrossRef]
- Miura, T.E.; Takihi, I.Y.; Maekawa, Y.H.; Chauffaille Mde, L.; Rizzatti, E.G.; Sandes, A.F. Iron staining in gammopathy-related crystal-storing histiocytosis: A misleading feature to the differential diagnosis with Gaucher’s disease. Mol. Genet. Metab. 2013, 110, 414–415. [Google Scholar] [CrossRef]
- Kawano, N.; Beppu, K.; Oyama, M.; Himeji, D.; Yoshida, S.; Kuriyama, T.; Ono, N.; Masuyama, H.; Yamashita, K.; Yamaguchi, K.; et al. Successful surgical treatment for pulmonary crystal-storing histiocytosis following the onset of gastric non-hodgkin lymphoma. J. Clin. Exp. Hematop. 2013, 53, 241–245. [Google Scholar] [CrossRef][Green Version]
- Johnson, M.; Mazariegos, J.; Lewis, P.J.; Pomakova, D. Crystal storing histiocytosis presenting as a temporal lobe mass lesion. Surg. Neurol. Int. 2013, 4, 112. [Google Scholar] [CrossRef] [PubMed]
- Duquesne, A.; Werbrouck, A.; Fabiani, B.; Denoyer, A.; Cervera, P.; Verpont, M.C.; Bender, S.; Piedagnel, R.; Brocheriou, I.; Ronco, P.; et al. Complete remission of monoclonal gammopathy with ocular and periorbital crystal storing histiocytosis and Fanconi syndrome. Hum. Pathol. 2013, 44, 927–933. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.M.; da Cunha Santos, G.; Boerner, S.L.; Bailey, D.J.; Geddie, W.R. Negative images of crystalline immunoglobulin in crystal storing histiocytosis: A potential cytologic mimic of mycobacteria in smears. Diagn. Cytopathol. 2012, 40, 916–919. [Google Scholar] [CrossRef]
- Hu, X.; Liu, J.; Bai, C.; Wang, J.; Song, X. Bortezomib combined with thalidomide and dexamethasone is effective for patient with crystal-storing histiocytosis associated with monoclonal gammopathy of undermined significance. Eur. J. Haematol. 2012, 89, 183–184. [Google Scholar] [CrossRef]
- Dogan, S.; Barnes, L.; Cruz-Vetrano, W.P. Crystal-storing histiocytosis: Report of a case, review of the literature (80 cases) and a proposed classification. Head Neck Pathol. 2012, 6, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Da Cruz Perez, D.E.; Silva-Sousa, Y.T.; de Andrade, B.A.; Rizo, V.H.; Almeida, L.Y.; Leon, J.E.; de Almeida, O.P. Crystal-storing histiocytosis: A rare lesion in periapical pathology. Ann. Diagn. Pathol. 2012, 16, 527–531. [Google Scholar] [CrossRef]
- Costanzi, C.; Bourdette, D.; Parisi, J.E.; Woltjer, R.; Rodriguez, F.; Steensma, D.; Lucchinetti, C.F. Crystal-storing histiocytosis: An unusual relapsing inflammatory CNS disorder. Mult. Scler. Relat. Disord. 2012, 1, 95–99. [Google Scholar] [CrossRef][Green Version]
- Lesesve, J.F.; Bronowicki, J.P.; Galed-Placed, I. Crystal-storing histiocytosis in ascites from a patient with IgM kappa lymphoplasmacytic lymphoma. Cytopathology 2011, 22, 207–208. [Google Scholar] [CrossRef]
- Khurram, S.A.; McPhaden, A.; Hislop, W.S.; Hunter, K.D. Crystal storing histiocytosis of the tongue as the initial presentation of multiple myeloma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 494–496. [Google Scholar] [CrossRef]
- Kaminsky, I.A.; Wang, A.M.; Olsen, J.; Schechter, S.; Wilson, J.; Olson, R. Central nervous system crystal-storing histiocytosis: Neuroimaging, neuropathology, and literature review. Am. J. Neuroradiol. 2011, 32, E26–E28. [Google Scholar] [CrossRef]
- Gao, F.F.; Khalbuss, W.E.; Austin, R.M.; Monaco, S.E. Cytomorphology of crystal storing histiocytosis in the breast associated with lymphoma: A case report. Acta Cytol. 2011, 55, 302–306. [Google Scholar] [CrossRef]
- Todd, W.U.; Drabick, J.J.; Benninghoff, M.G.; Frauenhoffer, E.E.; Zander, D.S. Pulmonary crystal-storing histiocytosis diagnosed by computed tomography-guided fine-needle aspiration. Diagn. Cytopathol. 2010, 38, 274–278. [Google Scholar] [CrossRef]
- Qureshi, A.; Kashif, M. Crystal-storing histiocytosis. Blood 2010, 115, 2568. [Google Scholar] [CrossRef][Green Version]
- El Hamel, C.; Thierry, A.; Trouillas, P.; Bridoux, F.; Carrion, C.; Quellard, N.; Goujon, J.M.; Aldigier, J.C.; Gombert, J.M.; Cogne, M.; et al. Crystal-storing histiocytosis with renal Fanconi syndrome: Pathological and molecular characteristics compared with classical myeloma-associated Fanconi syndrome. Nephrol. Dial. Transpl. 2010, 25, 2982–2990. [Google Scholar] [CrossRef] [PubMed]
- Sailey, C.J.; Alexiev, B.A.; Gammie, J.S.; Pinell-Salles, P.; Stafford, J.L.; Burke, A. Crystal-storing histiocytosis as a cause of symptomatic cardiac mass. Arch. Pathol. Lab. Med. 2009, 133, 1861–1864. [Google Scholar] [CrossRef] [PubMed]
- Laszlo, R.; Degrell, P.; Kellermayer, M.; Bollmann, D.; Egyed, M.; Seress, L.; Pajor, L. Crystal-storing histiocytosis associated with only one of two consecutive, but genetically unrelated B-cell lymphomas. Pathol. Res. Pract. 2009, 205, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Farooq, U.; Bayerl, M.G.; Abendroth, C.S.; Verma, N.; Talamo, G. Renal crystal storing histiocytosis in a patient with multiple myeloma. Ann. Hematol. 2009, 88, 807–809. [Google Scholar] [CrossRef] [PubMed]
- De Alba Campomanes, A.G.; Rutar, T.; Crawford, J.B.; Seiff, S.; Goodman, D.; Grenert, J. Crystal-storing histiocytosis and crystalline keratopathy caused by monoclonal gammopathy of undetermined significance. Cornea 2009, 28, 1081–1084. [Google Scholar] [CrossRef]
- Keane, C.; Gill, D. Multi-organ involvement with crystal-storing histiocytosis. Br. J. Haematol. 2008, 141, 750. [Google Scholar] [CrossRef]
- Kar, R.; Dutta, S.; Bhargava, R.; Tyagi, S. Crystal storing histiocytosis: A rare presentation of plasma cell myeloma. Indian J. Hematol. Blood Transfus. 2008, 24, 63–66. [Google Scholar] [CrossRef][Green Version]
- Kusakabe, T.; Watanabe, K.; Mori, T.; Iida, T.; Suzuki, T. Crystal-storing histiocytosis associated with MALT lymphoma of the ocular adnexa: A case report with review of literature. Virchows Arch. 2007, 450, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Joo, M.; Kwak, J.E.; Chang, S.H.; Kim, H.; Chi, J.G.; Moon, Y.S.; Kim, K.M. Localized gastric crystal-storing histiocytosis. Histopathology 2007, 51, 116–119. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.B.; Aronoff, B.; Siegel, D.; D’Agati, V.D. Dysproteinemia-related nephropathy associated with crystal-storing histiocytosis. Kidney Int. 2006, 70, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Pock, L.; Stuchlik, D.; Hercogova, J. Crystal storing histiocytosis of the skin associated with multiple myeloma. Int. J. Derm. 2006, 45, 1408–1411. [Google Scholar] [CrossRef]
- Pitman, S.D.; Wang, J.; Serros, E.R.; Zuppan, C. A 70-year-old woman with acute renal failure. Crystal-storing histiocytosis. Arch. Pathol. Lab. Med. 2006, 130, 1077–1078. [Google Scholar] [CrossRef]
- Galed-Placed, I. Immunoglobulin crystal-storing histiocytosis in a pleural effusion from a woman with IgA kapa multiple myeloma: A case report. Acta Cytol. 2006, 50, 539–541. [Google Scholar] [CrossRef]
- Fairweather, P.M.; Williamson, R.; Tsikleas, G. Pulmonary extranodal marginal zone lymphoma with massive crystal storing histiocytosis. Am. J. Surg. Pathol. 2006, 30, 262–267. [Google Scholar] [CrossRef]
- De Lastours, V.; Papo, T.; Cazals-Hatem, D.; Eden, A.; Feydy, A.; Belmatoug, N.; Chauveheid, M.P.; Lidove, O.; Fantin, B. Bone involvement in generalized crystal-storing histiocytosis. J. Rheumatol. 2006, 33, 2354–2358. [Google Scholar]
- Tholouli, E.; Krebs, M.; Reeve, R.; Houghton, J.B. Crystal-storing histiocytosis in a patient with IgG kappa multiple myeloma. Br. J. Haematol. 2005, 128, 412. [Google Scholar] [CrossRef]
- Zioni, F.; Giovanardi, P.; Bozzoli, M.; Artusi, T.; Bonacorsi, G.; Sighinolfi, P. Massive bone marrow crystal-storing histiocytosis in a patient with IgA-lambda multiple myeloma and extensive extramedullary disease. A case report. Tumori J. 2004, 90, 348–351. [Google Scholar] [CrossRef]
- Papla, B.; Spolnik, P.; Rzenno, E.; Zdunczyk, A.; Rudzki, Z.; Okon, K.; Szczepanski, W.; Dabros, W.; Stachura, J. Generalized crystal-storing histiocytosis as a presentation of multiple myeloma: A case with a possible pro-aggregation defect in the immunoglobulin heavy chain. Virchows Arch. 2004, 445, 83–89. [Google Scholar] [CrossRef]
- Sun, Y.; Tawfiqul, B.; Valderrama, E.; Kline, G.; Kahn, L.B. Pulmonary crystal-storing histiocytosis and extranodal marginal zone B-cell lymphoma associated with a fibroleiomyomatous hamartoma. Ann. Diagn. Pathol. 2003, 7, 47–53. [Google Scholar] [CrossRef]
- Sethi, S.; Cuiffo, B.P.; Pinkus, G.S.; Rennke, H.G. Crystal-storing histiocytosis involving the kidney in a low-grade B-cell lymphoproliferative disorder. Am. J. Kidney Dis. 2002, 39, 183–188. [Google Scholar] [CrossRef]
- Coupland, S.E.; Foss, H.D.; Hummel, M.; Stein, H. Extranodal marginal zone B-cell lymphoma of the lacrimal gland associated with crystal-storing histiocytosis. Ophthalmology 2002, 109, 105–110. [Google Scholar] [CrossRef]
- Jones, D.; Bhatia, V.K.; Krausz, T.; Pinkus, G.S. Crystal-storing histiocytosis: A disorder occurring in plasmacytic tumors expressing immunoglobulin kappa light chain. Hum. Pathol. 1999, 30, 1441–1448. [Google Scholar] [CrossRef]
- Prasad, M.L.; Charney, D.A.; Sarlin, J.; Keller, S.M. Pulmonary immunocytoma with massive crystal storing histiocytosis: A case report with review of literature. Am. J. Surg. Pathol. 1998, 22, 1148–1153. [Google Scholar] [CrossRef]
- Garcia, J.F.; Sanchez, E.; Lloret, E.; Martin, J.; Piris, M.A. Crystal-storing histiocytosis and immunocytoma associated with multifocal fibrosclerosis. Histopathology 1998, 33, 459–464. [Google Scholar] [CrossRef]
- Bosman, C.; Camassei, F.D.; Boldrini, R.; Piro, F.R.; Saponara, M.; Romeo, R.; Corsi, A. Solitary crystal-storing histiocytosis of the tongue in a patient with rheumatoid arthritis and polyclonal hypergammaglobulinemia. Arch. Pathol. Lab. Med. 1998, 122, 920–924. [Google Scholar]
- Llobet, M.; Castro, P.; Barcelo, C.; Trull, J.M.; Campo, E.; Bernado, L. Massive crystal-storing histiocytosis associated with low-grade malignant B-cell lymphoma of MALT-type of the parotid gland. Diagn. Cytopathol. 1997, 17, 148–152. [Google Scholar] [CrossRef]
- Kaufmann, O.; Hansen, A.; Deicke, P.; Burmester, G.R.; Dietel, M. Subcutaneous crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma (immunocytoma). Pathol. Res. Pract. 1996, 192, 1148–1151. [Google Scholar] [CrossRef]
- Jones, D.; Renshaw, A.A. Recurrent crystal-storing histiocytosis of the lung in a patient without a clonal lymphoproliferative disorder. Arch. Pathol. Lab. Med. 1996, 120, 978–980. [Google Scholar]
- Harada, M.; Shimada, M.; Fukayama, M.; Kaneko, T.; Kitazume, K.; Weiss, S.W. Crystal-storing histiocytosis associated with lymphoplasmacytic lymphoma mimicking Weber-Christian disease: Immunohistochemical, ultrastructural, and gene-rearrangement studies. Hum. Pathol. 1996, 27, 84–87. [Google Scholar] [CrossRef]
- Friedman, M.T.; Molho, L.; Valderrama, E.; Kahn, L.B. Crystal-storing histiocytosis associated with a lymphoplasmacytic neoplasm mimicking adult rhabdomyoma: A case report and review of the literature. Arch. Pathol. Lab. Med. 1996, 120, 1133–1136. [Google Scholar] [PubMed]
- Kapadia, S.B.; Enzinger, F.M.; Heffner, D.K.; Hyams, V.J.; Frizzera, G. Crystal-storing histiocytosis associated with lymphoplasmacytic neoplasms. Report of three cases mimicking adult rhabdomyoma. Am. J. Surg. Pathol. 1993, 17, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hishida, A.; Honda, N.; Ito, I.; Shirasawa, H.; Nagase, M. Crystal-storing histiocytosis and crystalline tissue deposition in multiple myeloma. Arch. Pathol. Lab. Med. 1991, 115, 351–354. [Google Scholar] [PubMed]
- Takahashi, K.; Naito, M.; Takatsuki, K.; Kono, F.; Chitose, M.; Ooshima, S.; Mori, N.; Sakuma, H.; Uchino, F. Multiple myeloma, IgA kappa type, accompanying crystal-storing histiocytosis and amyloidosis. Acta Pathol. Jpn. 1987, 37, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Alayed, K.M.; Alabdulaali, M.K.; Alkhairy, K.S.; Elnour, S.; Alhajjaj, A. Aggressive systemic mastocytosis with Charcot-Leyden crystals-associated crystal storing histiocytosis in bone marrow. Pathology 2010, 42, 85–87. [Google Scholar] [CrossRef]
- Lewis, J.T.; Candelora, J.N.; Hogan, R.B.; Briggs, F.R.; Abraham, S.C. Crystal-storing histiocytosis due to massive accumulation of charcot-leyden crystals: A unique association producing colonic polyposis in a 78-year-old woman with eosinophilic colitis. Am. J. Surg. Pathol. 2007, 31, 481–485. [Google Scholar] [CrossRef]
- Gebrail, F.; Knapp, M.; Perotta, G.; Cualing, H. Crystalline histiocytosis in hereditary cysinosis. Arch. Pathol. Lab. Med. 2002, 126, 1135. [Google Scholar] [CrossRef]
- Weiss, S.W.; Enzinger, F.M.; Johnson, F.B. Silica reaction simulating fibrous histiocytoma. Cancer 1978, 42, 2738–2743. [Google Scholar] [CrossRef]
- Balakrishna, J.; Chen, A.; Urken, M. Crystal storing histiocytosis clinically mimicking metastatic carcinoma: Report of a case and reviews of literature. Head Neck 2016, 38, E95–E98. [Google Scholar] [CrossRef] [PubMed]
- Pais, A.V.; Pereira, S.; Garg, I.; Stephen, J.; Antony, M.; Inchara, Y.K. Intra-abdominal, crystal-storing histiocytosis due to clofazimine in a patient with lepromatous leprosy and concurrent carcinoma of the colon. Lepr. Rev. 2004, 75, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Sukpanichnant, S.; Hargrove, N.S.; Kachintorn, U.; Manatsathit, S.; Chanchairujira, T.; Siritanaratkul, N.; Akaraviputh, T.; Thakerngpol, K. Clofazimine-induced crystal-storing histiocytosis producing chronic abdominal pain in a leprosy patient. Am. J. Surg. Pathol. 2000, 24, 129–135. [Google Scholar] [CrossRef]
- Ganz, T. Anemia of Inflammation. N. Engl. J. Med. 2019, 381, 1148–1157. [Google Scholar] [CrossRef] [PubMed]
- Pasqualetti, P.; Festuccia, V.; Collacciani, A.; Casale, R. The natural history of monoclonal gammopathy of undetermined significance. A 5- to 20-year follow-up of 263 cases. Acta Haematol 1997, 97, 174–179. [Google Scholar] [CrossRef]
- Amir, G.; Ron, N. Pulmonary pathology in Gaucher’s disease. Hum. Pathol. 1999, 30, 666–670. [Google Scholar] [CrossRef]



| Analysis | Values | References |
|---|---|---|
| Hemoglobin (g/dL) | 10.1 | 11.7–15.3 |
| EVF | 0.33 | 0.35–0.46 |
| CRP (mg/L) | 18 | <5 |
| SR (mm/t) | 69 | 1–30 |
| Creatinine (µmol/t) | 78 | 45–90 |
| Protein (g/L) | 69 | 62–78 |
| IgG (g/L) | 16.1 | 6.0–15.3 |
| IgA (g/L) | 0.76 | 0.8–4.0 |
| IgM (g/L) | 0.51 | 0.3–2.30 |
| Kappa free light chains (mg/L) | 54.0 | 6.7–22.4 |
| Lambda free light chains (mg/L) | 25.0 | 8.3–27.0 |
| Ratio kappa/lambda free light chains | 2.16 | 0.31–1.56 |
| S-protein electrophoresis | Monoclonal band | |
| S-immunofixation | Monoclonal band type IgG kappa. | |
| M-protein (mg/L) | 7.1 | 0 |
| Case nr. | Organs/Tissue Sites | Age | Sex | Type | LP–PCD | Material within Histiocytes (ISH) | Serum Immunofixation | Ref. | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Skin | Bonemarrow | Kidney | GI-Tract | Liver | Abdomen unsp. | Heart | Thymus | Breast | Lung | Spleen | Head an Neck | Lymph Node | ||||||||
| 1 | 71 | M | L | MGUS | NA | IgG kappa | [6] | |||||||||||||
| 2 | 57 | M | G | LPL | IgM/IgG/IgD kappa/lambda | IgG kappa | [7] | |||||||||||||
| 3 | 56 | F | L | MM | Kappa | IgG kappa | [8] | |||||||||||||
| 4 | 56 | M | L | MALT lymphoma | Kappa | NA | [9] | |||||||||||||
| 5 | 75 | F | G | MM | Kappa | IgG kappa | [10] | |||||||||||||
| 6 | 66 | F | L | MM | Kappa | IgA kappa | [11] | |||||||||||||
| 7 | 86 | M | L | MZL | Negative | IgM | ||||||||||||||
| 8 | 68 | F | L | NA | Kappa | Kappa | [12] | |||||||||||||
| 9 | 49 | M | G | MM | NA | NA | ||||||||||||||
| 10 | 74 | F | G | DLBCL | IgM kappa | IgM kappa | [13] | |||||||||||||
| 11 | 83 | F | L | MM | Kappa | NA | [14] | |||||||||||||
| 12 | 73 | F | G | MGUS | NA | IgG kappa | [15] | |||||||||||||
| 13 | 79 | M | L | MM | Negative | NA | [16] | |||||||||||||
| 14 | 71 | M | L | LPL | IgM Kappa | IgM kappa | [17] | |||||||||||||
| 15 | 67 | M | L | MGUS | IgG kappa | IgG kappa | ||||||||||||||
| 16 | 74 | M | L | MM | IgG kappa | IgG kappa | ||||||||||||||
| 17 | 65 | M | G | None | Negative | IgG kappa | [18] | |||||||||||||
| 18 | 72 | F | L | MM | Kappa | Kappa | [19] | |||||||||||||
| 19 | 36 | M | L | None | Kappa | Negative | [1] | |||||||||||||
| 20 | 63 | M | L | B-cell lymphoma | NA | IgM Kappa | [2] | |||||||||||||
| 21 | 40 | M | L | MM | Kappa | Kappa | ||||||||||||||
| 22 | 71 | M | G | MM | Kappa | IgG kappa | ||||||||||||||
| 23 | 65 | F | L | MM | Kappa | IgA Kappa | ||||||||||||||
| 24 | 70 | M | G | MM | NA | IgG Kappa | ||||||||||||||
| 25 | 73 | M | L | MGUS | NA | IgG Kappa | ||||||||||||||
| 26 | 56 | M | L | MM | NA | IgG Kappa | ||||||||||||||
| 27 | 60 | M | G | MM | Kappa | IgG kappa | [20] | |||||||||||||
| 28 | 48 | M | L | MM | Kappa | Kappa | [21] | |||||||||||||
| 29 | 70 | M | L | MM | NA | IgG kappa | [22] | |||||||||||||
| 30 | 36 | F | L | MALT lymphoma | Kappa | IgG | [23] | |||||||||||||
| 31 | 27 | F | L | EMZL | Lambda | Lambda | [24] | |||||||||||||
| 32 | 67 | M | L | MGRS | Kappa | IgG kappa | [25] | |||||||||||||
| 33 | 51 | M | G | MM | IgG/lambda/kappa | IgG lambda | [26] | |||||||||||||
| 34 | 75 | M | L | MM | IgG kappa | IgG kappa | ||||||||||||||
| 35 | 46 | F | L | MM | NA | IgA kappa | ||||||||||||||
| 36 | 74 | M | L | LPL | IgM | IgM | ||||||||||||||
| 37 | 63 | M | L | LPL | Lambda | IgM lambda | ||||||||||||||
| 38 | 79 | M | L | LPL | Kappa | IgM kappa | ||||||||||||||
| 39 | 43 | M | G | EMZL | IgA kappa | Negative | ||||||||||||||
| 40 | 63 | F | L | EMZL | IgM lambda | NA | ||||||||||||||
| 41 | 50 | F | L | EMZL | IgM lambda | NA | ||||||||||||||
| 42 | 33 | F | L | EMZL | Kappa | NA | ||||||||||||||
| 43 | 73 | F | L | EMZL | NA | NA | ||||||||||||||
| 44 | 58 | M | L | EMZL | NA | NA | ||||||||||||||
| 45 | 68 | F | L | SMZL | Lambda | Negative | ||||||||||||||
| 46 | 56 | M | L | None | NA | Negative | [27] | |||||||||||||
| 47 | 58 | M | L | EMZL | Kappa | NA | [28] | |||||||||||||
| 48 | 46 | M | L | MM | Lambda | NA | [29] | |||||||||||||
| 49 | 62 | F | L | EMZL | IgM | NA | [30] | |||||||||||||
| 50 | 80 | F | L | LPL | NA | IgM kappa | [31] | |||||||||||||
| 51 | 52 | M | L | MM | Negative | IgG kappa | ||||||||||||||
| 52 | 57 | F | L | MM | Kappa | IgA kappa | [32] | |||||||||||||
| 53 | 53 | F | G | LPL | NA | IgM lambda | [33] | |||||||||||||
| 54 | 69 | M | G | MGUS | NA | Kappa | [34] | |||||||||||||
| 55 | NA | M | L | None | NA | NA | [35] | |||||||||||||
| 56 | 71 | F | L | MALT lymphoma | IgG kappa | Negative | [36] | |||||||||||||
| 57 | 43 | F | L | MGUS | Neg | IgD kappa | [37] | |||||||||||||
| 58 | 91 | F | G | EMZL | IgM/IgG/kappa | IgM kappa | [38] | |||||||||||||
| 59 | 77 | M | L | MZL | Kappa | Negative | [39] | |||||||||||||
| 60 | 53 | M | L | MALT lymphoma | NA | NA | [40] | |||||||||||||
| 61 | 38 | F | L | MM | IgA kappa | IgA kappa | [41] | |||||||||||||
| 62 | 30 | F | L | None | NA | Negative | [42] | |||||||||||||
| 63 | 54 | F | L | MALT lymphoma | Kappa | NA | [43] | |||||||||||||
| 64 | 72 | F | L | BPDCN | NA | Negative | [44] | |||||||||||||
| 65 | 32 | M | L | EMZL | NA | NA | [45] | |||||||||||||
| 66 | 55 | F | L | None | IgA/IgG/IgM/kappa/lambda | Negative | [46] | |||||||||||||
| 67 | 54 | F | L | MGUS | Kappa | NA | [47,48] | |||||||||||||
| 68 | 89 | F | L | MZL | Kappa | NA | ||||||||||||||
| 69 | 50 | F | L | MZL | Kappa | NA | ||||||||||||||
| 70 | 63 | M | L | MM | Kappa | NA | ||||||||||||||
| 71 | 68 | M | L | MGUS | IgG kappa | IgG kappa | ||||||||||||||
| 72 | 78 | F | L | MGUS | NA | NA | [49] | |||||||||||||
| 73 | 80 | M | L | None | NA | Negative | [50] | |||||||||||||
| 74 | 20 | F | L | None | Lambda | NA | [51] | |||||||||||||
| 75 | 62 | F | G | MGUS | NA | IgG kappa | [52] | |||||||||||||
| 76 | 64 | M | L | EMZL | NA | NA | [53] | |||||||||||||
| 77 | 57 | F | L | EMZL | NA | NA | ||||||||||||||
| 78 | 48 | F | G | MGUS | Kappa | IgG kappa | [54] | |||||||||||||
| 79 | 51 | F | L | MGUS | IgM/IgG | NA | [55] | |||||||||||||
| 80 | 38 | M | L | None | IgG/kappa/lambda | Negative | [56] | |||||||||||||
| 81 | 32 | F | L | None | Kappa | NA | [57] | |||||||||||||
| 82 | 50 | F | L | LPL | NA | IgM kappa | [58] | |||||||||||||
| 83 | 67 | M | L | MM | IgG | IgG | [59] | |||||||||||||
| 84 | 27 | F | L | None | NA | NA | [60] | |||||||||||||
| 85 | 54 | F | L | EMZL | Kappa | NA | [61] | |||||||||||||
| 86 | 75 | F | L | MGUS | NA | IgG kappa | [62] | |||||||||||||
| 87 | 63 | M | L | MM | NA | IgG kappa | [63] | |||||||||||||
| 88 | 53 | F | L | MALT lymphoma | Non-crystallized IgG kappa | IgA kappa | [5] | |||||||||||||
| 89 | 52 | M | L | MGUS | NA | IgM kappa | [64] | |||||||||||||
| 90 | 70 | M | L | DLBCL | NA | IgM kappa | ||||||||||||||
| 91 | 65 | M | L | MGUS | NA | IgG kappa | ||||||||||||||
| 92 | 64 | M | L | MG | Negative | IgG kappa | [65] | |||||||||||||
| 93 | 76 | F | L | MZL | IgM lambda | IgM lambda | [66] | |||||||||||||
| 94 | 66 | M | G | MM | NA | IgG kappa | [67] | |||||||||||||
| 95 | 66 | M | L | MGUS | Lambda | IgG lambda | [68] | |||||||||||||
| 96 | 65 | M | G | None | NA | Kappa | [69] | |||||||||||||
| 97 | 54 | M | L | MM | NA | IgG kappa | [70] | |||||||||||||
| 98 | 81 | F | L | MALT lymphoma | IgM kappa | Negative | [71] | |||||||||||||
| 99 | 56 | F | L | None | Kappa/lambda | Negative | [72] | |||||||||||||
| 100 | 52 | M | L | MM | Non-crystallized kappa | NA | [4] | |||||||||||||
| 101 | 41 | M | L | MM | NA | IgD kappa | [73] | |||||||||||||
| 102 | 62 | F | L | MM | Kappa | IgG kappa | [74] | |||||||||||||
| 103 | 70 | F | L | None | Kappa | Kappa | [75] | |||||||||||||
| 104 | 79 | F | L | MM | IgA kappa | IgA kappa | [76] | |||||||||||||
| 105 | 69 | F | L | MALT lymphoma | NA | Negative | [77] | |||||||||||||
| 106 | 49 | M | G | MGUS | Kappa | IgG kappa | [78] | |||||||||||||
| 107 | 72 | F | G | MM | Kappa | IgA kappa | ||||||||||||||
| 108 | 62 | F | L | MM | NA | IgG kappa | [79] | |||||||||||||
| 109 | 74 | F | L | MM | Negative | IgA lambda | [80] | |||||||||||||
| 110 | 51 | M | G | MM | IgG kappa | Kappa | [81] | |||||||||||||
| 111 | 59 | M | L | EMZL | IgG/IgM/kappa/lambda | NA | [82] | |||||||||||||
| 112 | 58 | M | L | MZL | Lambda | IgM lambda | [83] | |||||||||||||
| 113 | 73 | M | G | MM | IgA kappa | IgA kappa | [3] | |||||||||||||
| 114 | 62 | F | L | EMZL | Kappa | Negative | [84] | |||||||||||||
| 115 | 48 | M | L | MM | IgA kappa | IgA kappa | [85] | |||||||||||||
| 116 | 53 | M | L | MM | IgA kappa | Negative | ||||||||||||||
| 117 | 50 | M | G | MM | IgG kappa | Negative | ||||||||||||||
| 118 | 77 | F | L | MM | Kappa | Negative | ||||||||||||||
| 119 | 66 | M | L | MGUS | IgA kappa | IgA kappa | ||||||||||||||
| 120 | 66 | M | G | LPL | IgM kappa | Negative | ||||||||||||||
| 121 | 68 | F | L | LPL | IgM kappa | IgM/IgG/kappa | ||||||||||||||
| 122 | 53 | F | L | LPL | IgM kappa | IgM kappa | ||||||||||||||
| 123 | 70 | M | G | LPL | IgM kappa | NA | ||||||||||||||
| 124 | 70 | M | G | LPL | IgM lambda | NA | ||||||||||||||
| 125 | 35 | F | L | LPL | IgA kappa | IgG lambda | ||||||||||||||
| 126 | 54 | F | L | None | IgG lambda | NA | ||||||||||||||
| 127 | 72 | F | L | LPL | IgM kappa | NA | [86] | |||||||||||||
| 128 | 44 | M | G | LPL | IgG kappa | IgG kappa | [87] | |||||||||||||
| 129 | 73 | F | L | None | IgG/kappa/lambda | IgG | [88] | |||||||||||||
| 130 | 81 | F | L | MALT lymphoma | IgM lambda | NA | [89] | |||||||||||||
| 131 | 61 | F | L | LPL | IgM/IgG/kappa/lambda | IgM kappa | [90] | |||||||||||||
| 132 | 54 | F | L | None | IgA/IgM/IgG/kappa/lambda | NA | [91] | |||||||||||||
| 133 | 46 | M | L | LPL | Negative | IgG/IgM/lambda | [92] | |||||||||||||
| 134 | 49 | M | L | B-cell lymphoma | NA | NA | [93] | |||||||||||||
| 135 | 78 | F | G | LPL | Negative | IgM kappa | [94] | |||||||||||||
| 136 | 77 | F | G | LPL | IgM kappa | NA | ||||||||||||||
| 137 | 18 | F | L | LPL | Negative | NA | ||||||||||||||
| 138 | 75 | M | G | MM | IgG kappa | NA | [95] | |||||||||||||
| 139 | 60 | M | G | MM | IgA kappa | NA | [96] | |||||||||||||
| 140 | 75 | F | L | MGUS | Non-crystallized kappa | IgG kappa | Pres | |||||||||||||
| Number (n) | Percentage (%) | |
|---|---|---|
| Sex | ||
| Men | 71 | 51 |
| Women | 69 | 49 |
| Etiology | ||
| Localized | 108 | 77 |
| Generalized | 32 | 23 |
| 1. SH with underlying LP–PCD | 122 | 87 |
| MGUS/MGRS | 21 | 15 |
| MM | 43 | 31 |
| LPL | 21 | 15 |
| DLBCL | 2 | 1 |
| BPDCN | 1 | 1 |
| MALT lymphoma | 10 | 7 |
| EMZL/SMZL | 23 | 16 |
| B-cell lymphoma not specified | 2 | 1 |
| 2. SH without underlying LP–PCD | 17 | 12 |
| 3. SH with unknown history | 1 | 1 |
| According to Crystal | According to Etiology |
|---|---|
| Associated with LP–PCD |
|
|
|
|
|
|
| Autoimmune |
|
|
| |
| Drugs | |
| |
| |
| Reactive-inflammatory | |
| |
| Metabolic | |
|
| New Classification |
|---|
| Immunoglobulin Storing Histiocytosis |
|
|
| Non-immunoglobulin Crystallized Storing Histiocytosis |
|
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiese-Hansen, H.; Leh, F.; Lodvir Hemsing, A.; Reikvam, H. Immunoglobulin-Storing Histiocytosis: A Case Based Systemic Review. J. Clin. Med. 2021, 10, 1834. https://doi.org/10.3390/jcm10091834
Wiese-Hansen H, Leh F, Lodvir Hemsing A, Reikvam H. Immunoglobulin-Storing Histiocytosis: A Case Based Systemic Review. Journal of Clinical Medicine. 2021; 10(9):1834. https://doi.org/10.3390/jcm10091834
Chicago/Turabian StyleWiese-Hansen, Hanne, Friedemann Leh, Anette Lodvir Hemsing, and Håkon Reikvam. 2021. "Immunoglobulin-Storing Histiocytosis: A Case Based Systemic Review" Journal of Clinical Medicine 10, no. 9: 1834. https://doi.org/10.3390/jcm10091834
APA StyleWiese-Hansen, H., Leh, F., Lodvir Hemsing, A., & Reikvam, H. (2021). Immunoglobulin-Storing Histiocytosis: A Case Based Systemic Review. Journal of Clinical Medicine, 10(9), 1834. https://doi.org/10.3390/jcm10091834







