Use of the Renal Artery Doppler to Identify Small for Gestational Age Fetuses at Risk for Adverse Neonatal Outcomes
Abstract
1. Introduction
2. Methods
Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baschat, A.A.; Gembruch, U.; Harman, C.R. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound Obstet. Gynecol. 2001, 18, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, H.S.; Kaufman, T.; Keil, L.C.; Rudolph, A.M. Responses to acute hypoxemia in fetal sheep at 0.6-0.7 gestation. Am. J. Physiol. Circ. Physiol. 1989, 256, H613–H620. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.; Lang, U. Foetal circulatory responses to arrest of uterine blood flow in sheep: Effects of chemical sympathectomy. J. Dev. Physiol. 1992, 17, 75–86. [Google Scholar]
- Malamitsi-Puchner, A.; Nikolaou, K.; Puchner, K.-P. Intrauterine Growth Restriction, Brain-Sparing Effect, and Neurotrophins. Ann. N. Y. Acad. Sci. 2006, 1092, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.M.; Mitchell, M.D.; Kumar, S.S. The physiology of intrapartum fetal compromise at term. Am. J. Obstet. Gynecol. 2020, 222, 17–26. [Google Scholar] [CrossRef]
- Jensen, A.; Hohmann, M.; Künzel, W. Redistribution of fetal circulation during repeated asphyxia in sheep: Effects on skin blood flow, transcutaneous PO2, and plasma catecholamines. J. Dev. Physiol. 1987, 9, 41–55. [Google Scholar] [PubMed]
- Walker, A.M.; Cannata, J.; Dowling, M.H.; Ritchie, B.; Maloney, J.E. Sympathetic and Parasympathetic Control of Heart Rate in Unanaesthetized Fetal and Newborn Lambs. Neonatology 1978, 33, 135–143. [Google Scholar] [CrossRef]
- Trudinger, B.J.; Giles, W.B.; Cook, C.M. Flow velocity waveforms in the maternal uteroplacental and fetal umbilical placental circulations. Am. J. Obstet. Gynecol. 1985, 152, 155–163. [Google Scholar] [CrossRef]
- Giles, W.B.; Trudinger, B.J.; Baird, P.J. Fetal umbilical artery flow velocity waveforms and placental resistance: Pathological correlation. BJOG Int. J. Obstet. Gynaecol. 1985, 92, 31–38. [Google Scholar] [CrossRef]
- Lees, C.; Marlow, N.; Arabin, B.; Bilardo, C.M.; Brezinka, C.; Derks, J.B.; Duvekot, J.; Frusca, T.; Diemert, A.; Ferrazzi, E.; et al. Perinatal morbidity and mortality in early-onset fetal growth restriction: Cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (TRUFFLE). Ultrasound Obstet. Gynecol. 2013, 42, 400–408. [Google Scholar] [CrossRef]
- Benavides-Serralde, A.; Scheier, M.; Cruz-Martinez, R.; Crispi, F.; Figueras, F.; Gratacos, E.; Hernandez-Andrade, E. Changes in Central and Peripheral Circulation in Intrauterine Growth-Restricted Fetuses at Different Stages of Umbilical Artery Flow Deterioration: New Fetal Cardiac and Brain Parameters. Gynecol. Obstet. Investig. 2011, 71, 274–280. [Google Scholar] [CrossRef]
- Turan, O.M.; Turan, S.; Gungor, S.; Berg, C.; Moyano, D.; Gembruch, U.; Nicolaides, K.H.; Harman, C.R.; Baschat, A.A. Progression of Doppler abnormalities in intrauterine growth restriction. Ultrasound Obstet. Gynecol. 2008, 32, 160–167. [Google Scholar] [CrossRef]
- Flood, K.; Unterscheider, J.; Daly, S.; Geary, M.P.; Kennelly, M.M.; McAuliffe, F.M.; O’Donoghue, K.; Hunter, A.; Morrison, J.J.; Burke, G.; et al. The role of brain sparing in the prediction of adverse outcomes in intrauterine growth restriction: Results of the multicenter PORTO Study. Am. J. Obstet. Gynecol. 2014, 211, 288.e1–288.e5. [Google Scholar] [CrossRef]
- Cruz-Martínez, R.; Figueras, F.; Hernandez-Andrade, E.; Oros, D.; Gratacos, E. Fetal Brain Doppler to Predict Cesarean Delivery for Nonreassuring Fetal Status in Term Small-for-Gestational-Age Fetuses. Obstet. Gynecol. 2011, 117, 618–626. [Google Scholar] [CrossRef] [PubMed]
- Khalil, A.; Morales-Roselló, J.; Townsend, R.; Morlando, M.; Papageorghiou, A.; Bhide, A.; Thilaganathan, B. Value of third-trimester cerebroplacental ratio and uterine artery Doppler indices as predictors of stillbirth and perinatal loss. Ultrasound Obstet. Gynecol. 2016, 47, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Devore, G.R. The importance of the cerebroplacental ratio in the evaluation of fetal well-being in SGA and AGA fetuses. Am. J. Obstet. Gynecol. 2015, 213, 5–15. [Google Scholar] [CrossRef]
- Cruz-Martinez, R.; Savchev, S.; Cruz-Lemini, M.; Mendez, A.; Gratacos, E.; Figueras, F. Clinical utility of third-trimester uterine artery Doppler in the prediction of brain hemodynamic deterioration and adverse perinatal outcome in small-for-gestational-age fetuses. Ultrasound Obstet. Gynecol. 2014, 45, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Flatley, C.; Greer, R.M.; Kumar, S. Magnitude of change in fetal cerebroplacental ratio in third trimester and risk of adverse pregnancy outcome. Ultrasound Obstet. Gynecol. 2017, 50, 514–519. [Google Scholar] [CrossRef]
- Gramellini, D.; Folli, M.C.; Raboni, S.; Vadora, E.; Merialdi, A. Cerebral-Umbilical Doppler Ratio As a Predictor of Adverse Perinatal Outcome. Obstet. Gynecol. 1992, 79, 416–420. [Google Scholar] [CrossRef]
- Rial-Crestelo, M.; Martinez-Portilla, R.J.; Cancemi, A.; Caradeux, J.; Fernandez, L.; Peguero, A.; Gratacos, E.; Figueras, F. Added value of cerebro-placental ratio and uterine artery Doppler at routine third trimester screening as a predictor of SGA and FGR in non-selected pregnancies. J. Matern. Neonatal Med. 2018, 32, 2554–2560. [Google Scholar] [CrossRef]
- Morris, R.; Say, R.; Robson, S.; Kleijnen, J.; Khan, K. Systematic review and meta-analysis of middle cerebral artery Doppler to predict perinatal wellbeing. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 165, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz, R.; Kingdom, J.; Geary, M.; Rodeck, C. Fetal cerebral blood flow redistribution in late gestation: Identification of compromise in small fetuses with normal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 2000, 15, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Oros, D.; Figueras, F.; Cruz-Martinez, R.; Meler, E.; Munmany, M.; Gratacos, E. Longitudinal changes in uterine, umbilical and fetal cerebral Doppler indices in late-onset small-for-gestational age fetuses. Ultrasound Obstet. Gynecol. 2010, 37, 191–195. [Google Scholar] [CrossRef]
- ACOG. Practice Bulletin No. 175: Ultrasound in Pregnancy. Obstet. Gynecol. 2016, 128, e241–e256. [Google Scholar] [CrossRef] [PubMed]
- Hadlock, F.P.; Harrist, R.; Sharman, R.S.; Deter, R.L.; Park, S.K. Estimation of fetal weight with the use of head, body, and femur measurements—A prospective study. Am. J. Obstet. Gynecol. 1985, 151, 333–337. [Google Scholar] [CrossRef]
- Bhide, A.; Acharya, G.; Bilardo, C.M.; Brezinka, C.; Cafici, D.; Hernandez-Andrade, E.; Kalache, K.; Kingdom, J.; Kiserud, T.; Lee, W.; et al. ISUOG Practice Guidelines: Use of Doppler ultrasonography in obstetrics. Ultrasound Obstet. Gynecol. 2013, 41, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Baschat, A.A.; Gembruch, U. The cerebroplacental Doppler ratio revisited. Ultrasound Obstet. Gynecol. 2003, 21, 124–127. [Google Scholar] [CrossRef]
- Acharya, G.; Wilsgaard, T.; Berntsen, G.K.R.; Maltau, J.M.; Kiserud, T. Reference ranges for serial measurements of umbilical artery Doppler indices in the second half of pregnancy. Am. J. Obstet. Gynecol. 2005, 192, 937–944. [Google Scholar] [CrossRef]
- Ebbing, C.; Rasmussen, S.; Kiserud, T. Middle cerebral artery blood flow velocities and pulsatility index and the cerebroplacental pulsatility ratio: Longitudinal reference ranges and terms for serial measurements. Ultrasound Obstet. Gynecol. 2007, 30, 287–296. [Google Scholar] [CrossRef]
- Contag, S.; Patel, P.; Payton, S.; Crimmins, S.; Goetzinger, K.R. Renal artery Doppler compared with the cerebral placental ratio to identify fetuses at risk for adverse neonatal outcome. J. Matern. Neonatal Med. 2021, 34, 532–540. [Google Scholar] [CrossRef]
- Lewis, A.B.; Evans, W.N.; Sischo, W. Plasma Catecholamine Responses to Hypoxemia in Fetal Lambs. Neonatology 1982, 41, 115–122. [Google Scholar] [CrossRef]
- Nicolaides, K.H.; Bilardo, C.M.; Soothill, P.W.; Campbell, S. Absence of end diastolic frequencies in umbilical artery: A sign of fetal hypoxia and acidosis. BMJ 1988, 297, 1026–1027. [Google Scholar] [CrossRef] [PubMed]
- Karsdorp, V.; van Vugt, J.; van Geijn, H.; Kostense, P.; Arduim, D.; Montenegro, N.; Todros, T. Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet 1994, 344, 1664–1668. [Google Scholar] [CrossRef]
- Giussani, D.A. The fetal brain sparing response to hypoxia: Physiological mechanisms. J. Physiol. 2016, 594, 1215–1230. [Google Scholar] [CrossRef] [PubMed]
- Giussani, D.A.; Gardner, D.S.; Cox, D.T.; Fletcher, A.J.W. Purinergic contribution to circulatory, metabolic, and adrenergic responses to acute hypoxemia in fetal sheep. Am. J. Physiol. Integr. Comp. Physiol. 2001, 280, R678–R685. [Google Scholar] [CrossRef]
- Gardner, D.S.; Fletcher, A.J.W.; Bloomfield, M.R.; Fowden, A.L.; Giussani, D.A. Effects of prevailing hypoxaemia, acidaemia or hypoglycaemia upon the cardiovascular, endocrine and metabolic responses to acute hypoxaemia in the ovine fetus. J. Physiol. 2002, 540, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.J.W.; Gardner, D.S.; Edwards, C.M.B.; Fowden, A.L.; Giussani, D.A. Development of the ovine fetal cardiovascular defense to hypoxemia towards full term. Am. J. Physiol. Circ. Physiol. 2006, 291, H3023–H3034. [Google Scholar] [CrossRef]
- Assali, N.S.; Brinkman, C.R., III; Woods, J.R., Jr.; Dandavino, A.; Nuwayhid, B. Development of neurohumoral control of fetal, neonatal, and adult cardiovascular functions. Am. J. Obstet. Gynecol. 1977, 129, 748–759. [Google Scholar] [CrossRef]
- Barrett, C.T.; Heymann, M.A.; Rudolph, A.M. Alpha and beta adrenergic receptor activity in fetal sheep. Am. J. Obstet. Gynecol. 1972, 112, 1114–1121. [Google Scholar] [CrossRef]
- Wyse, D.G.; Van Petten, G.R.; Harris, W.H. Responses to electrical stimulation, noradrenaline, serotonin, and vasopressin in the isolated ear artery of the developing lamb and ewe. Can. J. Physiol. Pharmacol. 1977, 55, 1001–1006. [Google Scholar] [CrossRef]
- Galan, H.L.; Anthony, R.V.; Rigano, S.; Parker, T.A.; De Vrijer, B.; Ferrazzi, E.; Wilkening, R.B.; Regnault, T.R. Fetal hypertension and abnormal Doppler velocimetry in an ovine model of intrauterine growth restriction. Am. J. Obstet. Gynecol. 2005, 192, 272–279. [Google Scholar] [CrossRef]
- Rhee, C.J.; Fraser, C.D.; Kibler, K.; Easley, R.B.; Andropoulos, D.B.; Czosnyka, M.; Varsos, G.V.; Smielewski, P.; Rusin, C.G.; Brady, K.M.; et al. The Ontogeny of Cerebrovascular Pressure Autoregulation in Premature Infants. Pain 2016, 122, 151–155. [Google Scholar] [CrossRef]
- Kennelly, M.M.; Farah, N.; Hogan, J.; Reilly, A.; Turner, M.J.; Stuart, B. Longitudinal study of aortic isthmus Doppler in appropriately grown and small-for-gestational-age fetuses with normal and abnormal umbilical artery Doppler. Ultrasound Obstet. Gynecol. 2011, 39, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, A.M. Distribution and regulation of blood flow in the fetal and neonatal lamb. Circ. Res. 1985, 57, 811–821. [Google Scholar] [CrossRef]
- Baschat, A.A. Fetal growth restriction—From observation to intervention. J. Périnat. Med. 2010, 38, 239–246. [Google Scholar] [CrossRef]
- Kiserud, T.; Chedid, G.; Rasmussen, S. Foramen ovale changes in growth-restricted fetuses. Ultrasound Obstet. Gynecol. 2004, 24, 141–146. [Google Scholar] [CrossRef]
- Anderson, P.A.; Kleinman, C.S.; Lister, G.; Talner, S. Cardiovascular function during development and the response to hypoxia. In Fetal and Neonatal Physiology, 3rd ed.; Polin, F., Fox, W., Abman, S., Eds.; Saunders: Philadelphia, PA, USA, 2004; Volume 1, pp. 635–668. [Google Scholar]
- Gardner, D.S.; Fowden, A.L.; Giussani, D.A. Adverse Intrauterine Conditions Diminish the Fetal Defense Against Acute Hypoxia by Increasing Nitric Oxide Activity. Circulation 2002, 106, 2278–2283. [Google Scholar] [CrossRef]
- Gardner, D.S.; Giussani, D.A. Enhanced umbilical blood flow during acute hypoxemia after chronic umbilical cord compression: A role for nitric oxide. Circulation 2003, 108, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Arduini, D.; Rizzo, G. Fetal renal artery velocity waveforms and amniotic fluid volume in growth-retarded and post-term fetuses. Obstet. Gynecol. 1991, 77, 370–373. [Google Scholar]
- Stigter, R.H.; Mulder, E.J.H.; Bruinse, H.W.; Visser, G.H.A. Doppler studies on the fetal renal artery in the severely growth-restricted fetus. Ultrasound Obstet. Gynecol. 2001, 18, 141–145. [Google Scholar] [CrossRef] [PubMed]
- Figueira, C.O.; Surita, F.G.; Dertkigil, M.S.; Pereira, S.L.; Bennini, J.R.; Morais, S.S.; Cecatti, J.G. Longitudinal reference intervals for Doppler velocimetric parameters of the fetal renal artery correlated with amniotic fluid index among low-risk pregnancies. Int. J. Gynecol. Obstet. 2015, 131, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Mari, G.; Kirshon, B.; Abuhamad, A. Fetal renal artery flow velocity waveforms in normal pregnancies and pregnancies complicated by polyhydramnios and oligohydramnios. Obstet. Gynecol. 1993, 81, 560–564. [Google Scholar] [PubMed]
- Vyas, S.; Nicolaides, K.; Campbell, S. Renal artery flow-velocity waveforms in normal and hypoxemic fetuses. Am. J. Obstet. Gynecol. 1989, 161, 168–172. [Google Scholar] [CrossRef]
- Iura, T.; Makinoda, S.; Fujita, S.; Matsuzawa, S.; Waseda, T.; Ohshima, K.; Tomizawa, H. Analysis of Renal Artery Hemodynamics in Normal Fetuses Using the Color Doppler Method. Fetal Diagn. Ther. 2005, 20, 86–90. [Google Scholar] [CrossRef]
- Konje, J.C.; Abrams, K.R.; Taylor, D.J. Normative values of Doppler velocimetry of five major fetal arteries as determined by color power angiography. Acta Obstet. Gynecol. Scand. 2005, 84, 230–237. [Google Scholar] [CrossRef] [PubMed]
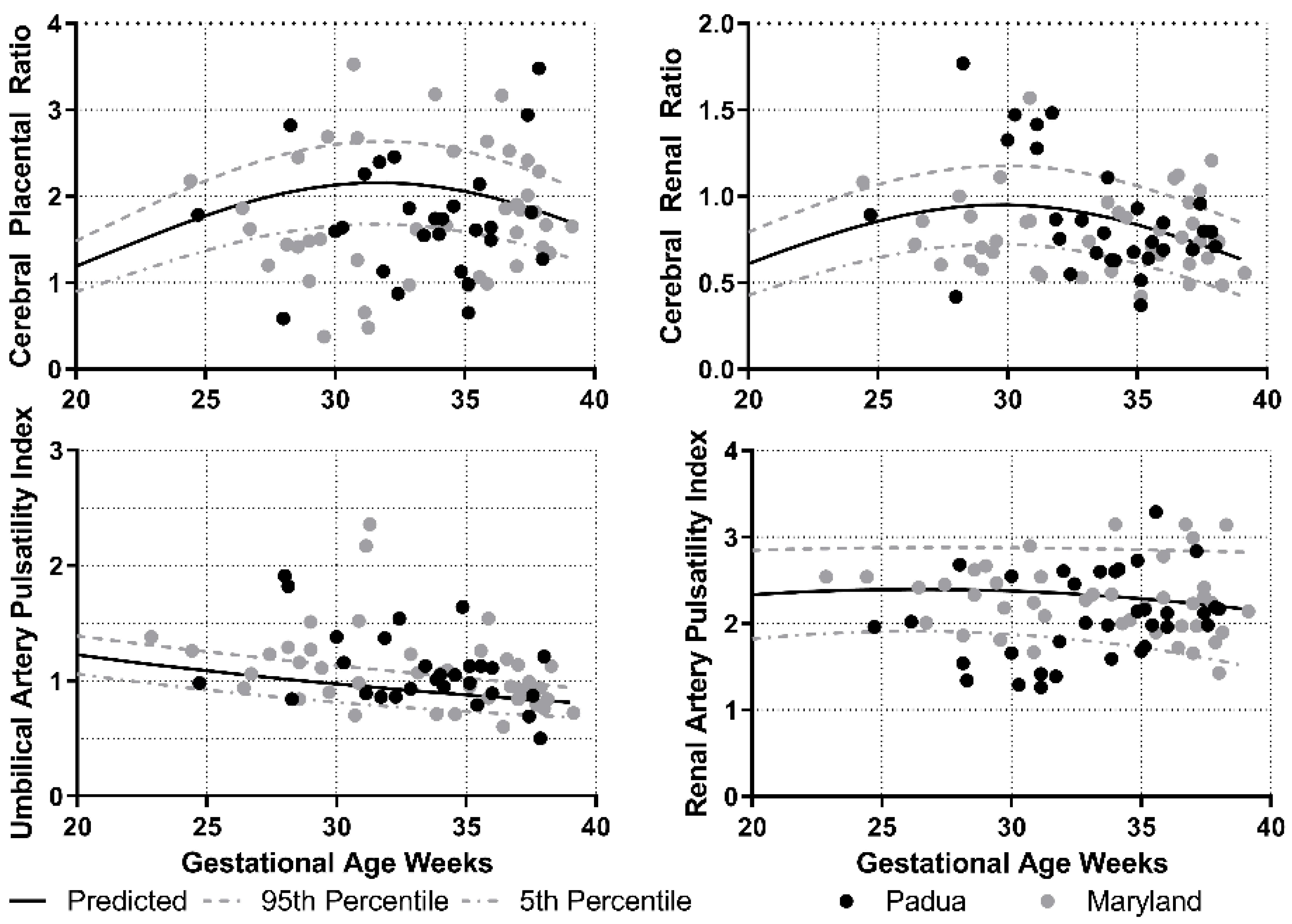

| All (92) | |
|---|---|
| Variable | Median (range) |
| Gravida | 2 (1–3) |
| Para | 1 (0–2) |
| BMI | 30.5 (23.3–37.3) |
| EFW | 1932 (1275–2183) |
| Percentile | 7 (4–9) |
| AFI | 13.1 (11.1–16.7) |
| Gestational age at scan | 34 (32–36) |
| Scan to delivery time | 2.14 (0.9–4.0) |
| Number (%) | |
| Preeclampsia | 15 (16) |
| Chronic hypertension | 6 (6.5) |
| Gestational diabetes | 7 (7.6) |
| Total (92) | |
|---|---|
| Variable | Median (range) |
| Gestational age at birth | 37 (34–39) |
| Birthweight | 2305 (1630–2660) |
| NICU length of stay | 4 (0–25) |
| Number (%) | |
| Mode of delivery | |
| Vaginal | 35 (39) |
| Cesarean | 52 (58) |
| Operative vaginal | 2 (2) |
| Unknown | 3 (2) |
| Indication for delivery: non-reassuring fetal status | 27 (30) |
| Neonatal outcomes | |
| Apgar <7 at 5 min | 7 (8) |
| Umbilical artery pH <7.2 | 24 (26) |
| Umbilical artery BE >8 | 19 (21) |
| Abnormal pH and BE 2 | 19 (21) |
| NICU admission >48 h | 37 (40) |
| Neonatal ventilation >6 h | 9 (10) |
| Neonatal complication 3 | 49 (53) |
| Maryland | Padua | ||
|---|---|---|---|
| Test | Mean (SD) | Mean (SD) | p-Values |
| Umbilical artery pulsatility index | 1.15 (0.52 | 1.10 (0.33) | 0.6 |
| Renal artery pulsatility index | 2.29 (0.42) | 2.07 (0.50) | 0.04 |
| Cerebral–placental ratio | 1.76 (0.72) | 1.74 (0.68) | 0.93 |
| Cerebral–renal ratio | 0.79 (0.23) | 0.88 (0.34) | 0.24 |
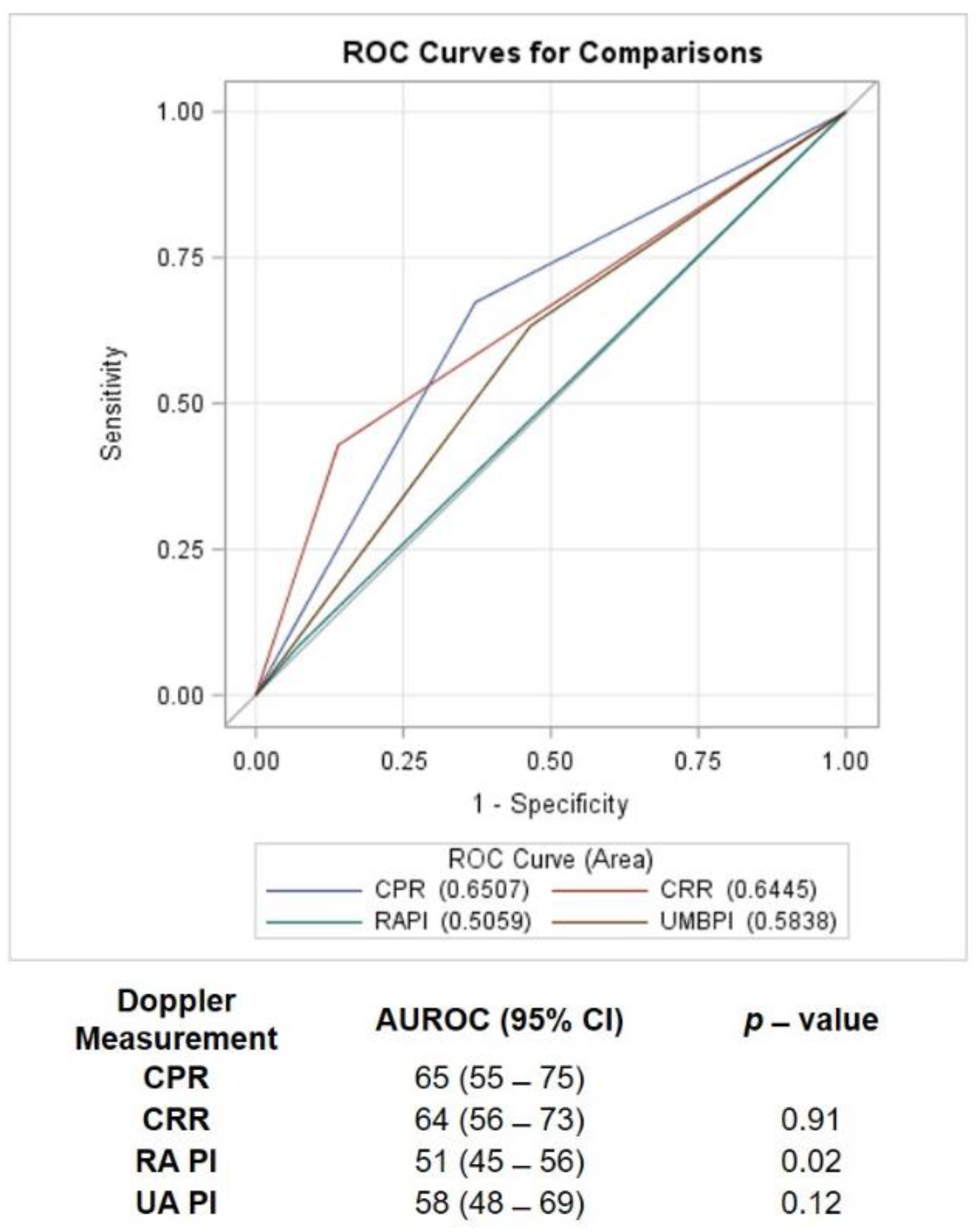
| CPR | CRR | Renal PI | Umbilical PI | |
|---|---|---|---|---|
| Sensitivity (%) | 67 (54–80) | 43 (29–57) | 8 (2–20) | 63 (50–77) |
| Specificity (%) | 63 (48–77) | 86 (76–96) | 93 (81–99) | 54 (39–68) |
| False positive rate (%) | 37 (23–52) | 14 (5–28) | 7 (2–19) | 47 (32–61) |
| False negative rate (%) | 33 (20–46) | 57 (43–71) | 92 (80–98) | 37 (23–50) |
| Positive predictive value (%) | 67 (54–80) | 78 (62–93) | 57 (18–90) | 61 (47–74) |
| Negative predictive value (%) | 63 (48–77) | 57 (45–69) | 47 (37–58) | 56 (41–71) |
| Positive likelihood ratio | 1.8 (1.2–2.8) | 1.8 (1.3–2.5) | 1.1 (0.6–2.1) | 1.4 (0.9–2.1) |
| Negative likelihood ratio | 0.6 (0.4–0.9) | 0.6 (0.4–0.8) | 0.9 (0.5–1.8) | 0.7 (0.5–1.1) |
| McNemar’s p-value | Reference | <0.01 | <0.01 | 0.9 |
| Cohen’s kappa value | Reference | 0.6 (0.5–0.7) | −0.04 (−0.1–0.03) | 0.5 (0.3–0.6) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Contag, S.; Visentin, S.; Goetzinger, K.; Cosmi, E. Use of the Renal Artery Doppler to Identify Small for Gestational Age Fetuses at Risk for Adverse Neonatal Outcomes. J. Clin. Med. 2021, 10, 1835. https://doi.org/10.3390/jcm10091835
Contag S, Visentin S, Goetzinger K, Cosmi E. Use of the Renal Artery Doppler to Identify Small for Gestational Age Fetuses at Risk for Adverse Neonatal Outcomes. Journal of Clinical Medicine. 2021; 10(9):1835. https://doi.org/10.3390/jcm10091835
Chicago/Turabian StyleContag, Stephen, Silvia Visentin, Katherine Goetzinger, and Erich Cosmi. 2021. "Use of the Renal Artery Doppler to Identify Small for Gestational Age Fetuses at Risk for Adverse Neonatal Outcomes" Journal of Clinical Medicine 10, no. 9: 1835. https://doi.org/10.3390/jcm10091835
APA StyleContag, S., Visentin, S., Goetzinger, K., & Cosmi, E. (2021). Use of the Renal Artery Doppler to Identify Small for Gestational Age Fetuses at Risk for Adverse Neonatal Outcomes. Journal of Clinical Medicine, 10(9), 1835. https://doi.org/10.3390/jcm10091835





