Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability
Abstract
:1. Introduction
2. Materials and Methods
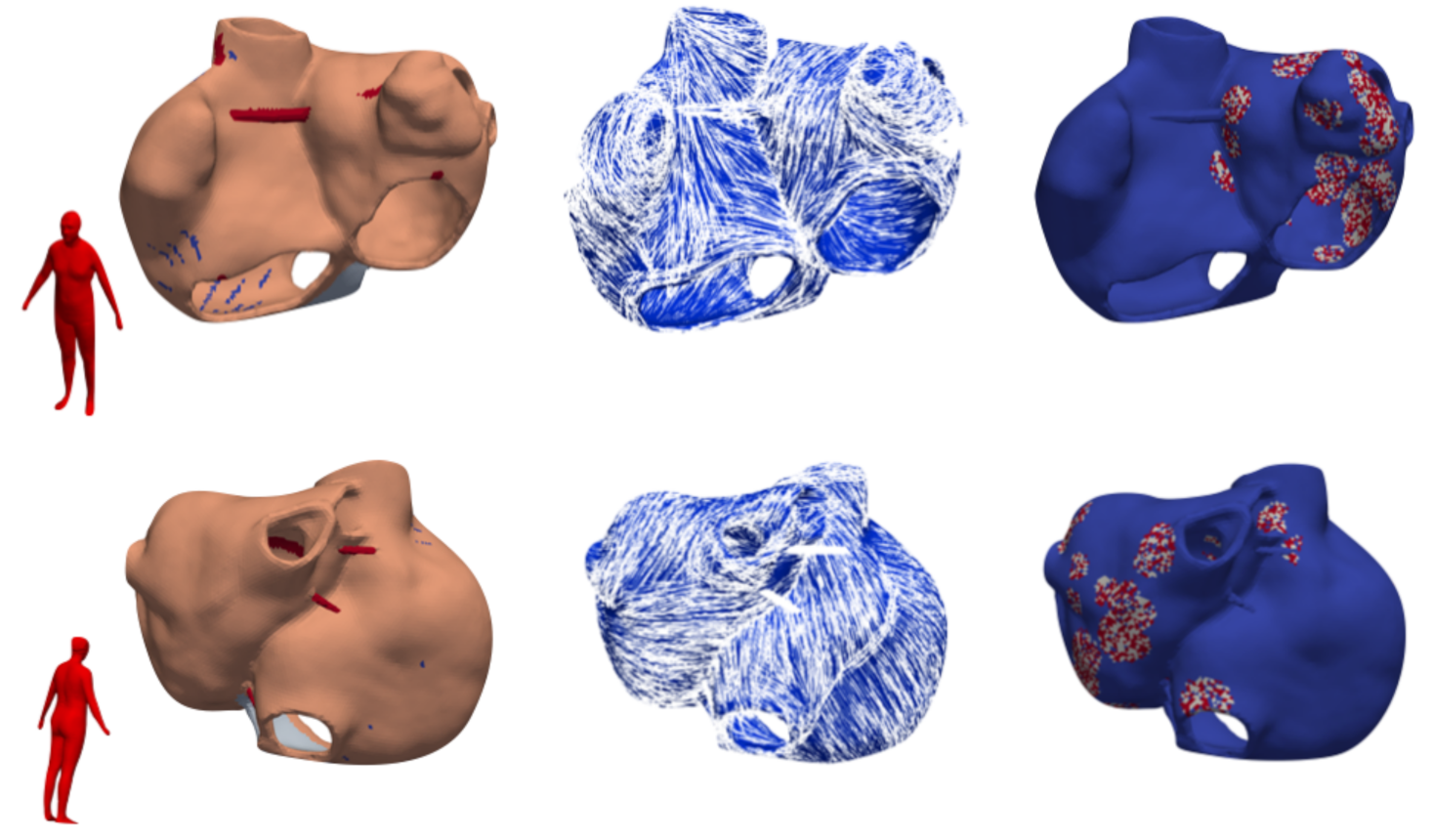
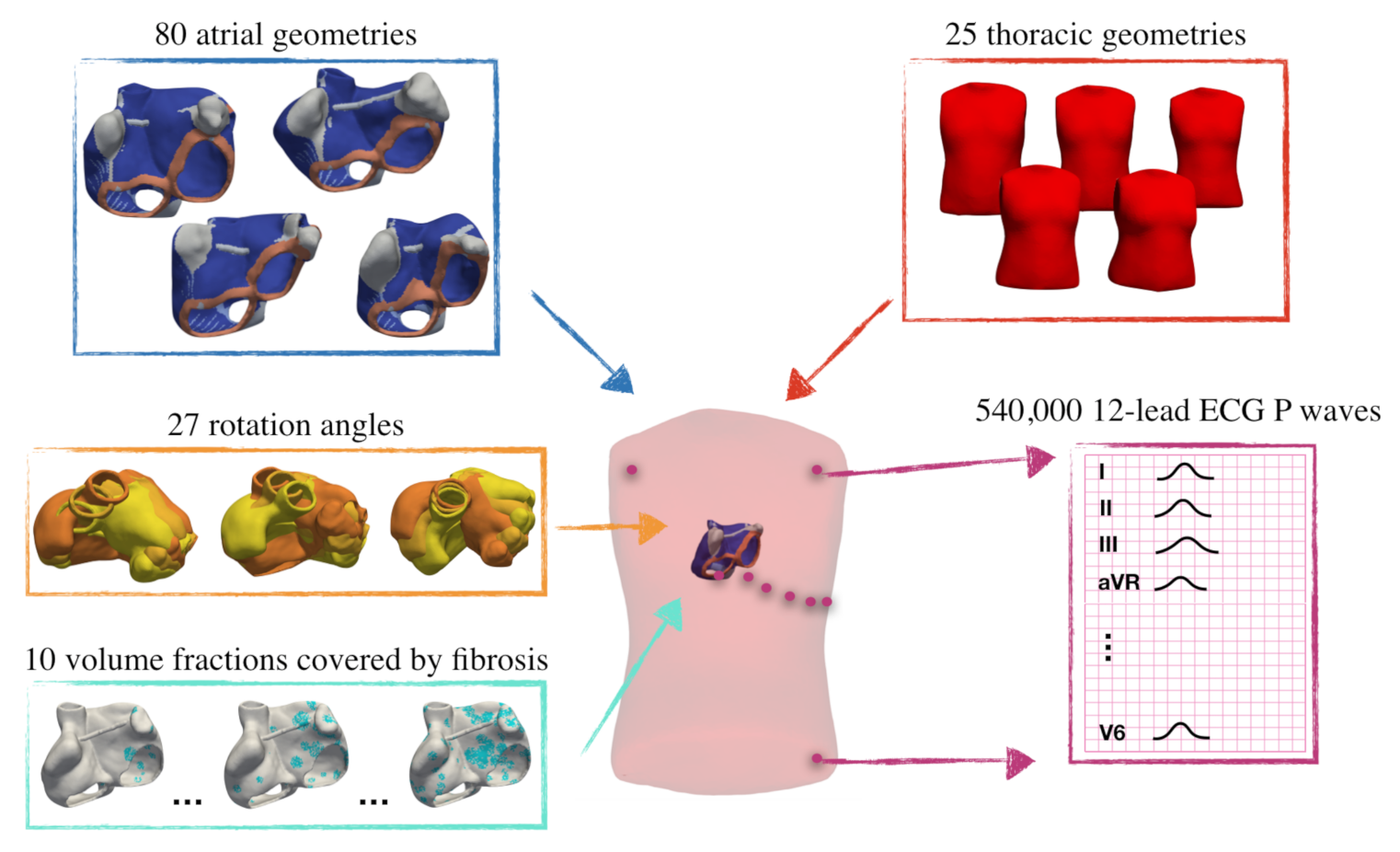
2.1. Database
2.2. Modeling Methodology of Fibrotic Tissue
2.3. Electrophysiological Simulations
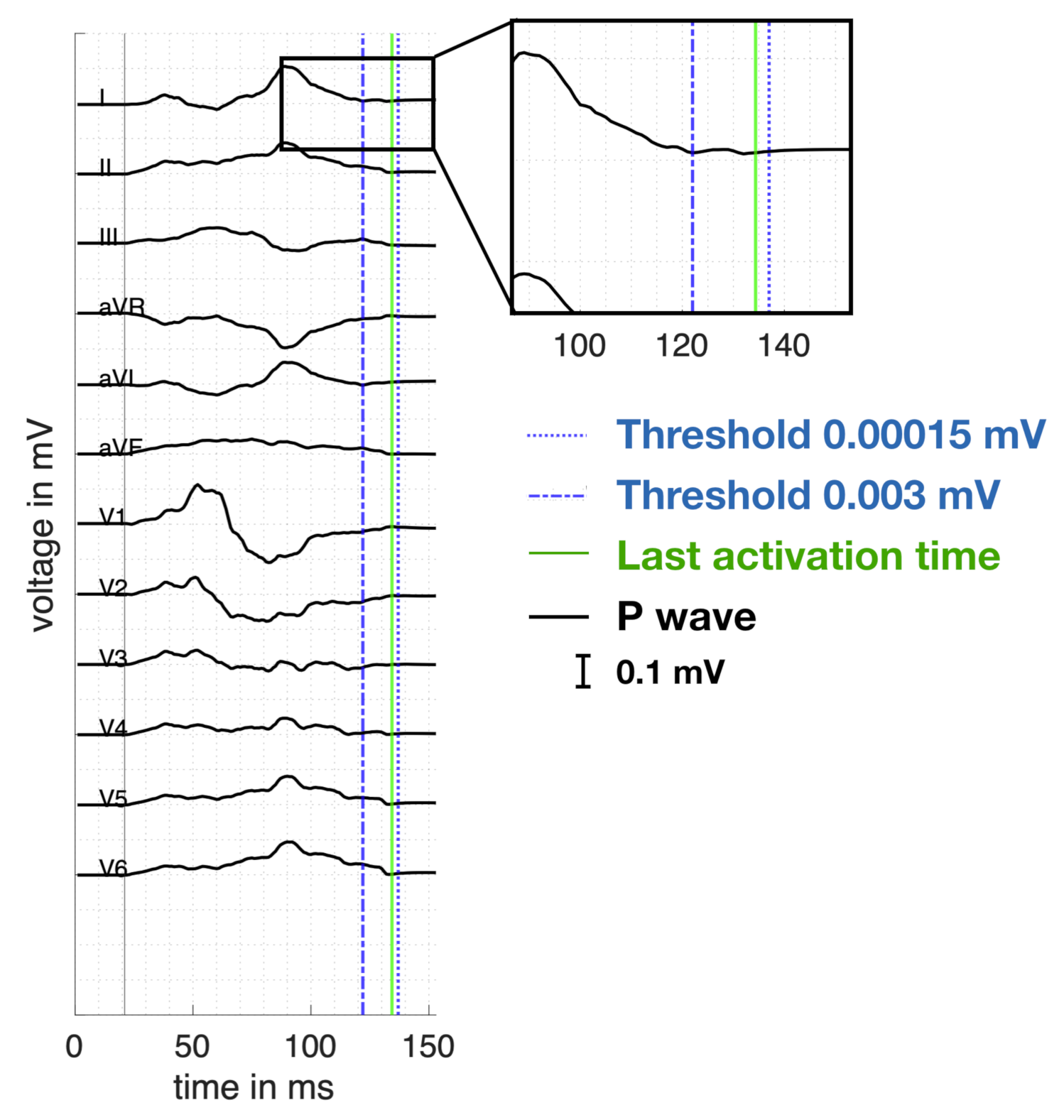
2.4. ECG Analysis and Feature Extraction
2.5. Regression Using Neural Networks
3. Results
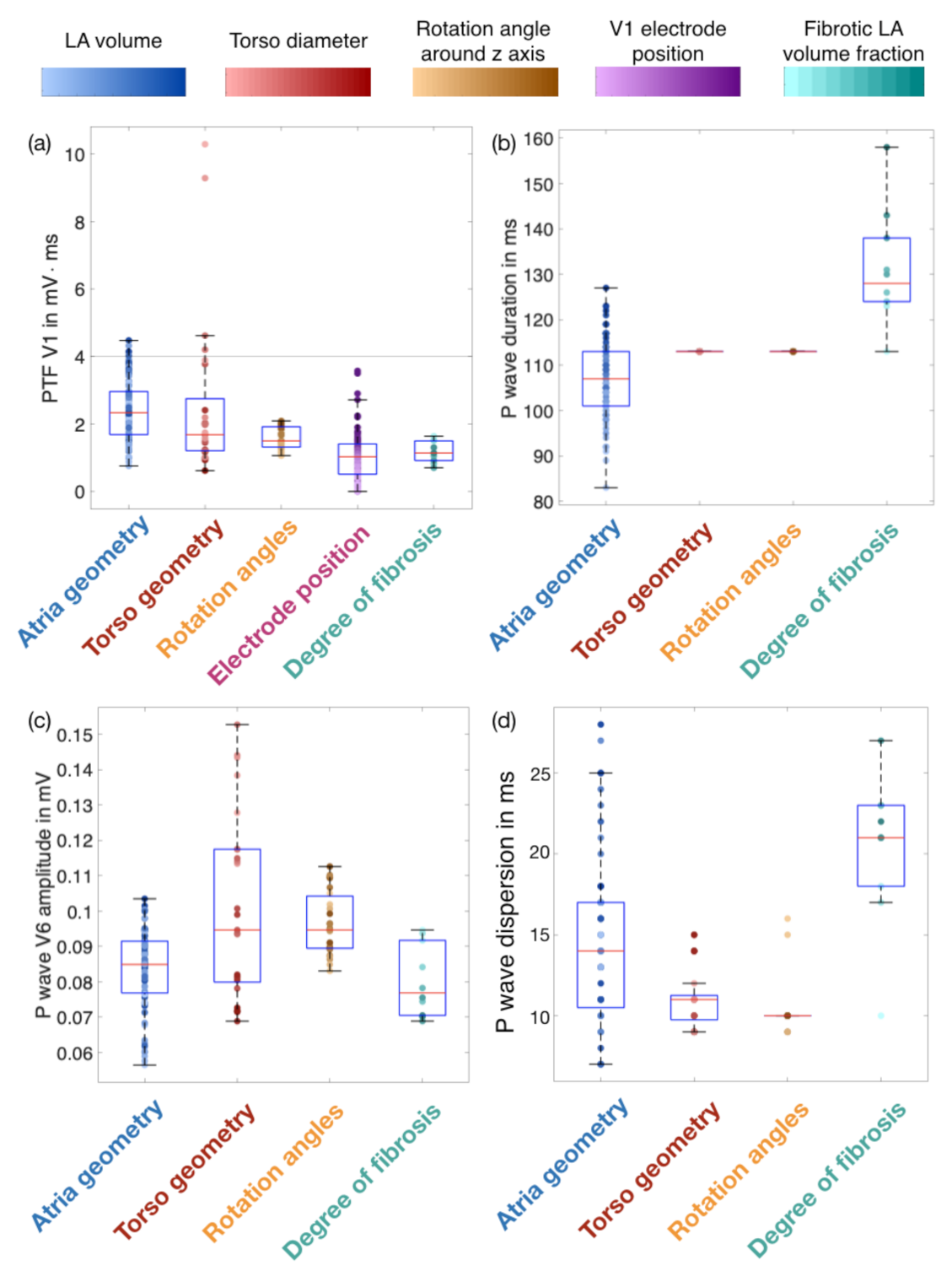
3.1. Influence of Geometries, Rotation Angles and Electrode Positions on P wave Features
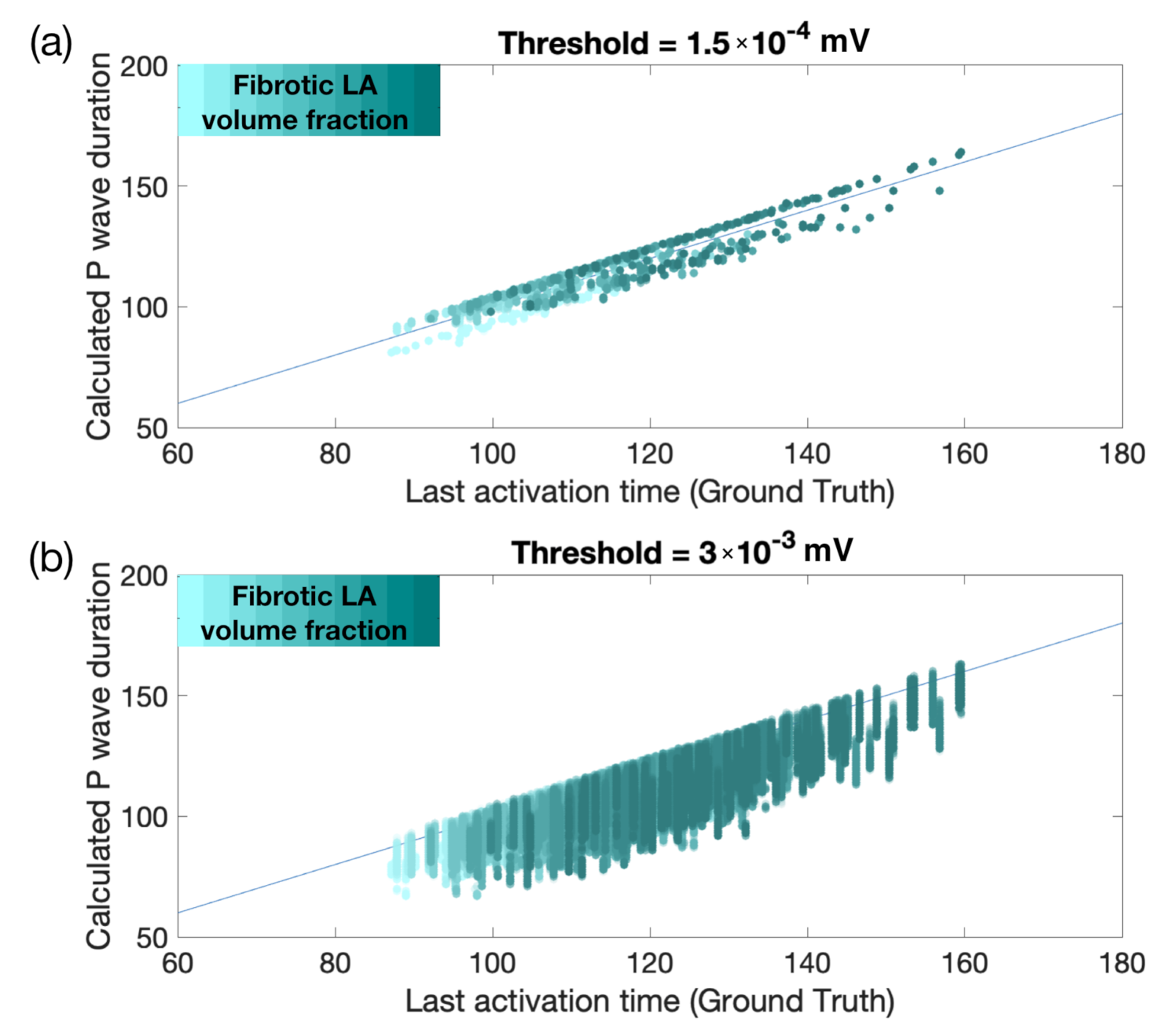
3.2. Effect of the Fibrotic LA Volume Fraction on P Wave Features
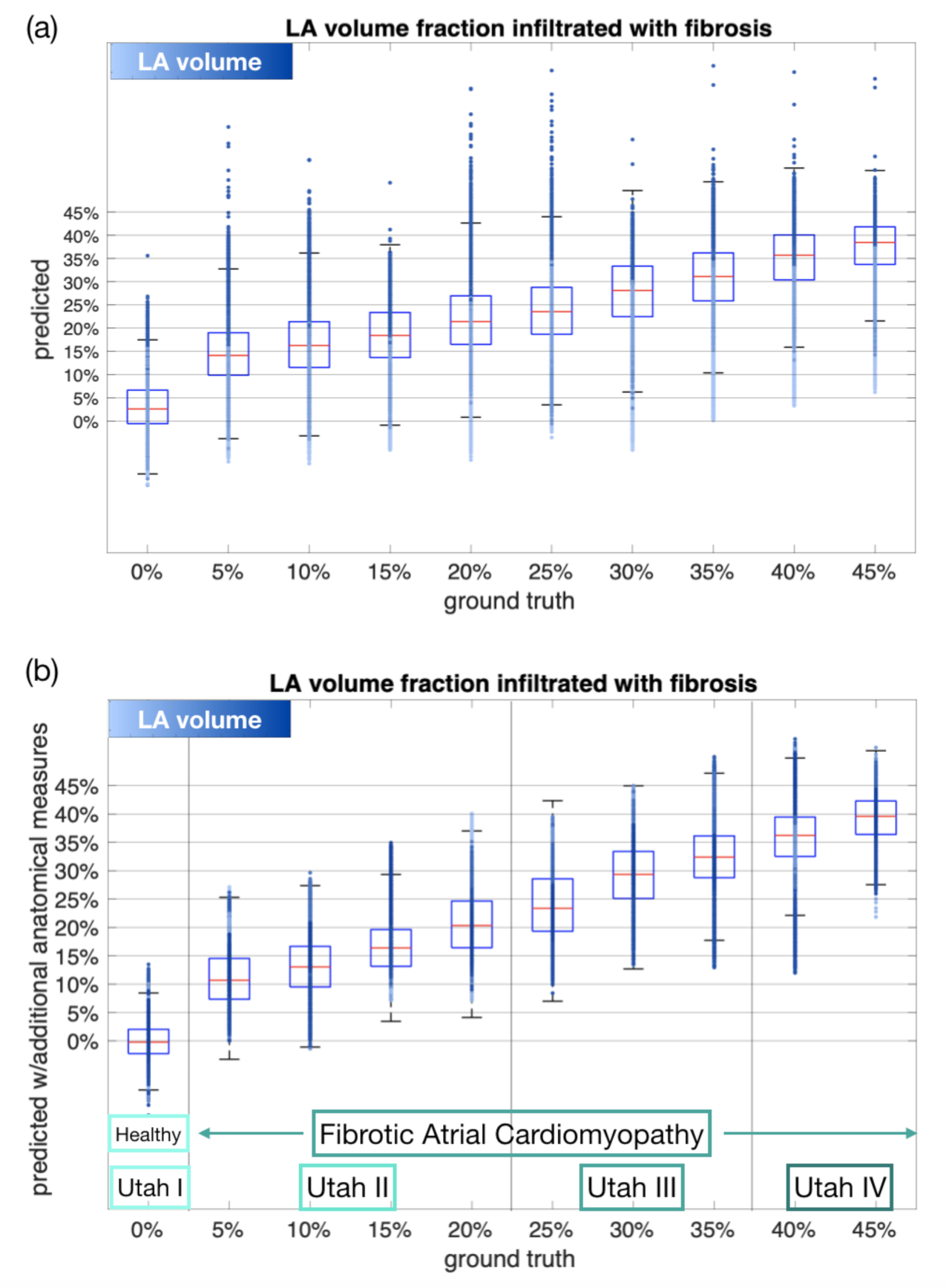
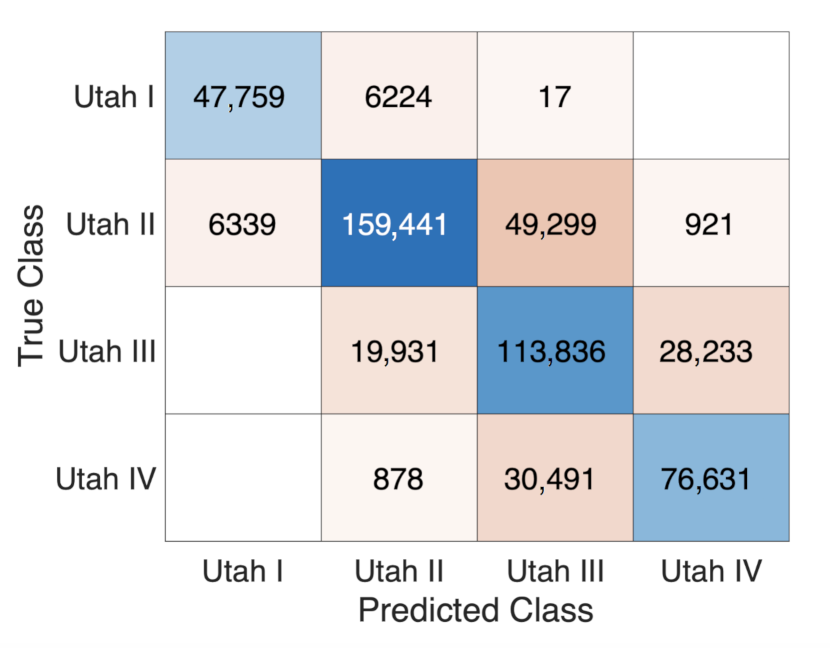
3.3. Estimating the Amount of Fibrosis with Neural Networks
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AR | anisotropy ratio |
| CV | conduction velocity |
| ECG | electrocardiogram |
| FAM | fibrotic atrial cardiomyopathy |
| LA | left atrium |
| LAT | local activation time |
| MRI | magnetic resonance imaging |
| NN | neural network |
| PTF V1 | P wave terminal force in lead V1 |
| PWD | P wave duration |
| RMSE | root mean square error |
| SR | sinus rhythm |
Appendix A. Calculation of the PWD
References
- Zoni-Berisso, M.; Lercari, F.; Carazza, T.; Domenicucci, S. Epidemiology of atrial fibrillation: European perspective. Clin. Epidemiol. 2014, 6, 213–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schotten, U.; Dobrev, D.; Platonov, P.G.; Kottkamp, H.; Hindricks, G. Current controversies in determining the main mechanisms of atrial fibrillation. J. Intern. Med. 2016, 279, 428–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijman, J.; Linz, D.; Schotten, U. Dynamics of Atrial Fibrillation Mechanisms and Comorbidities. Annu. Rev. Physiol. 2021, 83. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, T.; Niwano, S.; Niwano, H.; Igarashi, T.; Fujiishi, T.; Ishizue, N.; Oikawa, J.; Satoh, A.; Kurokawa, S.; Hatakeyama, Y.; et al. Prediction of new onset atrial fibrillation through P wave analysis in 12 lead ECG. Int. Heart J. 2014, 55, 422–427. [Google Scholar] [CrossRef] [Green Version]
- Jadidi, A.; Müller-Edenborn, B.; Chen, J.; Keyl, C.; Weber, R.; Allgeier, J.; Moreno-Weidmann, Z.; Trenk, D.; Neumann, F.J.; Lehrmann, H.; et al. The Duration of the Amplified Sinus-P-Wave Identifies Presence of Left Atrial Low Voltage Substrate and Predicts Outcome after Pulmonary Vein Isolation in Patients with Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 531–543. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Marrouche, N.F. Recurrence Post-Atrial Fibrillation Ablation: Think Outside the Pulmonary Veins. Circ. Arrhythmia Electrophysiol. 2018, 11, e006379. [Google Scholar] [CrossRef]
- Nairn, D.; Lehrmann, H.; Müller-Edenborn, B.; Schuler, S.; Arentz, T.; Dössel, O.; Jadidi, A.; Loewe, A. Comparison of Unipolar and Bipolar Voltage Mapping for Localization of Left Atrial Arrhythmogenic Substrate in Patients with Atrial Fibrillation. Front. Physiol. 2020, 11. [Google Scholar] [CrossRef]
- Jadidi, A.; Nothstein, M.; Chen, J.; Lehrmann, H.; Dössel, O.; Allgeier, J.; Trenk, D.; Neumann, F.J.; Loewe, A.; Müller-Edenborn, B.; et al. Specific Electrogram Characteristics Identify the Extra-Pulmonary Vein Arrhythmogenic Sources of Persistent Atrial Fibrillation-Characterization of the Arrhythmogenic Electrogram Patterns During Atrial Fibrillation and Sinus Rhythm. Sci. Rep. 2020, 10, 9147. [Google Scholar] [CrossRef]
- Caixal, G.; Alarcón, F.; Althoff, T.F.; Nuñez-Garcia, M.; Benito, E.M.; Borràs, R.; Perea, R.J.; Prat-González, S.; Garre, P.; Soto-Iglesias, D.; et al. Accuracy of left atrial fibrosis detection with cardiac magnetic resonance: Correlation of late gadolinium enhancement with endocardial voltage and conduction velocity. EP Eur. 2020, 23, 380–388. [Google Scholar] [CrossRef]
- Karim, R.; Housden, R.J.; Balasubramaniam, M.; Chen, Z.; Perry, D.; Uddin, A.; Al-Beyatti, Y.; Palkhi, E.; Acheampong, P.; Obom, S.; et al. Evaluation of current algorithms for segmentation of scar tissue from late gadolinium enhancement cardiovascular magnetic resonance of the left atrium: An open-access grand challenge. J. Cardiovasc. Magn. Reson. 2013, 15, 105. [Google Scholar] [CrossRef] [Green Version]
- Nairn, D.; Hunyar, D.; Sánchez, J.; Dössel, O.; Loewe, A. Inverse Relationship Between Electrode Size and Voltage During Intracardiac Mapping. In Proceedings of the Computing in Cardiology, Rimini, Italy, 13–16 September 2020. [Google Scholar]
- Müller-Edenborn, B.; Chen, J.; Allgeier, J.; Didenko, M.; Moreno-Weidmann, Z.; Neumann, F.J.; Lehrmann, H.; Weber, R.; Arentz, T.; Jadidi, A. Amplified sinus-P-wave reveals localization and extent of left atrial low-voltage substrate: Implications for arrhythmia freedom following pulmonary vein isolation. EP Eur. 2019, 22, 240–249. [Google Scholar] [CrossRef]
- Jadidi, A.; Müller-Edenborn, B.; Chen, J.; Keyl, C.; Weber, R.; Allgeier, J.; Moreno-Weidmann, Z.; Trenk, D.; Neumann, F.J.; Lehrmann, H.; et al. Amplified Sinus P-Wave Duration in Persistent Atrial Fibrillation. JACC Clin. Electrophysiol. 2018. [Google Scholar] [CrossRef]
- Andlauer, R.; Seemann, G.; Baron, L.; Dössel, O.; Kohl, P.; Platonov, P.; Loewe, A. Influence of left atrial size on P-wave morphology: Differential effects of dilation and hypertrophy. Europace 2018, 20, iii36–iii44. [Google Scholar] [CrossRef]
- Nakatani, Y.; Sakamoto, T.; Yamaguchi, Y.; Tsujino, Y.; Kataoka, N.; Kinugawa, K. P-wave vector magnitude predicts the left atrial low-voltage area in patients with paroxysmal atrial fibrillation. J. Electrocardiol. 2020, 59, 35–40. [Google Scholar] [CrossRef]
- Lankveld, T.; Zeemering, S.; Scherr, D.; Kuklik, P.; Hoffmann, B.A.; Willems, S.; Pieske, B.; Haïssaguerre, M.; Jaïs, P.; Crijns, H.J.; et al. Atrial Fibrillation Complexity Parameters Derived From Surface ECGs Predict Procedural Outcome and Long-Term Follow-Up of Stepwise Catheter Ablation for Atrial Fibrillation. Circ. Arrhythmia Electrophysiol. 2016, 9, e003354. [Google Scholar] [CrossRef] [Green Version]
- Tse, G.; Lakhani, I.; Zhou, J.; Li, K.H.C.; Lee, S.; Liu, Y.; Leung, K.S.K.; Liu, T.; Baranchuk, A.; Zhang, Q. P-Wave Area Predicts New Onset Atrial Fibrillation in Mitral Stenosis: A Machine Learning Approach. Front. Bioeng. Biotechnol. 2020, 8, 479. [Google Scholar] [CrossRef]
- Nagel, C.; Schuler, S.; Dössel, O.; Loewe, A. A bi-atrial statistical shape model for large-scale in silico studies of human atria: Model development and application to ECG simulations. arXiv 2021, arXiv:2102.10838. [Google Scholar]
- Rasmussen, M.U.; Fabricius-Bjerre, A.; Kumarathurai, P.; Larsen, B.S.; Domínguez, H.; Kanters, J.K.; Sajadieh, A. Common source of miscalculation and misclassification of P-wave negativity and P-wave terminal force in lead V1. J. Electrocardiol. 2019, 53, 85–88. [Google Scholar] [CrossRef]
- Wachter, A.; Loewe, A.; Krueger, M.W.; Dössel, O.; Seemann, G. Mesh structure-independent modeling of patient-specific atrial fiber orientation. De Gruyter 2015, 1, 409–412. [Google Scholar] [CrossRef] [Green Version]
- Nagel, C.; Schuler, S.; Dössel, O.; Loewe, A. A Bi-atrial Statistical Shape Model and 100 Volumetric Anatomical Models of the Atria. Available online: https://zenodo.org/record/4309958#.YHg-eOgzZPY (accessed on 23 February 2021). [CrossRef]
- Pishchulin, L.; Wuhrer, S.; Helten, T.; Theobalt, C.; Schiele, B. Building statistical shape spaces for 3D human modeling. Pattern Recognit. 2017, 67, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Odille, F.; Liu, S.; van Dam, P.; Felblinger, J.; Odille, F.; Liu, S.; van Dam, P.; Felblinger, J. Statistical Variations of Heart Orientation in Healthy Adults. In Proceedings of the 2017 Computing in Cardiology Conference (CinC), Rennes, France, 24–27 September 2017; Volume 44. [Google Scholar] [CrossRef]
- Akoum, N.; McGann, C.; Vergara, G.; Badger, T.; Ranjan, R.; Mahnkopf, C.; Kholmovski, E.; Macleod, R.; Marrouche, N. Atrial fibrosis quantified using late gadolinium enhancement MRI is associated with sinus node dysfunction requiring pacemaker implant. J. Cardiovasc. Electrophysiol. 2012, 23, 44–50. [Google Scholar] [CrossRef] [Green Version]
- Nezlobinsky, T.; Solovyova, O.; Panfilov, A.V. Anisotropic conduction in the myocardium due to fibrosis: The effect of texture on wave propagation. Sci. Rep. 2020, 10, 764. [Google Scholar] [CrossRef]
- Higuchi, K.; Cates, J.; Gardner, G.; Morris, A.; Burgon, N.S.; Akoum, N.; Marrouche, N.F. The Spatial Distribution of Late Gadolinium Enhancement of Left Atrial Magnetic Resonance Imaging in Patients with Atrial Fibrillation. JACC Clin. Electrophysiol. 2018, 4, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Vigmond, E.; Pashaei, A.; Amraoui, S.; Cochet, H.; Hassaguerre, M. Percolation as a mechanism to explain atrial fractionated electrograms and reentry in a fibrosis model based on imaging data. Heart Rhythm 2016, 13, 1536–1543. [Google Scholar] [CrossRef]
- Xintarakou, A.; Tzeis, S.; Psarras, S.; Asvestas, D.; Vardas, P. Atrial fibrosis as a dominant factor for the development of atrial fibrillation: Facts and gaps. EP Eur. 2020, 22, 342–351. [Google Scholar] [CrossRef]
- Roney, C.H.; Bayer, J.D.; Zahid, S.; Meo, M.; Boyle, P.M.J.; Trayanova, N.A.; Haïssaguerre, M.; Dubois, R.; Cochet, H.; Vigmond, E.J. Modelling methodology of atrial fibrosis affects rotor dynamics and electrograms. EP Eur. 2016, 18, iv146–iv155. [Google Scholar] [CrossRef] [PubMed]
- Azzolin, L.; Schuler, S.; Loewe, A.; Dössel, O. A Reproducible Protocol to Assess Arrhythmia Vulnerability in Silico: Pacing at the End of the Effective Refractory Period. medRxiv 2021. [Google Scholar] [CrossRef]
- McDowell, K.S.; Vadakkumpadan, F.; Blake, R.; Blauer, J.; Plank, G.; Macleod, R.S.; Trayanova, N.A. Methodology for patient-specific modeling of atrial fibrosis as a substrate for atrial fibrillation. J. Electrocardiol. 2012, 45, 640–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, Z.; Kirby, R.M.; Whitaker, R.T. A Fast Iterative Method for Solving the Eikonal Equation on Tetrahedral Domains. SIAM J. Sci. Comput. 2013, 35, c473–c494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loewe, A.; Krueger, M.W.; Holmqvist, F.; Dössel, O.; Seemann, G.; Platonov, P.G. Influence of the earliest right atrial activation site and its proximity to interatrial connections on P-wave morphology. Europace 2016, 18, iv35–iv43. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, M.; Ramirez, R.J.; Nattel, S. Ionic mechanisms underlying human atrial action potential properties: Insights from a mathematical model. Am. J. Physiol. 1998, 275, H301–H321. [Google Scholar] [CrossRef]
- Kahlmann, W.; Poremba, E.; Potyagaylo, D.; Dössel, O.; Loewe, A. Modelling of patient-specific Purkinje activation based on measured ECGs. Curr. Dir. Biomed. Eng. Gruyter 2017, 3, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Malmivuo, J.; Plonsey, R. Source-Field Models. In Bioelectromagnetism; Oxford University Press: Oxford, UK, 1995; pp. 148–158. [Google Scholar]
- Luongo, G.; Schuler, S.; Rivolta, M.W.; Dössel, O.; Sassi, R.; Loewe, A. Automatic ECG-based Discrimination of 20 Atrial Flutter Mechanisms: Influence of Atrial and Torso Geometries. In Proceedings of the Computing in Cardiology 2020, Rimini, Italy, 13–16 September 2020. [Google Scholar]
- Akoum, N.; Daccarett, M.; McGann, C.; Segerson, N.; Vergara, G.; Kuppahally, S.; Badger, T.; Burgon, N.; Haslam, T.; Kholmovski, E.; et al. Atrial fibrosis helps select the appropriate patient and strategy in catheter ablation of atrial fibrillation: A DE-MRI guided approach. J. Cardiovasc. Electrophysiol. 2011, 22, 16–22. [Google Scholar] [CrossRef]
- Conte, G.; Luca, A.; Yazdani, S.; Caputo, M.L.; Regoli, F.; Moccetti, T.; Kappenberger, L.; Vesin, J.M.; Auricchio, A. Usefulness of P-Wave Duration and Morphologic Variability to Identify Patients Prone to Paroxysmal Atrial Fibrillation. Am. J. Cardiol. 2017, 119, 275–279. [Google Scholar] [CrossRef]
- Neic, A.; Campos, F.O.; Prassl, A.J.; Niederer, S.A.; Bishop, M.J.; Vigmond, E.J.; Plank, G. Efficient computation of electrograms and ECGs in human whole heart simulations using a reaction-eikonal model. J. Comput. Phys. 2017, 346, 191–211. [Google Scholar] [CrossRef]
- Lenis, G.; Pilia, N.; Loewe, A.; Schulze, W.H.W.; Dössel, O. Comparison of Baseline Wander Removal Techniques considering the Preservation of ST Changes in the Ischemic ECG: A Simulation Study. Comput. Math. Methods Med. 2017, 2017, 9295029. [Google Scholar] [CrossRef]

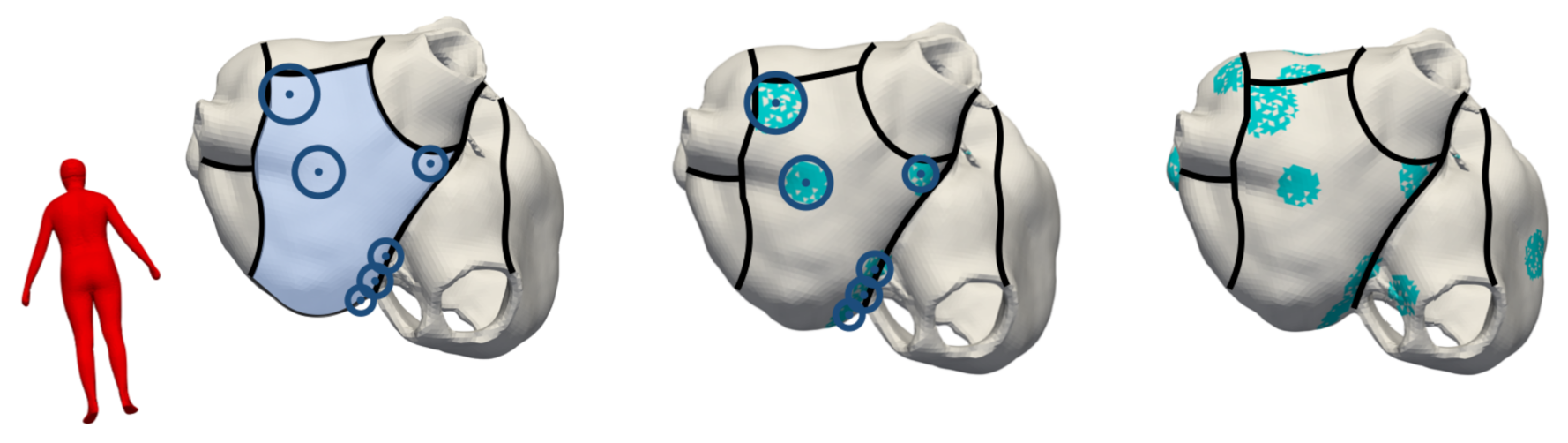


| Utah Stage | Fibrotic LA Volume Fraction | Fibrotic RA Volume Fraction |
|---|---|---|
| Utah I | 0–5% | 1.27 ± 0.38% |
| Utah II | 5–20% | 4.65 ± 0.70% |
| Utah III | 20–35% | 9.40 ± 2.16% |
| Utah IV | >35% | 12.66 ± 3.0% |
| Tissue Region | CV in m/s | Anisotropy Ratio (AR) |
|---|---|---|
| Bulk right and left atrium | 0.591 | 2.090 |
| Crista terminalis | 0.591 | 2.843 |
| Pectinate muscles | 0.461 | 3.780 |
| Inter-atrial connections | 0.645 | 3.339 |
| Inferior isthmus | 0.540 | 1 |
| Fibrosis (non-conductive) | 0 | NA |
| Fibrosis (slow conducting) | 0.2 × CV | 2.5 × AR |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagel, C.; Luongo, G.; Azzolin, L.; Schuler, S.; Dössel, O.; Loewe, A. Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability. J. Clin. Med. 2021, 10, 1797. https://doi.org/10.3390/jcm10081797
Nagel C, Luongo G, Azzolin L, Schuler S, Dössel O, Loewe A. Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability. Journal of Clinical Medicine. 2021; 10(8):1797. https://doi.org/10.3390/jcm10081797
Chicago/Turabian StyleNagel, Claudia, Giorgio Luongo, Luca Azzolin, Steffen Schuler, Olaf Dössel, and Axel Loewe. 2021. "Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability" Journal of Clinical Medicine 10, no. 8: 1797. https://doi.org/10.3390/jcm10081797
APA StyleNagel, C., Luongo, G., Azzolin, L., Schuler, S., Dössel, O., & Loewe, A. (2021). Non-Invasive and Quantitative Estimation of Left Atrial Fibrosis Based on P Waves of the 12-Lead ECG—A Large-Scale Computational Study Covering Anatomical Variability. Journal of Clinical Medicine, 10(8), 1797. https://doi.org/10.3390/jcm10081797






