Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics
Abstract
1. Introduction
2. Experimental Section
2.1. Animals
2.2. Experimental Design
2.3. Behavioral Tests
2.4. ELISA
2.5. Real-Time Polymerase Chain Reaction (PCR)
2.6. Metabolomic Analysis
2.6.1. Saliva Sample Collection
2.6.2. Saliva Metabolomics
2.7. Statistical Analyses
3. Results
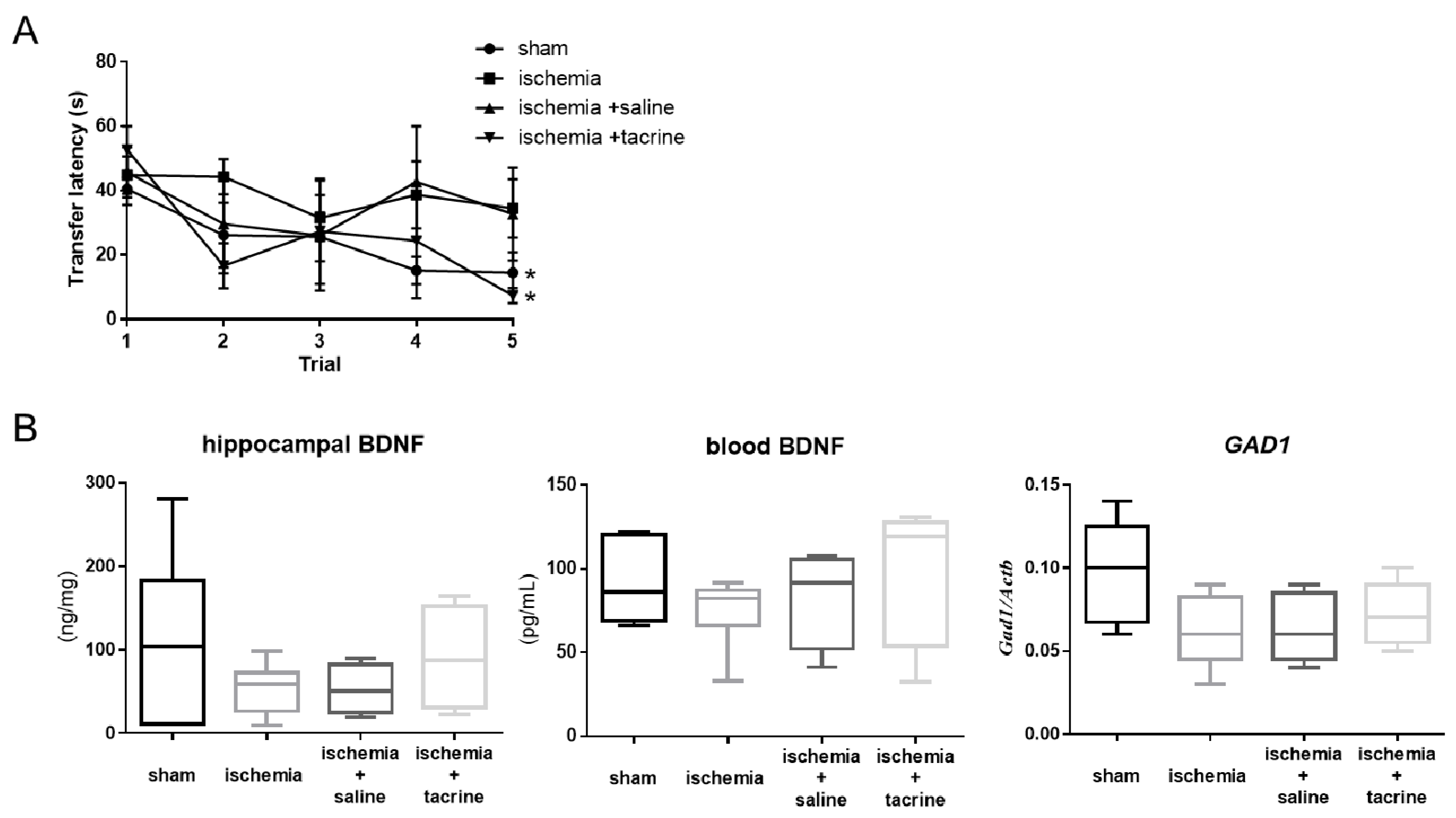
3.1. Effects of Cerebral Ischemia on Learning and Memory Performance
3.2. Cerebral Ischemia Affected BDNF-Associated Factors in the Hippocampus
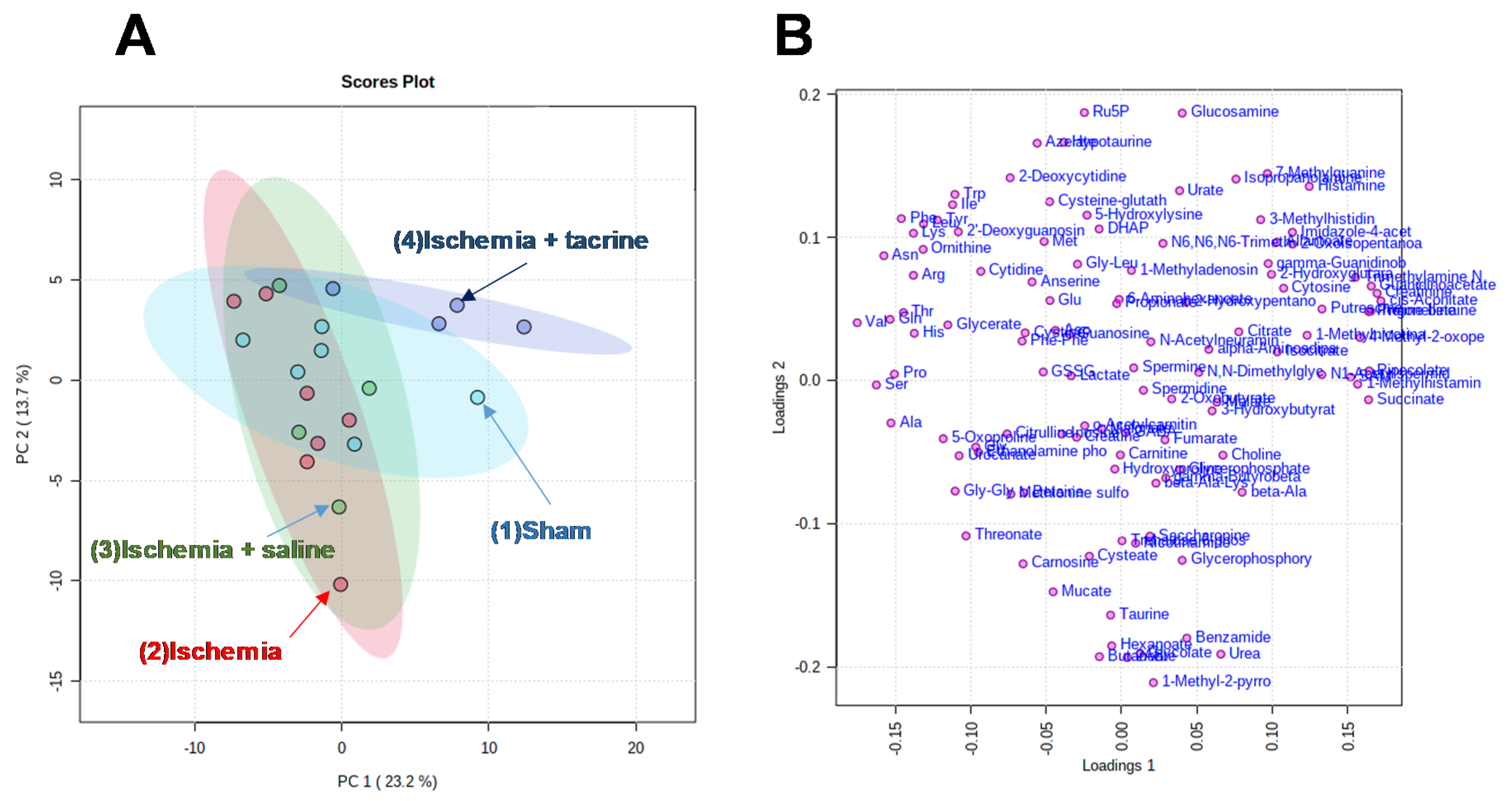
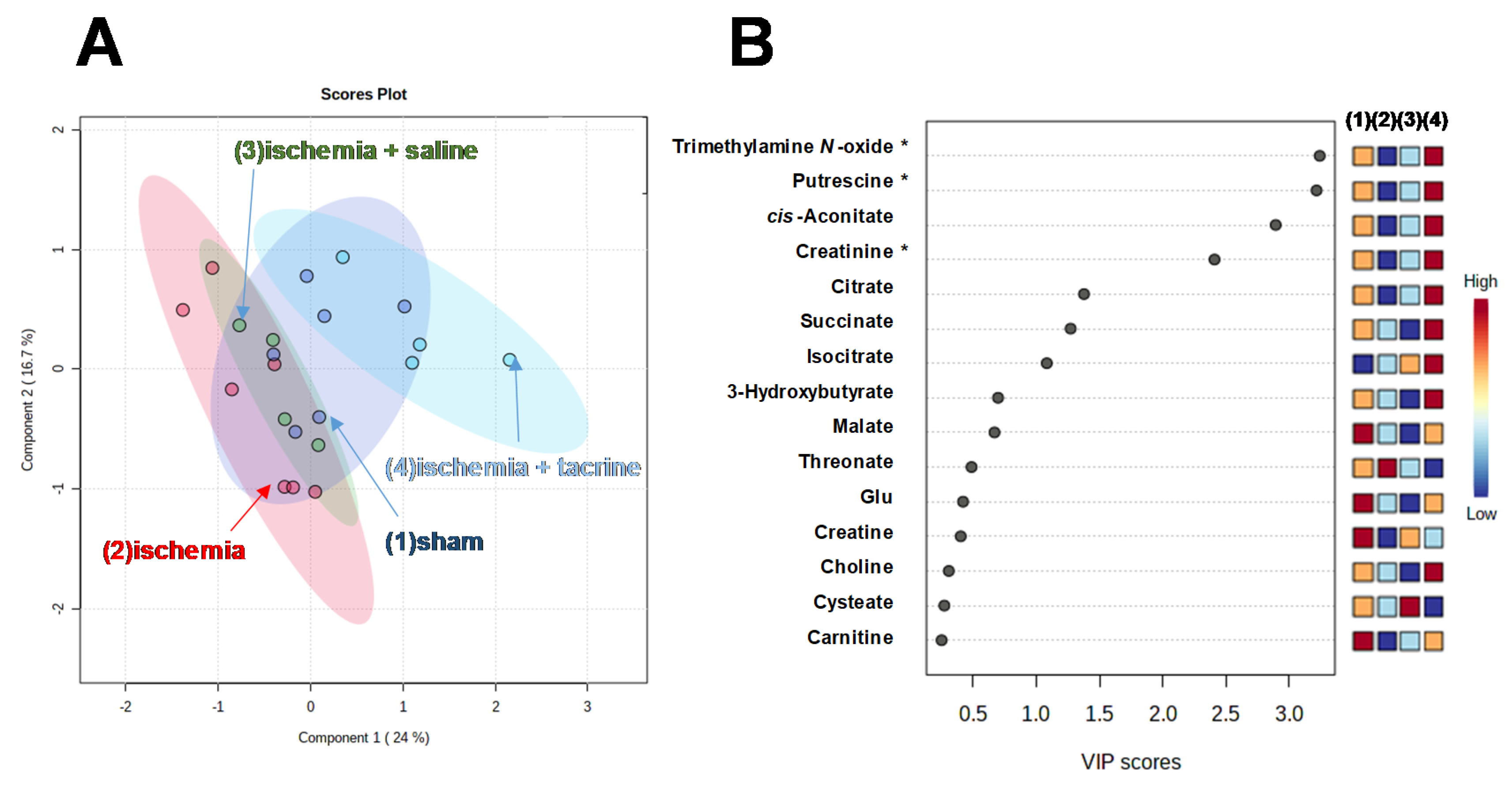
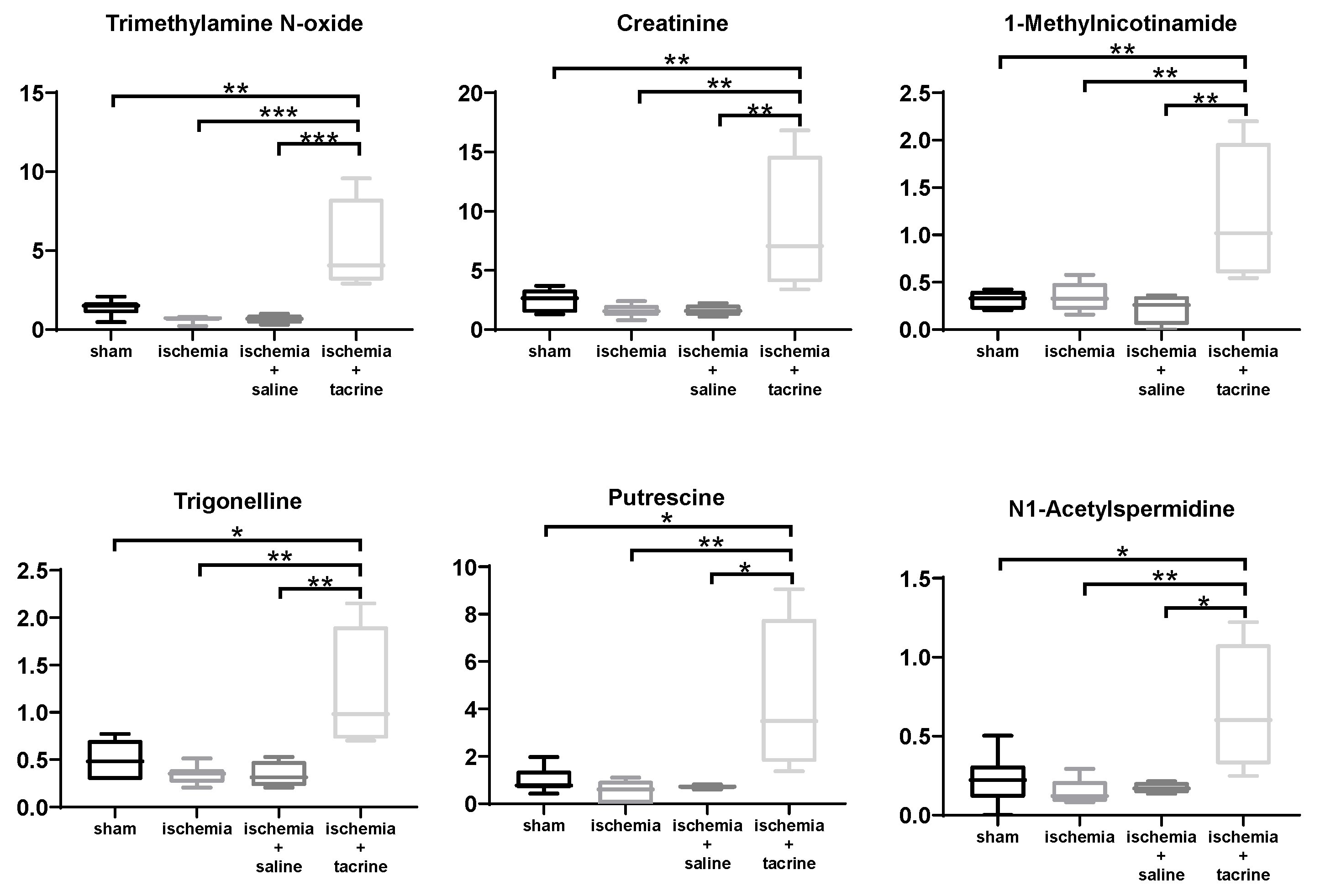
3.3. Effects of Cerebral Ischemia on Salivary Metabolite Profiles
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wortmann, M. Dementia: A global health priority-highlights from an ADI and World Health Organization report. Alzheimers Res. Ther. 2012, 4, 1–3. [Google Scholar] [CrossRef]
- Wang, Z.; Fan, J.; Wang, J.; Li, Y.; Duan, D.; Du, G.; Wang, Q. Chronic cerebral hypoperfusion induces long-lasting cognitive deficits accompanied by long-term hippocampal silent synapses increase in rats. Behav. Brain Res. 2016, 301, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Iadecola, C. The Pathobiology of Vascular Dementia. Neuron 2013, 80, 844–866. [Google Scholar] [CrossRef]
- Moorhouse, P.; Rockwood, K. Vascular cognitive impairment: Current concepts and clinical developments. Lancet Neurol. 2008, 7, 246–255. [Google Scholar] [CrossRef]
- Wallin, A.; Sjögren, M.; Edman, Å.; Blennow, K.; Regland, B. Symptoms, vascular risk factors and blood-brain barrier function in relation to CT white-matter changes in dementia. Eur. Neurol. 2000, 44, 229–235. [Google Scholar] [CrossRef]
- Jiwa, N.S.; Garrard, P.; Hainsworth, A.H. Experimental models of vascular dementia and vascular cognitive impairment: A systematic review. J. Neurochem. 2010, 115, 814–828. [Google Scholar] [CrossRef]
- Zhang, N.; Xing, M.; Wang, Y.; Tao, H.; Cheng, Y. Repetitive transcranial magnetic stimulation enhances spatial learning and synaptic plasticity via the VEGF and BDNF–NMDAR pathways in a rat model of vascular dementia. Neuroscience 2015, 311, 284–291. [Google Scholar] [CrossRef]
- Farkas, E.; Luiten, P.G.; Bari, F. Permanent, bilateral common carotid artery occlusion in the rat: A model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res. Rev. 2007, 54, 162–180. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Meyer, E.; da Silva, F.F.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments. Eur. J. Neurosci. 2021, CB2. [Google Scholar] [CrossRef]
- Nygren, J.; Kokaia, M.; Wieloch, T. Decreased expression of brain-derived neurotrophic factor in BDNF+/− mice is associated with enhanced recovery of motor performance and increased neuroblast number following experimental stroke. J. Neurosci. Res. 2006, 84, 626–631. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, W.; Wang, Z.; Guo, P.; Li, L.; Li, N. Metabolic profiling of the effects of ginsenoside Re in an Alzheimer’s disease mouse model. Behav. Brain Res. 2018, 337, 160–172. [Google Scholar] [CrossRef]
- Truiti, M.T.; Soares, L.; Longhini, R.; Milani, H.; Nakamura, C.V.; Mello, J.C.P.; De Oliveira, R.M.W. Trichilia catigua ethyl-acetate fraction protects against cognitive impairments and hippocampal cell death induced by bilateral common carotid occlusion in mice. J. Ethnopharmacol. 2015, 172, 232–237. [Google Scholar] [CrossRef]
- Zhao, Q.; Murakami, Y.; Tohda, M.; Watanabe, H.; Matsumoto, K. Preventive Effect of Chotosan, a Kampo Medicine, on Transient Ischemia-Induced Learning Deficit Is Mediated by Stimulation of Muscarinic M1 But Not Nicotinic Receptor. Biol. Pharm. Bull. 2005, 28, 1873–1878. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Afshar, S.; Shahidi, S.; Rohani, A.H.; Komaki, A.; Asl, S.S. The effect of NAD-299 and TCB-2 on learning and memory, hippocampal BDNF levels and amyloid plaques in Streptozotocin-induced memory deficits in male rats. Psychopharmacology 2018, 235, 2809–2822. [Google Scholar] [CrossRef] [PubMed]
- Monteggia, L.M.; Barrot, M.; Powell, C.M.; Berton, O.; Galanis, V.; Gemelli, T.; Meuth, S.; Nagy, A.; Greene, R.W.; Nestler, E.J. Essential role of brain-derived neurotrophic factor in adult hippocampal function. Proc. Natl. Acad. Sci. USA 2004, 101, 10827–10832. [Google Scholar] [CrossRef]
- Lim, Y.Y.; Rainey-Smith, S.; Lim, Y.; Laws, S.M.; Gupta, V.; Porter, T.; Bourgeat, P.; Ames, D.; Fowler, C.; Salvado, O.; et al. BDNF Val66Met in preclinical Alzheimer’s disease is associated with short-term changes in episodic memory and hippocampal volume but not serum mBDNF. Int. Psychogeriatr. 2017, 29, 1825–1834. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.D.; Duman, R.S. Peripheral BDNF Produces Antidepressant-Like Effects in Cellular and Behavioral Models. Neuropsychopharmacology 2010, 35, 2378–2391. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ad, I.; Portnoy, M.; Tarasenko, I.; Bidder, M.; Kramer, M.; Taler, M.; Weizman, A. A novel analog of olanzapine linked to sarcosinyl moiety (PGW5) demonstrates high efficacy and good safety profile in mouse models of schizophrenia. Eur. Neuropsychopharmacol. 2014, 24, 425–436. [Google Scholar] [CrossRef]
- Makinson, R.; Lundgren, K.H.; Seroogy, K.B.; Herman, J.P. Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiol. Behav. 2015, 146, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Subburaju, S.; Benes, F.M. Induction of the GABA Cell Phenotype: An In Vitro Model for Studying Neurodevelopmental Disorders. PLoS ONE 2012, 7, e33352. [Google Scholar] [CrossRef] [PubMed]
- Siegmund, K.D.; Connor, C.M.; Campan, M.; Long, T.I.; Weisenberger, D.J.; Biniszkiewicz, D.; Jaenisch, R.; Laird, P.W.; Akbarian, S. DNA Methylation in the Human Cerebral Cortex Is Dynamically Regulated throughout the Life Span and Involves Differentiated Neurons. PLoS ONE 2007, 2, e895. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, Y.; Dong, B.; Zheng, H.; Lin, X.; Du, Y.; Li, X.; Zhao, L.; Gao, H. Metabonomic profiles delineate potential role of glutamate-glutamine cycle in db/db mice with diabetes-associated cognitive decline. Mol. Brain 2016, 9, 40. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, R.; Li, Y.; Li, M.; Zhang, Y.; Jiang, L.; Fan, J.; Wang, Q.; Yang, D. Plasma Neurofilament Light Chain as a Predictive Biomarker for Post-stroke Cognitive Impairment: A Prospective Cohort Study. Front. Aging Neurosci. 2021, 13, 631738. [Google Scholar] [CrossRef]
- Bouftas, M. A Systematic Review on the Feasibility of Salivary Biomarkers for Alzheimer’s Disease. J. Prev. Alzheimers Dis. 2020, 8, 1–8. [Google Scholar] [CrossRef]
- Ashton, N.J.; Ide, M.; Zetterberg, H.; Blennow, K. Salivary Biomarkers for Alzheimer’s Disease and Related Disorders. Neurol. Ther. 2019, 8, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Guo, J.-P.; Kennedy, K.; McGeer, E.G.; McGeer, P.L. A Method for Diagnosing Alzheimer’s Disease Based on Salivary Amyloid-β Protein 42 Levels. J. Alzheimer’s Dis. 2016, 55, 1175–1182. [Google Scholar] [CrossRef]
- Hayashi, T.; To, M.; Saruta, J.; Sato, C.; Yamamoto, Y.; Kondo, Y.; Shimizu, T.; Kamata, Y.; Tsukinoki, K. Salivary lactoferrin is transferred into the brain via the sublingual route. Biosci. Biotechnol. Biochem. 2017, 81, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Takai, N.; Eto, K.; Uchihashi, K.; Yamaguchi, M.; Nishikawa, Y. Correlation of haloperidol levels between submandibular saliva and brain in the rat. Arch. Oral Biol. 2006, 51, 567–572. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Saruta, J.; Matsuki, C.; To, M.; Onuma, H.; Kaneko, M.; Soga, T.; Tomita, M.; Tsukinoki, K. Physiological and environmental parameters associated with mass spectrometry-based salivary metabolomic profiles. Metabolomics 2012, 9, 454–463. [Google Scholar] [CrossRef]
- Sugimoto, M. Salivary metabolomics for cancer detection. Expert Rev. Proteom. 2020, 1–10. [Google Scholar] [CrossRef]
- François, M.; Bull, C.F.; Fenech, M.F.; Leifert, W.R. Current State of Saliva Biomarkers for Aging and Alzheimer’s Disease. Curr. Alzheimer Res. 2018, 16, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Wong, D.T.; Hirayama, A.; Soga, T.; Tomita, M. Capillary electrophoresis mass spectrometry-based saliva metabolomics identified oral, breast and pancreatic cancer-specific profiles. Metabolomics 2009, 6, 78–95. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Lee, M.; Kennedy, K.; McGeer, E.G.; Geer, M. Saliva Diagnosis as a Disease Predictor. J. Clin. Med. 2020, 9, 377. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Shi, J.; Lee, M.; Arnold, L.; Al-Hasan, Y.; Heim, J.; McGeer, P. Salivary beta amyloid protein levels are detectable and differentiate patients with Alzheimer’s disease dementia from normal controls: Preliminary findings. BMC Neurol. 2018, 18, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Sui, Y.-T.; Peskind, E.R.; Li, G.; Hwang, H.; Devic, I.; Ginghina, C.; Edgar, J.S.; Pan, C.; Goodlett, D.R.; et al. Salivary Tau Species are Potential Biomarkers of Alzheimer’s Disease. J. Alzheimers Dis. 2011, 27, 299–305. [Google Scholar] [CrossRef]
- Saruta, J.; To, M.; Sugimoto, M.; Yamamoto, Y.; Shimizu, T.; Nakagawa, Y.; Inoue, H.; Saito, I.; Tsukinoki, K. Salivary Gland Derived BDNF Overexpression in Mice Exerts an Anxiolytic Effect. Int. J. Mol. Sci. 2017, 18, 1902. [Google Scholar] [CrossRef]
- Ralbovsky, N.M.; Halámková, L.; Wall, K.; Anderson-Hanley, C.; Lednev, I.K. Screening for Alzheimer’s Disease Using Saliva: A New Approach Based on Machine Learning and Raman Hyperspectroscopy. J. Alzheimers Dis. 2019, 71, 1351–1359. [Google Scholar] [CrossRef]
- Itoh, J.; Nabeshima, T.; Kameyama, T. Utility of an elevated plus-maze for dissociation of amnesic and behavioral effects of drugs in mice. Eur. J. Pharmacol. 1991, 194, 71–76. [Google Scholar] [CrossRef]
- Saruta, J.; Lee, T.; Shirasu, M.; Takahashi, T.; Sato, C.; Sato, S.; Tsukinoki, K. Chronic stress affects the expression of brain-derived neurotrophic factor in rat salivary glands. Stress 2010, 13, 53–60. [Google Scholar] [CrossRef]
- Sugimoto, M.; Sakagami, H.; Yokote, Y.; Onuma, H.; Kaneko, M.; Mori, M.; Sakaguchi, Y.; Soga, T.; Tomita, M. Non-targeted metabolite profiling in activated macrophage secretion. Metabolomics 2011, 8, 624–633. [Google Scholar] [CrossRef]
- Sugimoto, M.; Ota, S.; Kaneko, M.; Enomoto, A.; Soga, T. Quantification of Salivary Charged Metabolites using Capillary Electrophoresis Time-of-flight-mass Spectrometry. Bio-Protocol 2020, 10, e3797. [Google Scholar] [CrossRef]
- Ishikawa, S.; Sugimoto, M.; Kitabatake, K.; Sugano, A.; Nakamura, M.; Kaneko, M.; Ota, S.; Hiwatari, K.; Enomoto, A.; Soga, T.; et al. Identification of salivary metabolomic biomarkers for oral cancer screening. Sci. Rep. 2016, 6, 31520. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, M.; Kawakami, M.; Robert, M.; Soga, T.; Tomita, M. Bioinformatics Tools for Mass Spectroscopy-Based Metabolomic Data Processing and Analysis. Curr. Bioinform. 2012, 7, 96–108. [Google Scholar] [CrossRef]
- Watanabe, H.; Zhao, Q.; Matsumoto, K.; Tohda, M.; Murakami, Y.; Zhang, S.-H.; Kang, T.-H.; Mahakunakorn, P.; Maruyama, Y.; Sakakibara, I.; et al. Pharmacological evidence for antidementia effect of Choto-san (Gouteng-san), a traditional Kampo medicine. Pharmacol. Biochem. Behav. 2003, 75, 635–643. [Google Scholar] [CrossRef]
- Itoh, J.; Nabeshima, T.; Kameyama, T. Utility of an elevated plus-maze for the evaluation of memory in mice: Effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology 1990, 101, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Godinho, J.; De Oliveira, R.M.W.; De Sa-Nakanishi, A.B.; Bacarin, C.C.; Huzita, C.H.; Longhini, R.; Mello, J.C.P.; Nakamura, C.V.; Previdelli, I.S.; Ribeiro, M.H.D.M.; et al. Ethyl-acetate fraction of Trichilia catigua restores long-term retrograde memory and reduces oxidative stress and inflammation after global cerebral ischemia in rats. Behav. Brain Res. 2018, 337, 173–182. [Google Scholar] [CrossRef]
- Takagi, M.; Ishigaki, Y.; Uno, K.; Sawada, S.; Imai, J.; Kaneko, K.; Hasegawa, Y.; Yamada, T.; Tokita, A.; Iseki, K.; et al. Cognitive dysfunction associated with anti-glutamic acid decarboxylase autoimmunity: A case-control study. BMC Neurol. 2013, 13, 76. [Google Scholar] [CrossRef]
- Passaro, A.; Nora, E.D.; Morieri, M.L.; Soavi, C.; Sanz, J.M.; Zurlo, A.; Fellin, R.; Zuliani, G. Brain-Derived Neurotrophic Factor Plasma Levels: Relationship with Dementia and Diabetes in the Elderly Population. J. Gerontol. Ser. A Boil. Sci. Med. Sci. 2014, 70, 294–302. [Google Scholar] [CrossRef]
- Kumari, E.; Li, K.; Yang, Z.; Zhang, T. Tacrine accelerates spatial long-term memory via improving impaired neural oscillations and modulating GAD isomers including neuro-receptors in the hippocampus of APP/PS1 AD mice. Brain Res. Bull. 2020, 161, 166–176. [Google Scholar] [CrossRef]
- Zhao, Q.; Murakami, Y.; Tohda, M.; Obi, R.; Shimada, Y.; Matsumoto, K. Chotosan, a Kampo Formula, Ameliorates Chronic Cerebral Hypoperfusion-Induced Deficits in Object Recognition Behaviors and Central Cholinergic Systems in Mice. J. Pharmacol. Sci. 2007, 103, 360–373. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Zhao, Q.; Harada, K.; Tohda, M.; Watanabe, H.; Matsumoto, K. Choto-san, a Kampo formula, improves chronic cerebral hypoperfusion-induced spatial learning deficit via stimulation of muscarinic M receptor. Pharmacol. Biochem. Behav. 2005, 81, 616–625. [Google Scholar] [CrossRef]
- Schwarz, C.; Horn, N.; Benson, G.; Calzado, I.W.; Wurdack, K.; Pechlaner, R.; Grittner, U.; Wirth, M.; Flöel, A. Spermidine intake is associated with cortical thickness and hippocampal volume in older adults. NeuroImage 2020, 221, 117132. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhang, H.; Zhang, J.; Yu, H.; Lin, Z.; Cai, Y. Spermidine Exhibits Protective Effects Against Traumatic Brain Injury. Cell. Mol. Neurobiol. 2020, 40, 927–937. [Google Scholar] [CrossRef]
- Weng, W.-C.; Huang, W.-Y.; Tang, H.-Y.; Cheng, M.-L.; Chen, K.-H. The Differences of Serum Metabolites Between Patients with Early-Stage Alzheimer’s Disease and Mild Cognitive Impairment. Front. Neurol. 2019, 10, 1223. [Google Scholar] [CrossRef] [PubMed]
- González-Domínguez, R.; García, A.; García-Barrera, T.; Barbas, C.; Gómez-Ariza, J.L. Metabolomic profiling of serum in the progression of Alzheimer’s disease by capillary electrophoresis-mass spectrometry. Electrophoresis 2014, 35, 3321–3330. [Google Scholar] [CrossRef]
- Tsuruoka, M.; Hara, J.; Hirayama, A.; Sugimoto, M.; Soga, T.; Shankle, W.R.; Tomita, M. Capillary electrophoresis-mass spectrometry-based metabolome analysis of serum and saliva from neurodegenerative dementia patients. Electrophoresis 2013, 34, 2865–2872. [Google Scholar] [CrossRef]
- Mu, R.-H.; Tan, Y.-Z.; Fu, L.-L.; Islam, M.N.; Hu, M.; Hong, H.; Tang, S.-S. 1-Methylnicotinamide attenuates lipopolysaccharide-induced cognitive deficits via targeting neuroinflammation and neuronal apoptosis. Int. Immunopharmacol. 2019, 77, 105918. [Google Scholar] [CrossRef]
- Schulman, A.N.; Dienstag, J.L.; Jackson, D.R.; Hoofnagle, J.H.; Gerety, R.J.; Purcell, R.H.; Barker, L.F. Hepatitis a Antigen Particles in Liver, Bile, and Stool of Chimpanzees. J. Infect. Dis. 1976, 134, 80–84. [Google Scholar] [CrossRef]
- Ray, M.T. Decreased BDNF, trkB-TK+ and GAD67 mRNA expression in the hippocampus of individuals with schizophrenia and mood disorders. J. Psychiatry Neurosci. 2011, 36, 195–203. [Google Scholar] [CrossRef]
- Nakagawasai, O.; Lin, J.-R.; Odaira, T.; Takahashi, K.; Nemoto, W.; Moriguchi, S.; Yabuki, Y.; Kobayakawa, Y.; Fukunaga, K.; Nakada, M.; et al. Scabronine G Methyl Ester Improves Memory-Related Behavior and Enhances Hippocampal Cell Proliferation and Long-Term Potentiation via the BDNF-CREB Pathway in Olfactory Bulbectomized Mice. Front. Pharmacol. 2020, 11, 583291. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
To, M.; Sugimoto, M.; Saruta, J.; Yamamoto, Y.; Sakaguchi, W.; Kawata, A.; Matsuo, M.; Tsukinoki, K. Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics. J. Clin. Med. 2021, 10, 1698. https://doi.org/10.3390/jcm10081698
To M, Sugimoto M, Saruta J, Yamamoto Y, Sakaguchi W, Kawata A, Matsuo M, Tsukinoki K. Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics. Journal of Clinical Medicine. 2021; 10(8):1698. https://doi.org/10.3390/jcm10081698
Chicago/Turabian StyleTo, Masahiro, Masahiro Sugimoto, Juri Saruta, Yuko Yamamoto, Wakako Sakaguchi, Akira Kawata, Masato Matsuo, and Keiichi Tsukinoki. 2021. "Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics" Journal of Clinical Medicine 10, no. 8: 1698. https://doi.org/10.3390/jcm10081698
APA StyleTo, M., Sugimoto, M., Saruta, J., Yamamoto, Y., Sakaguchi, W., Kawata, A., Matsuo, M., & Tsukinoki, K. (2021). Cognitive Dysfunction in a Mouse Model of Cerebral Ischemia Influences Salivary Metabolomics. Journal of Clinical Medicine, 10(8), 1698. https://doi.org/10.3390/jcm10081698






