Calcium Channel Blockers Are Associated with Nocturia in Men Aged 40 Years or Older
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. IPSS and Nocturia
2.3. Medications
2.4. Measurement of BP and Variation between Daytime and Morning BP
2.5. Covariates
2.6. Endpoints
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
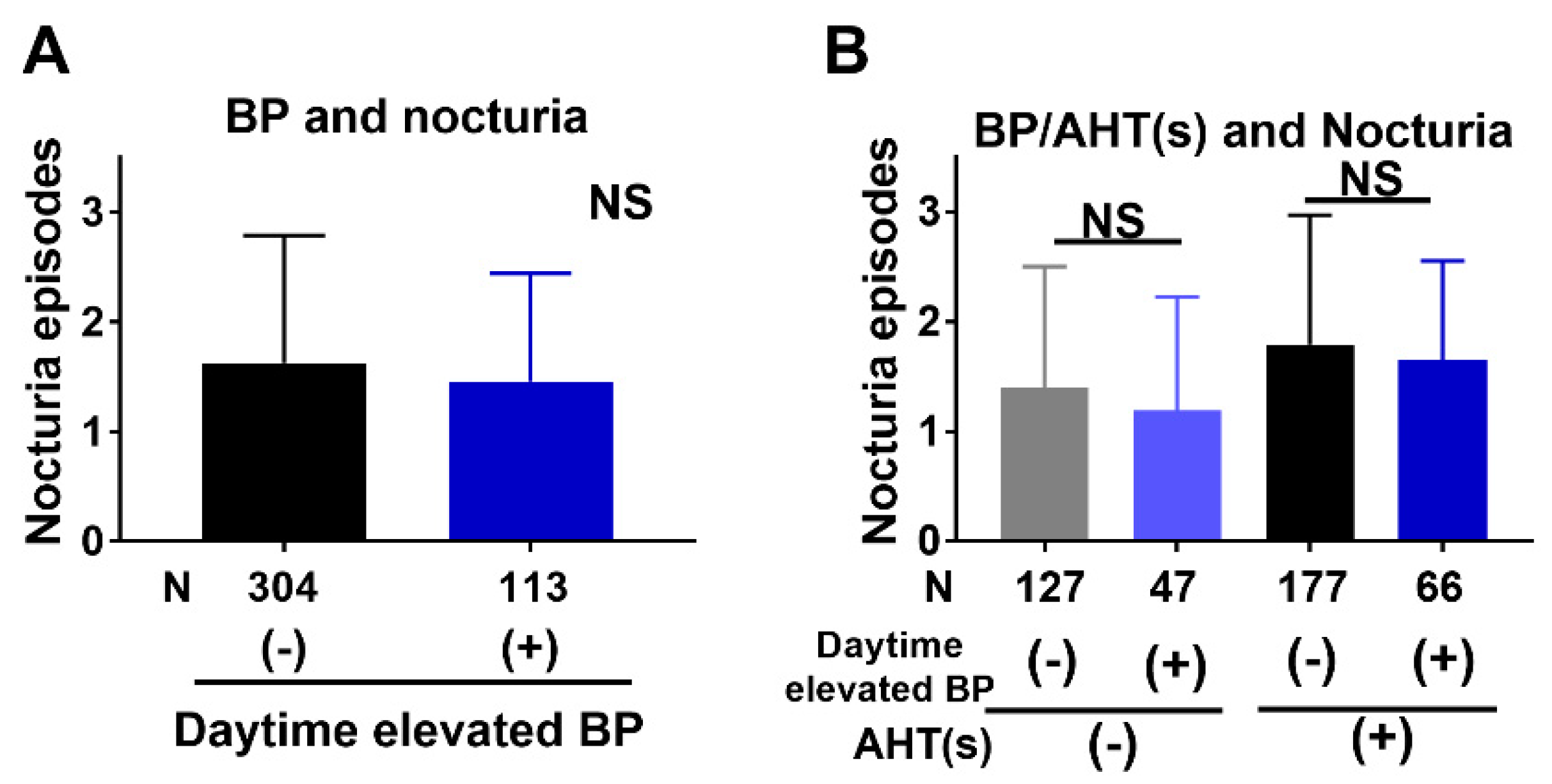
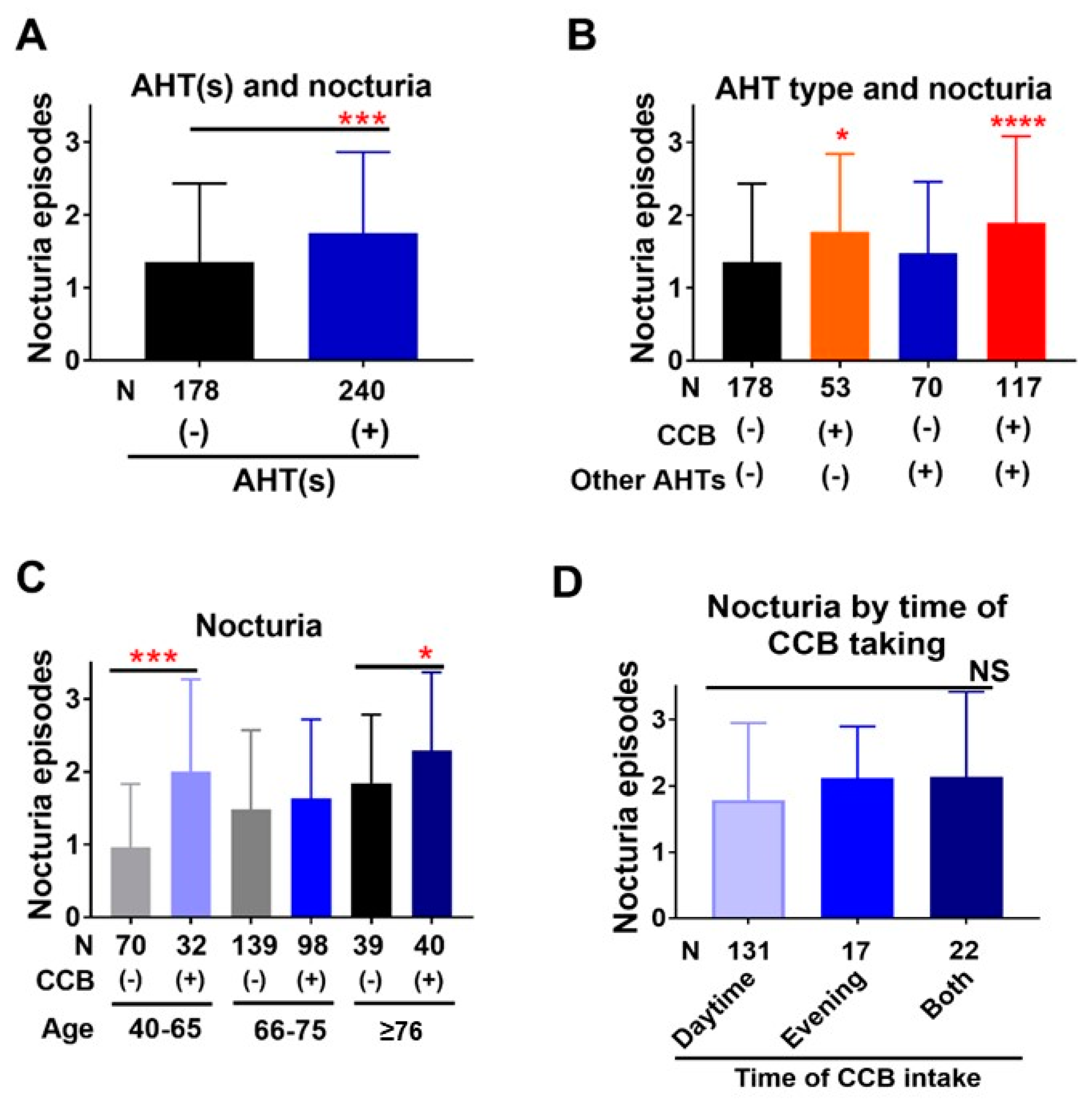
3.2. Association of Nocturia with BP and AHTs
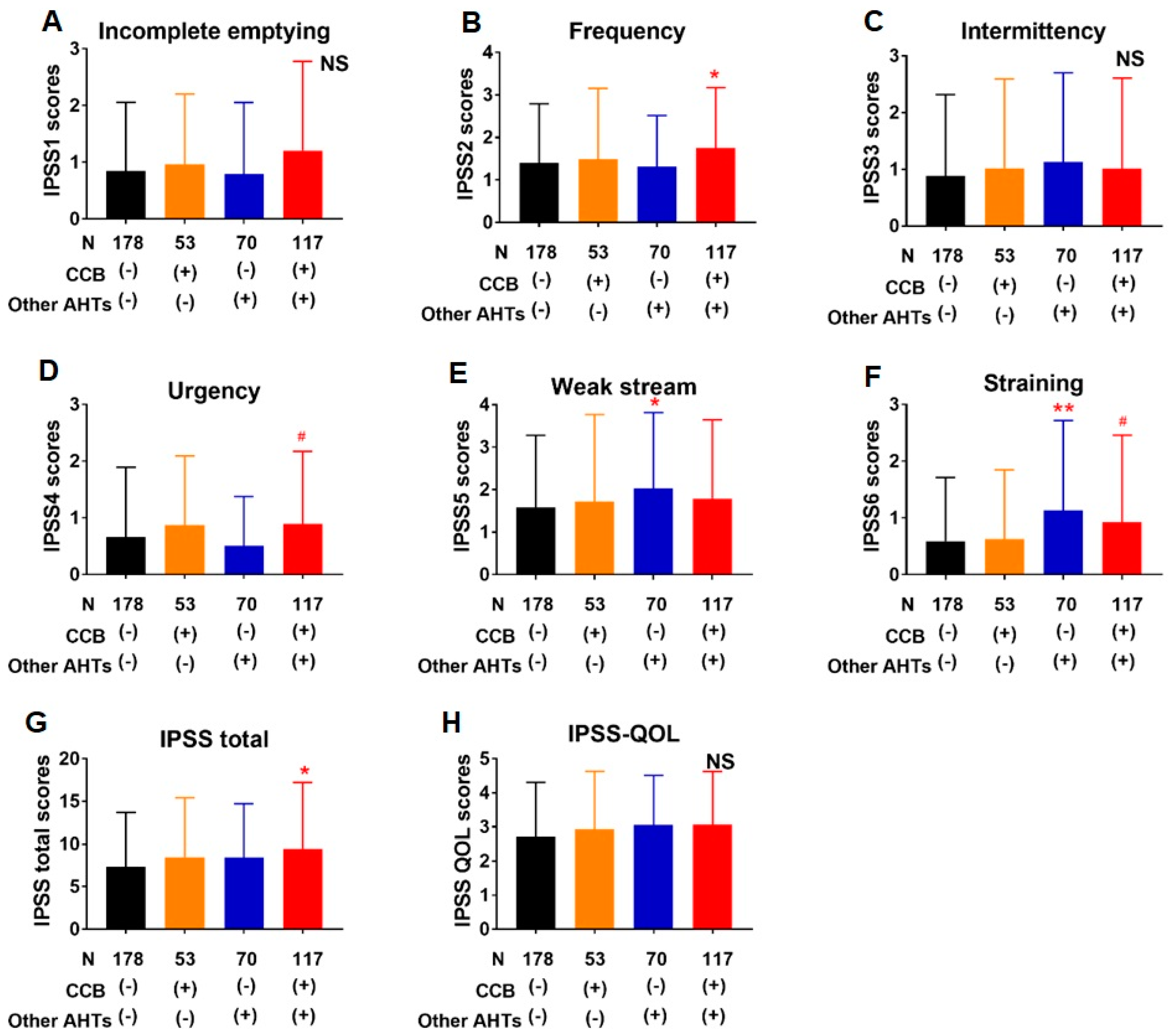
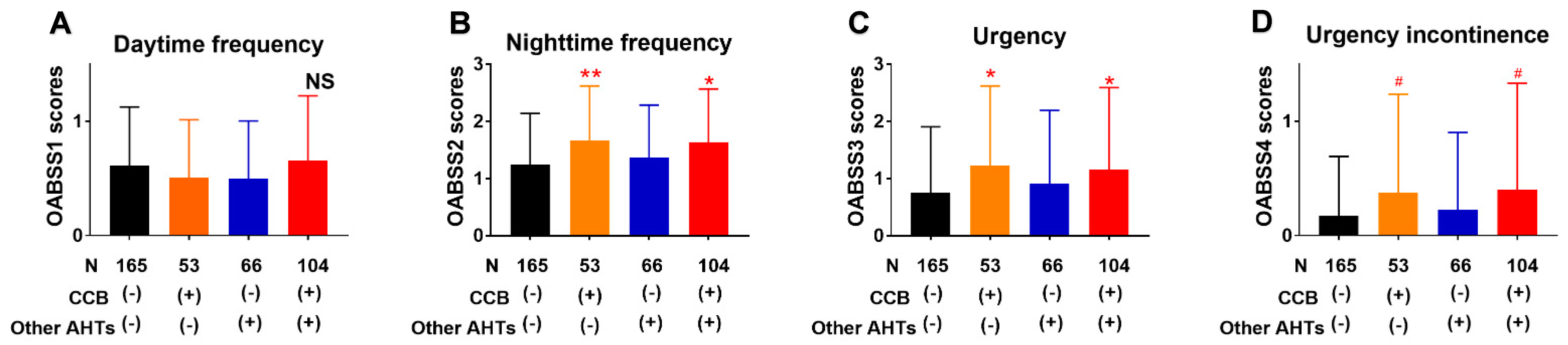
3.3. Association of Lower Urinary Tract Symptoms with AHTs
3.4. Correlations between Nocturia and Other Covariates
3.5. Factors Associated with Clinically Important Nocturia in Multivariate Analysis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Everaert, K.; Hervé, F.; Bosch, R.; Dmochowski, R.; Drake, M.; Hashim, H.; Chapple, C.; Van Kerrebroeck, P.; Mourad, S.; Abrams, P.; et al. International Continence Society consensus on the diagnosis and treatment of nocturia. Neurourol. Urodyn. 2019, 38, 478–498. [Google Scholar] [CrossRef]
- Hashim, K.; Blanker, M.H.; Drake, H.; Djurhuus, J.C.; Meijlink, J.; Morris, V.; Petros, P.; Wen, J.G.; Wein, A. ICS Terminology Working Group on Nocturia and Nocturnal Lower Urinary Tract Function. International Continence Society (ICS) report on the terminology for nocturia and nocturnal lower urinary tract function. Neurourol. Urodyn. 2019, 38, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Kurtzman, J.T.; Bergman, A.M.; Weiss, J.P. Nocturia in women. Curr. Opin. Urol. 2016, 26, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Schatzl, G.; Temml, C.; Schmidbauer, J.; Dolezal, B.; Haidinger, G.; Madersbacher, S. Cross-sectional study of nocturia in both sexes: Analysis of a voluntary health screening project. Urology 2000, 56, 71–75. [Google Scholar] [CrossRef]
- Holm-Larsen, T. The economic impact of nocturia. Neurourol. Urodyn. 2014, 33 (Suppl. 1), S10–S14. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Bang, W.; Kim, M.-S.; Park, B.; Kim, J.-H.; Choi, H.G. Nocturia Is Associated with Slipping and Falling. PLoS ONE 2017, 12, e0169690. [Google Scholar]
- Oelke, M.; Wiese, B.; Berges, R. Nocturia and its impact on health-related quality of life and health care seeking behaviour in German community-dwelling men aged 50 years or older. World J. Urol. 2014, 32, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Obayashi, K.; Saeki, K.; Negoro, H.; Kurumatani, N. Nocturia increases the incidence of depressive symptoms: A longitudinal study of the HEIJO-KYO cohort. BJU Int. 2017, 120, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Oelke, M.; Anderson, P.; Wood, R.; Holm-Larsen, T. Nocturia is often inadequately assessed, diagnosed and treated by physicians: Results of an observational, real-life practice database containing 8659 European and US-American patients. Int. J. Clin. Pract. 2016, 70, 940–949. [Google Scholar] [CrossRef]
- Gordon, D.J.; Emeruwa, J.E.; Weiss, J.P. Management Strategies for Nocturia. Curr. Urol. Rep. 2019, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Van Kerrebroeck, P. Nocturia: Current status and future perspectives. Curr. Opin. Obstet. Gynecol. 2011, 23, 376–385. [Google Scholar] [CrossRef]
- Yoshimura, K.; Oka, Y.; Kamoto, T.; Yoshimura, K.; Ogawa, O. Differences and associations between nocturnal voiding/nocturia and sleep disorders. BJU Int. 2009, 106, 232–237. [Google Scholar] [CrossRef]
- Sugaya, K.; Kadekawa, K.; Ikehara, A.; Nakayama, T.; Gakiya, M.; Nashiro, F.; Goya, M.; Hatano, T.; Ogawa, Y. Influence of hypertension on lower urinary tract symptoms in benign prostatic hyperplasia. Int. J. Urol. 2003, 10, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Terada, N.; Matsui, Y.; Terai, A.; Kinukawa, N.; Arai, Y. Prevalence of and risk factors for nocturia: Analysis of a health screening program. Int. J. Urol. 2004, 11, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, O.; Nishizawa, O.; Homma, Y.; Takeda, M.; Gotoh, M.; Kakizaki, H.; Akino, H.; Hayashi, K.; Yonemoto, K.; OASIS Project Group. Nocturnal Polyuria and Hypertension in Patients with Lifestyle Related Diseases and Overactive Bladder. J. Urol. 2017, 197, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Victor, R.G.; Li, N.; Blyler, C.A.; Mason, O.R.; Chang, L.C.; Moy, N.P.B.; Rashid, M.A.; Weiss, J.P.; Handler, J.; Brettler, J.W.; et al. Nocturia as an Unrecognized Symptom of Uncontrolled Hypertension in Black Men Aged 35 to 49 Years. J. Am. Heart Assoc. 2019, 8, e010794. [Google Scholar] [CrossRef]
- Hughes, J.D.; Coles, M.A.; Joyce, A. Calcium channel blocker associated lower urinary tract symptoms in males: An Australian retrospective observational study. Qual. Prim. Care 2011, 19, 223–231. [Google Scholar] [PubMed]
- Elhebir, E.S.; Hughes, J.D.; Hilmi, S.C. Calcium Antagonists Use and Its Association with Lower Urinary Tract Symptoms: A Cross-Sectional Study. PLoS ONE 2013, 8, e66708. [Google Scholar]
- Hall, S.A.; Chiu, G.R.; Kaufman, D.W.; Wittert, G.A.; Link, C.L.; McKinlay, J.B. Commonly used antihypertensives and lower urinary tract symptoms: Results from the Boston Area Community Health (BACH) Survey. BJU Int. 2012, 109, 1676–1684. [Google Scholar] [CrossRef]
- Lombardo, R.; Tubaro, A.; Burkhard, F. Nocturia: The Complex Role of the Heart, Kidneys, and Bladder. Eur. Urol. Focus 2020, 6, 534–536. [Google Scholar] [CrossRef]
- Salman, M.; Khan, A.H.; Sulaiman, S.A.S.; Khan, J.H.; Hussain, K.; Shehzadi, N. Effect of Calcium Channel Blockers on Lower Urinary Tract Symptoms: A Systematic Review. BioMed Res. Int. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Santiapillai, J.; Tadtayev, S.; Miles, A.; Arumainayagam, N.; Yeong, K.; Murray, P. Dihydropyridine calcium channel blockers and obstructive sleep apnea: Two underrecognized causes of nocturia? Neurourol. Urodyn. 2020, 39, 1612–1614. [Google Scholar] [CrossRef] [PubMed]
- Hollenberg, N.K.; Williams, G.H.; Anderson, R.; Akhras, K.S.; Bittman, R.M.; Krause, S.L. Symptoms and the distress they cause: Comparison of an aldosterone antagonist and a calcium channel blocking agent in patients with systolic hypertension. Arch. Int. Med. 2003, 163, 1543–1548. [Google Scholar] [CrossRef]
- Bulpitt, C.J.; Connor, M.; Schulte, M.; Fletcher, A.E. Bisoprolol and nifedipine retard in elderly hypertensive patients: Effect on quality of life. J. Hum. Hypertens. 2000, 14, 205–212. [Google Scholar] [CrossRef]
- Weiss, J.P.; Monaghan, T.F.; Epstein, M.R.; Lazar, J.M. Future Considerations in Nocturia and Nocturnal Polyuria. Urology 2019, 133S, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Ito, H.; Taga, M.; Tsuchiyama, K.; Akino, H.; Yokoyama, O. IPSS is lower in hypertensive patients treatedwith angiotensin-II receptor blocker: Posthoc analyses of a lower urinary tract symptoms population. Neurourol. Urodyn. 2013, 32, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Harikrishna, M.; Sripal, B.; Romero, J.; Htyte, N.; Berrios, R.S.; Makwana, H.; Messerli, F.H. Peripheral edema associated with calcium channel blockers: Incidence and withdrawal rate—A meta-analysis of randomized trials. J. Hypertens. 2011, 29, 1270–1280. [Google Scholar]
- Sugaya, K.; Nishijima, S.; Oda, M.; Owan, T.; Miyazato, M.; Ogawa, Y. Biochemical and body composition analysis of nocturia in the elderly. Neurourol. Urodyn. 2008, 27, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Blanker, M.H.; Bohnen, A.M.; Groneveld, F.P.; Bernsen, R.M.; Bosch, J.L.R. Normal voiding patterns and determinants of increased diurnal and nocturnal voiding frequency in elderly men. J. Urol. 2000, 164, 1201–1205. [Google Scholar] [CrossRef]
| n | % | |
|---|---|---|
| Age, mean (SD) | 69.4 | (7.60) |
| 40–65 | 102 | (24) |
| 66–75 | 237 | (57) |
| 76 or more | 79 | (19) |
| Diseases for hospitalization | ||
| Prostate cancer | 250 | (60) |
| Prostate cancer suspected | 86 | (21) |
| Renal cell carcinoma | 30 | (7) |
| Bladder tumor | 20 | (5) |
| Renal pelvic/ureteral Ca | 17 | (4) |
| Others | 12 | (3) |
| Taking AHTs | 240 | (57) |
| Daytime elevated BP | 113 | (27) |
| DM | 100 | (24) |
| Taking hypoglycemic agent | 61 | (15) |
| BMI, kg/m2, mean (SD) | 24.3 | (3.22) |
| eGFR, mL/min/1.73 m2, mean (SD) | 71.5 | (16.5) |
| Glycated hemoglobin, %, mean (SD) | 5.97 | (0.695) |
| IPSS | 40–65 Years (n = 102) | 66–75 Years (n = 237) | ≥76 Years (n = 79) | Total (n = 418) |
|---|---|---|---|---|
| IPSS total, mean ± SD | 6.87 ± 5.96 | 8.69 ± 7.38 | 8.62 ± 6.27 | 8.23 ± 6.88 |
| 0–7 | 64% | 57% | 53% | 58% |
| 8–19 | 32% | 34% | 39% | 35% |
| 20–35 | 4% | 10% | 8% | 8% |
| IPSS item 7 (Nocturia), mean ± SD | 1.28 ± 1.12 | 1.54 ± 1.09 | 2.08 ± 1.04 | 1.58 ± 1.11 |
| IPSS-QOL, mean ± SD | 2.69 ± 1.57 | 2.83 ± 1.57 | 3.36 ± 1.61 | 2.89 ± 1.59 |
| Variables | Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | (95% CI) | p Value | ||
| Age | years | 1.07 | (1.04–1.10) | <0.0001 | 1.06 | (1.03–1.10) | <0.0001 |
| BMI | kg/m2 | 1.04 | (0.90–1.19) | 0.632 | (-) | ||
| Daytime elevated BP | mmHg | 0.97 | (0.63–1.50) | 0.889 | (-) | ||
| Morning elevated BP | mmHg | 0.93 | (0.61–1.43) | 0.932 | (-) | ||
| Glycated Hb | % | 1.02 | (0.80–1.29) | 0.885 | (-) | ||
| eGFR | mL/min/ 1.73 m2 | 0.99 | (0.98–1.00) | 0.048 | 1.00 | (0.98–1.01) | 0.741 |
| Sum of IPSS item 1–6 | 1.05 | (1.01–1.07) | 0.006 | 1.05 | (1.02–1.09) | <0.0001 | |
| AHTs | CCB | 2.80 | (1.88–4.18) | <0.0001 | 2.68 | (1.69–4.26) | <0.0001 |
| ARB | 1.48 | (0.99–2.22) | 0.059 | 0.89 | (0.54–1.46) | 0.638 | |
| ACE-I | 0.72 | (0.34–1.51) | 0.385 | 1.05 | (0.46–2.37) | 0.916 | |
| β-blocker | 2.17 | (1.00–4.68) | 0.048 | 2.15 | (0.94–4.90) | 0.069 | |
| α-blocker | 1.86 | (0.87–3.98) | 0.107 | 1.59 | (0.68–3.72) | 0.282 | |
| Thiazide | 1.49 | (0.60–3.67) | 0.388 | 0.84 | (0.31–2.26) | 0.725 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Washino, S.; Ugata, Y.; Saito, K.; Miyagawa, T. Calcium Channel Blockers Are Associated with Nocturia in Men Aged 40 Years or Older. J. Clin. Med. 2021, 10, 1603. https://doi.org/10.3390/jcm10081603
Washino S, Ugata Y, Saito K, Miyagawa T. Calcium Channel Blockers Are Associated with Nocturia in Men Aged 40 Years or Older. Journal of Clinical Medicine. 2021; 10(8):1603. https://doi.org/10.3390/jcm10081603
Chicago/Turabian StyleWashino, Satoshi, Yusuke Ugata, Kimitoshi Saito, and Tomoaki Miyagawa. 2021. "Calcium Channel Blockers Are Associated with Nocturia in Men Aged 40 Years or Older" Journal of Clinical Medicine 10, no. 8: 1603. https://doi.org/10.3390/jcm10081603
APA StyleWashino, S., Ugata, Y., Saito, K., & Miyagawa, T. (2021). Calcium Channel Blockers Are Associated with Nocturia in Men Aged 40 Years or Older. Journal of Clinical Medicine, 10(8), 1603. https://doi.org/10.3390/jcm10081603






