Extracorporeal Membrane Oxygenation for Fulminant Myocarditis: Increase of Cardiac Enzyme and SOFA Score Is Associated with High Mortality
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Definitions and Outcomes
2.3. Statistical Analyses
3. Results
3.1. Baseline Characteristics
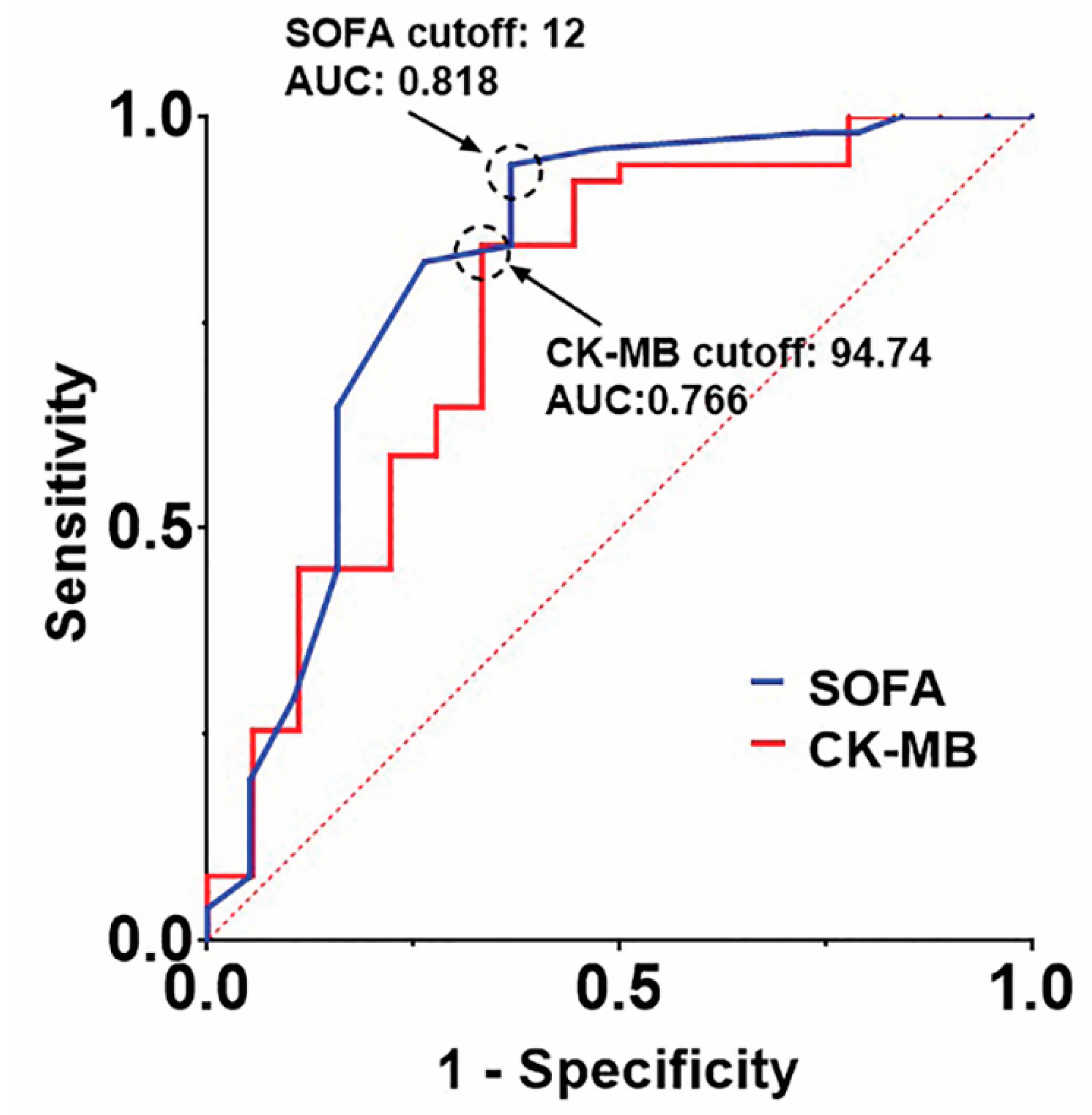
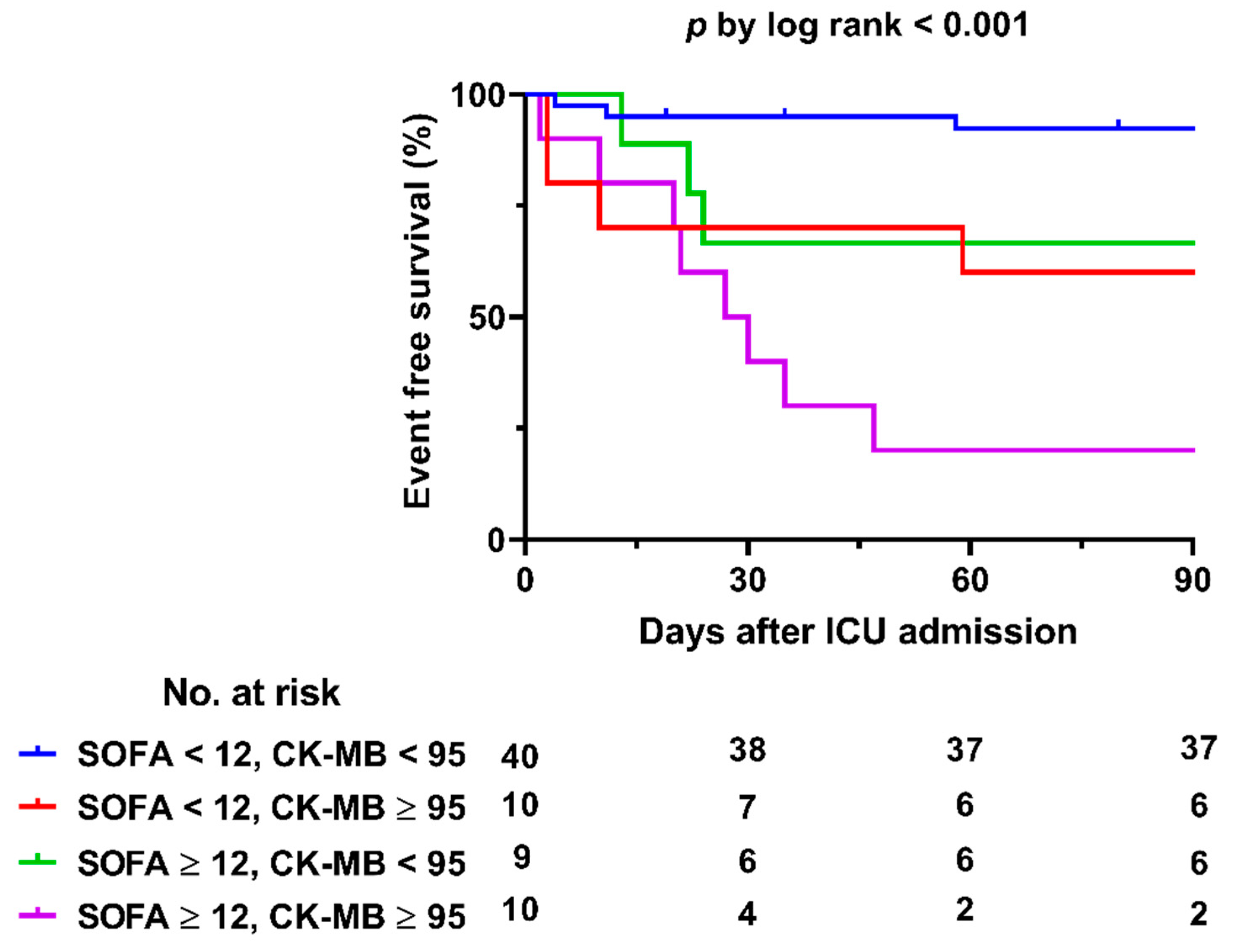
3.2. Clinical Outcomes and Predictors of In-Hospital Mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
References
- Thiagarajan, R.R.; Barbaro, R.P.; Rycus, P.T.; McMullan, D.M.; Conrad, S.A.; Fortenberry, J.D.; Paden, M.L.; ELSO member centers. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017, 63, 60–67. [Google Scholar] [CrossRef]
- Paden, M.L.; Conrad, S.A.; Rycus, P.T.; Thiagarajan, R.R.; ELSO Registry. Extracorporeal Life Support Organization Registry Report 2012. ASAIO J. 2013, 59, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Miyake, C.Y.; Teele, S.A.; Chen, L.; Motonaga, K.S.; Dubin, A.M.; Balasubramanian, S.; Balise, R.R.; Rosenthal, D.N.; Alexander, M.E.; Walsh, E.P.; et al. In-hospital arrhythmia development and outcomes in pediatric patients with acute myocarditis. Am. J. Cardiol. 2014, 113, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Sawamura, A.; Okumura, T.; Ito, M.; Ozaki, Y.; Ohte, N.; Amano, T.; Murohara, T.; CHANGE PUMP Investigators. Prognostic Value of Electrocardiography in Patients with Fulminant Myocarditis Supported by Percutaneous Venoarterial Extracorporeal Membrane Oxygenation- Analysis from the CHANGE PUMP Study. Circ. J. 2018, 82, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Tsuda, E.; Miyazaki, A.; Ishibashi-Ueda, H.; Yamada, O. Clinical characteristics and long-term outcome of acute myocarditis in children. Heart Vessels. 2013, 28, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; Chen, C.; Seubert, J.M.; Wang, D.W. Fulminant myocarditis: A comprehensive review from etiology to treatments and outcomes. Signal Transduct. Target Ther. 2020, 5, 287. [Google Scholar] [CrossRef]
- Caforio, A.; Sabine, P.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Heliö, T.; Heymans, S.; Jahns, R.; et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: A position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- McCarthy, R.E., 3rd; Boehmer, J.P.; Hruban, R.H.; Hutchins, G.M.; Kasper, E.K.; Hare, J.M.; Baughman, K.L. Long-term outcome of fulminant myocarditis as compared with acute (nonfulminant) myocarditis. N. Engl. J. Med. 2000, 342, 690–695. [Google Scholar] [CrossRef]
- Matsumoto, M.; Asaumi, Y.; Nakamura, Y.; Nakatani, T.; Nagai, T.; Kanaya, T.; Kawakami, S.; Honda, S.; Kataoka, Y.; Nakajima, S.; et al. Clinical determinants of successful weaning from extracorporeal membrane oxygenation in patients with fulminant myocarditis. ESC Heart Fail. 2018, 5, 675–684. [Google Scholar] [CrossRef]
- Ginsberg, F.; Parrillo, J.E. Fulminant myocarditis. Crit. Care Clin. 2013, 29, 465–483. [Google Scholar] [CrossRef]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; De Mendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Matics, T.J.; Sanchez-Pinto, L.N. Adaptation and Validation of a Pediatric Sequential Organ Failure Assessment Score and Evaluation of the Sepsis-3 Definitions in Critically Ill Children. JAMA Pediatr. 2017, 171, e172352. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Toda, K.; Miyagawa, S.; Yoshikawa, Y.; Hata, H.; Yoshioka, D.; Domae, K.; Tsukamoto, Y.; Sakata, Y.; Sawa, Y. Diagnosis, medical treatment, and stepwise mechanical circulatory support for fulminat myocarditis. J. Artif. Organs 2018, 21, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Diddle, J.W.; Almodovar, M.C.; Rajagopal, S.K.; Rycus, P.T.; Thiagarajan, R.R. Extracorporeal membrane oxygenation for the support of adults with acute myocarditis. Crit. Care Med. 2015, 43, 1016–1025. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.H.; Southern, D.; Wilton, S.B.; James, M.T.; Har, B.; Schnell, G.; van Diepen, S.; Grant, A.D.M. Comparative Prognostic Accuracy of Risk Prediction Models for Cardiogenic Shock. J. Intensive Care Med. 2020, 35, 1513–1519. [Google Scholar] [CrossRef]
- Ferreira, F.L.; Bota, D.P.; Bross, A.; Melot, C.; Vincent, J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001, 286, 1754–1758. [Google Scholar] [CrossRef] [PubMed]
- Raith, E.P.; Udy, A.A.; Bailey, M.; McGloughlin, S.; MacIsaac, C.; Bellomo, R.; Pilcher, D.V. Prognostic Accuracy of the SOFA Score, SIRS Criteria, and qSOFA Score for In-Hospital Mortality Among Adults with Suspected Infection Admitted to the Intensive Care Unit. JAMA 2017, 317, 290–300. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; von Birgelen, C.; Rademaker-Havinga, T.; Spitzer, E.; Kleiman, N.S.; Cohen, D.J.; Kennedy, K.F.; Camenzind, E.; Mauri, L.; et al. Impact of Periprocedural Myocardial Biomarker Elevation on Mortality Following Elective Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2019, 12, 1954–1962. [Google Scholar] [CrossRef]
- Brener, S.J.; Lytle, B.W.; Schneider, J.P.; Ellis, S.G.; Topol, E.J. Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J. Am. Coll. Cardiol. 2002, 40, 1961–1967. [Google Scholar] [CrossRef][Green Version]
- Brener, S.J.; Ellis, S.G.; Schneider, J.; Topol, E.J. Frequency and long-term impact of myonecrosis after coronary stenting. Eur. Heart J. 2002, 23, 869–876. [Google Scholar] [CrossRef]
- Hsu, K.H.; Chi, N.H.; Yu, H.Y.; Wang, C.H.; Huang, S.C.; Wang, S.S.; Ko, W.J.; Chen, Y.S. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: Analysis of a single center’s experience. Eur. J. Cardiothorac. Surg. 2011, 40, 682–688. [Google Scholar] [CrossRef]
- Ishida, K.; Wada, H.; Sakakura, K.; Kubo, N.; Ikeda, N.; Sugawara, Y.; Ako, J.; Momomura, S. Long-term follow-up on cardiac function following fulminant myocarditis requiring percutaneous extracorporeal cardiopulmonary support. Heart Vessel. 2013, 28, 86–90. [Google Scholar] [CrossRef]
- Mirabel, M.; Luyt, C.E.; Leprince, P.; Trouillet, J.L.; Leger, P.; Pavie, A.; Chastre, J.; Combes, A. Outcomes, long-term quality of life, and psychologic assessment of fulminant myocarditis patients rescued by mechanical circulatory support. Crit. Care Med. 2011, 39, 1029–1035. [Google Scholar] [CrossRef]
- Chong, S.Z.; Fang, C.Y.; Fang, H.Y.; Chen, H.C.; Chen, C.J.; Yang, C.H.; Hang, C.L.; Yip, H.K.; Wu, C.J.; Lee, W.C. Associations with the In-Hospital Survival Following Extracorporeal Membrane Oxygenation in Adult Acute Fulminant Myocarditis. J. Clin. Med. 2018, 7, 452. [Google Scholar] [CrossRef]
- Aoyama, N.; Izumi, T.; Hiramori, K.; Isobe, M.; Kawana, M.; Hiroe, M.; Hishida, H.; Kitaura, Y.; Imaizumi, T.; Japanese Investigators of Fulminant Myocarditis. National survey of fulminant myocarditis in Japan: Therapeutic guidelines and long-term prognosis of using percutaneous cardiopulmonary support for fulminant myocarditis (special report from a scientific committee). Circ. J. 2002, 66, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, R.; Centofanti, P.; Gelsomino, S.; Barili, F.; Di Mauro, M.; Orlando, P.; Botta, L.; Milazzo, F.; Actis Dato, G.; Casabona, R.; et al. Venoarterial Extracorporeal Membrane Oxygenation for Acute Fulminant Myocarditis in Adult Patients: A 5-Year Multi-Institutional Experience. Ann. Thorac. Surg. 2016, 101, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Ammirati, E.; Cipriani, M.; Lilliu, M.; Sormani, P.; Varrenti, M.; Raineri, C.; Petrella, D.; Garascia, A.; Pedrotti, P.; Roghi, A.; et al. Survival and Left Ventricular Function Changes in Fulminant Versus Nonfulminant Acute Myocarditis. Circulation 2017, 136, 529–545. [Google Scholar] [CrossRef]
- Chang, J.; Lin, M.; Chen, T.; Chen, D.; Chen, S.; Hsu, J.; Wang, P.; Lin, Y. Heart Failure and Mortality of Adult Survivors from Acute Myocarditis Requiring Intensive Care Treatment—A Nationwide Cohort Study. Int. J. Med. Sci. 2017, 14, 1241–1250. [Google Scholar] [CrossRef] [PubMed]

| ECMO (n = 71) | Non-ECMO (n = 29) | p -Value | |
|---|---|---|---|
| Patient demographics | |||
| Age (year) | 34 (19–46) | 14 (4–49.5) | 0.14 |
| Adult (≥18 year) | 41 (31–49) (n = 55) | 52 (39–56.50) (n = 13) | 0.069 |
| Pediatric (<18 year) | 4 (0–7.75) (n = 16) | 6 (1.5–10.75) (n = 16) | 0.364 |
| Gender, male | 25 (35.2%) | 19 (65.5%) | 0.006 |
| BSA (m2) | 1.60 (1.40–1.69) | 1.43 (0.68–1.72) | 0.137 |
| Smoking | 14 (20%) | 3 (10.3%) | 0.246 |
| Diabetes mellitus | 14 (19.7%) | 1 (3.4%) | 0.039 |
| Hypertension | 15 (21.1%) | 2 (6.9%) | 0.086 |
| Malignancy | 4 (5.6%) | 2 (6.9%) | 0.809 |
| Dyslipidemia | 3 (4.3%) | 1 (3.4%) | 0.857 |
| Chronic kidney disease a | 3 (4.3%) | 0 (0%) | 0.261 |
| Previous coronary artery diseases b | 4 (5.6%) | 0 (0%) | 0.192 |
| Cardiac arrest | 24 (33.8%) | 6 (20.7%) | 0.194 |
| ECPR c | 15 (21.1%) | 0 (0%) | NA |
| Data at ICU admission | |||
| Cardiac enzymes | |||
| Troponin I (ng/mL) | 16.96 (3.42–41.70) | 4.35 (1.25–21.15) | 0.007 |
| CK-MB (ng/mL) | 55.76 (23.96–107.36) | 24.59 (11.92–68.76) | 0.026 |
| NT-proBNP (pg/mL) * | 15618 (7582–32,400) | 7839 (3223–26,338) | 0.068 |
| WBC (×103/μL) | 12.79 (9.17–16.92) | 9.73 (8.11–13.86) | 0.037 |
| CRP (mg/dL) | 4.27 (1.32–11.34) | 2.71 (0.56–6.51) | 0.087 |
| Creatinine (mg/dL) | 1.04 (0.80–1.51) | 0.62 (0.48–1.04) | <0.001 |
| Lactic acid (mmol/L) | 5.09 (2.97–9.08) | 2.33 (1.41–3.81) | <0.001 |
| EF (%) at ICU admission | 20.0 (15.0–34.0) | 40.4 (36.1–58.5) | <0.001 |
| SOFA score d | 9 | 5 | <0.001 |
| Documented arrhythmia | 63 (90%) (n = 70) | 23 (79.3%) | 0.152 |
| Asystole | 5 (7.1%) | 0 (0%) | |
| Brady-arrhythmia e | 12 (17.1%) | 5 (17.2%) | |
| Tachy-arrhythmia f | 36 (51.4%) | 7 (24.1%) | |
| VT/VF g | 30 (42.9%) | 3 (10.3%) | |
| Widened QRS complex h | 3 (4.3%) | 5 (17.2%) | |
| Other arrhythmias i | 7 (10%) | 6 (20.7%) | |
| Mechanical ventilator | 62 (87.3%) | 11 (37.9%) | <0.001 |
| CRRT j | 27 (38.0%) | 1 (3.4%) | <0.001 |
| IABP k | 18 (25.4%) | 2 (6.9%) | 0.036 |
| All (n = 100) | ECMO (n = 71) | Non-ECMO (n = 29) | p-Value | |
|---|---|---|---|---|
| In-hospital mortality | 22 (22%) | 20 (28.2%) | 2 (6.9%) | 0.020 |
| Proportion of heart transplantation/VAD | 8 (8%) | 8 (11.3%) | 0 (0%) | 0.101 |
| Long-term outcomes (n = 78) | ||||
| Death after hospital discharge | 3 (3.8%) | 3 (5.9%) | 0 (0%) | 0.547 |
| Median NYHA class of the survivors | 1 | 1 | 1 | 0.453 |
| EF (%) at last echocardiography during follow-up | 61.7 (56–66.8) | 60 (52.5–65) | 63 (60.1–67.8) | 0.059 |
| Univariable Analysis | Multivariable Analysis | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Overall cohort | ||||
| Age | 1 (0.955–1.048) | 0.985 | ||
| Gender, male | 0.608 (0.094–3.947) | 0.602 | ||
| Deployment of ECMO | 0.563 (0.022–14.386) | 0.729 | ||
| CRRT | 2.889 (0.511–16.332) | 0.230 | ||
| Cardiac arrest | 1.140 (0.163–7.942) | 0.895 | ||
| EF (%) at ICU admission | 0.976 (0.919–1.036) | 0.419 | ||
| CRP | 1.003 (0.871–1.154) | 0.972 | ||
| Lactic acid | 1.106 (0.905–1.352) | 0.323 | ||
| CK-MB | 1.006 (0.997–1.015) | 0.212 | 1.006 (0.998–1.013) | 0.139 |
| SOFA score | 1.480 (1.044–2.098) | 0.028 | 1.715 (1.304–2.256) | <0.001 |
| ECMO group | ||||
| Age | 0.991 (0.950–1.034) | 0.678 | ||
| Gender, male | 0.334 (0.055–2.021) | 0.233 | ||
| CRRT | 2.543 (0.555–11.661) | 0.230 | ||
| Cardiac arrest | 1.028 (0.169–6.235) | 0.976 | ||
| CK-MB | 1.013 (1.003–1.023) | 0.011 | 1.014 (1.003–1.024) | 0.009 |
| SOFA score | 1.492 (1.089–2.046) | 0.013 | 1.499 (1.180–1.903) | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.I.; Chung, S.; Yang, J.-H.; Sung, K.; Kim, D.; Choi, J.-O.; Jeon, E.-S.; Yang, J.H.; Cho, Y.H. Extracorporeal Membrane Oxygenation for Fulminant Myocarditis: Increase of Cardiac Enzyme and SOFA Score Is Associated with High Mortality. J. Clin. Med. 2021, 10, 1526. https://doi.org/10.3390/jcm10071526
Lee YI, Chung S, Yang J-H, Sung K, Kim D, Choi J-O, Jeon E-S, Yang JH, Cho YH. Extracorporeal Membrane Oxygenation for Fulminant Myocarditis: Increase of Cardiac Enzyme and SOFA Score Is Associated with High Mortality. Journal of Clinical Medicine. 2021; 10(7):1526. https://doi.org/10.3390/jcm10071526
Chicago/Turabian StyleLee, Yun Im, Suryeun Chung, Ji-Hyuk Yang, Kiick Sung, Darae Kim, Jin-Oh Choi, Eun-Seok Jeon, Jeong Hoon Yang, and Yang Hyun Cho. 2021. "Extracorporeal Membrane Oxygenation for Fulminant Myocarditis: Increase of Cardiac Enzyme and SOFA Score Is Associated with High Mortality" Journal of Clinical Medicine 10, no. 7: 1526. https://doi.org/10.3390/jcm10071526
APA StyleLee, Y. I., Chung, S., Yang, J.-H., Sung, K., Kim, D., Choi, J.-O., Jeon, E.-S., Yang, J. H., & Cho, Y. H. (2021). Extracorporeal Membrane Oxygenation for Fulminant Myocarditis: Increase of Cardiac Enzyme and SOFA Score Is Associated with High Mortality. Journal of Clinical Medicine, 10(7), 1526. https://doi.org/10.3390/jcm10071526






