How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches
Abstract
1. Introduction
Overview of Gastric Cancer Classification and Relevance for Immunotherapy
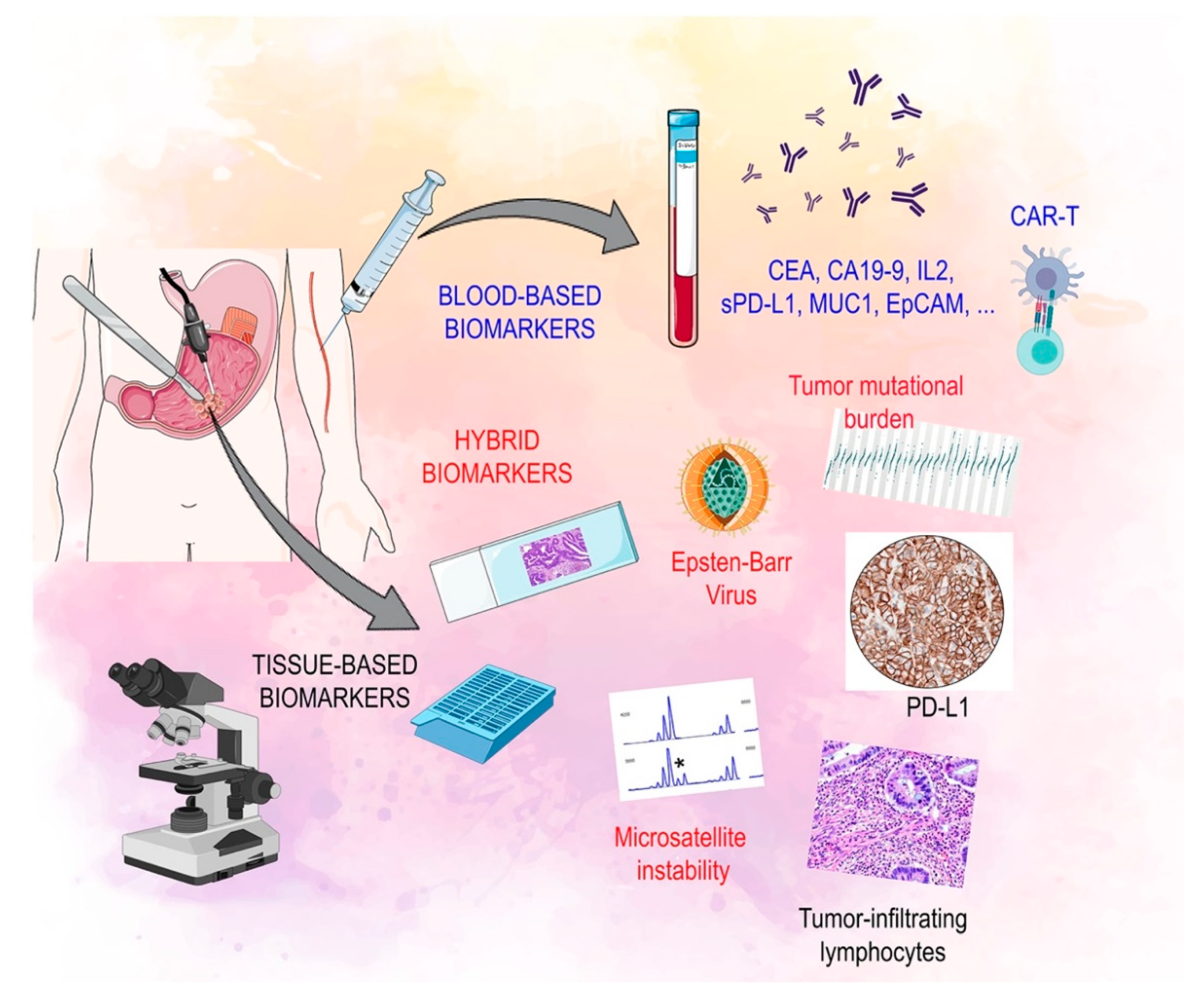
2. Biomarkers of Response to Immunotherapy in Gastric Cancer
2.1. Tissue Based Biomarkers
2.2. Circulating Biomarkers
3. Immunotherapy: From Landmark Trials to Clinical Practice and Future Perspectives
3.1. Non-Metastatic Disease
3.2. Metastatic Disease: 1st Line Treatment
3.3. Metastatic Disease: Second Line Treatment and Beyond
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Global Cancer Observatory: Gastric Cancer. Available online: https://gco.iarc.fr/ (accessed on 23 December 2020).
- Laurén, P. The two histological main types of gastric carcinoma: Diffuse and so-called intestinal-type carcinoma. Acta Pathol. Microbiol. Scand. 1965, 64, 31–49. [Google Scholar] [CrossRef]
- Marqués-Lespier, J.M.; González-Pons, M.; Cruz-Correa, M. Current Perspectives on Gastric Cancer. Gastroenterol. Clin. North Am. 2016, 45, 413–428. [Google Scholar] [CrossRef]
- Ma, J.; Shen, H.; Kapesa, L.; Zeng, S. Lauren classification and individualized chemotherapy in gastric cancer. Oncol. Lett. 2016, 11, 2959–2964. [Google Scholar] [CrossRef]
- Blair, V.R.; McLeod, M.; Carneiro, F.; Coit, D.G.; D’Addario, J.L.; van Dieren, J.M.; Harris, K.L.; Hoogerbrugge, N.; Oliveira, C.; van der Post, R.S.; et al. Hereditary diffuse gastric cancer: Updated clinical practice guidelines. Lancet Oncol. 2020, 21, e386–e397. [Google Scholar] [CrossRef]
- Berlth, F. Pathohistological classification systems in gastric cancer: Diagnostic relevance and prognostic value. World, J. Gastroenterol. 2014, 20, 5679. [Google Scholar] [CrossRef] [PubMed]
- Bosman, F.T.; Carneiro, F.; Hruban, R.H.; Theise, N.D. WHO Classification of Tumours of the Digestive System; World Health Organization: Geneva, Switzerland, 2010; ISBN 9789283224327. [Google Scholar]
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef]
- Ling, Y.; Watanabe, Y.; Nagahashi, M.; Shimada, Y.; Ichikawa, H.; Wakai, T.; Okuda, S. Genetic profiling for diffuse type and genomically stable subtypes in gastric cancer. Comput. Struct. Biotechnol. J. 2020, 18, 3301–3308. [Google Scholar] [CrossRef]
- Rodriquenz, M.G.; Roviello, G.; D’Angelo, A.; Lavacchi, D.; Roviello, F.; Polom, K. MSI and EBV Positive Gastric Cancer’s Subgroups and Their Link with Novel Immunotherapy. J. Clin. Med. 2020, 9, 1427. [Google Scholar] [CrossRef]
- Li, X.; Wu, W.K.K.; Xing, R.; Wong, S.H.; Liu, Y.; Fang, X.; Zhang, Y.; Wang, M.; Wang, J.; Li, L.; et al. Distinct Subtypes of Gastric Cancer Defined by Molecular Characterization Include Novel Mutational Signatures with Prognostic Capability. Cancer Res. 2016, 76, 1724–1732. [Google Scholar] [CrossRef]
- Leung, S.Y.; Yuen, S.T.; Chung, L.P.; Chu, K.M.; Chan, A.S.Y.; Ho, J.C.I. hMLH1 Promoter Methylation and Lack of hMLH1 Expression in Sporadic Gastric Carcinomas with High-Frequency Microsatellite Instability. Cancer Res. 1999, 59, 159–164. [Google Scholar]
- Pietrantonio, F.; Miceli, R.; Raimondi, A.; Kim, Y.W.; Kang, W.K.; Langley, R.E.; Choi, Y.Y.; Kim, K.-M.; Nankivell, M.G.; Morano, F.; et al. Individual Patient Data Meta-Analysis of the Value of Microsatellite Instability As a Biomarker in Gastric Cancer. J. Clin. Oncol. 2019, 37, 3392–3400. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.R.; Kemberling, H.; Eyring, A.D.; Skora, A.D.; Luber, B.S.; Azad, N.S.; Laheru, D.; et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N. Engl. J. Med. 2015, 372, 2509–2520. [Google Scholar] [CrossRef]
- Eichelberg, M.R.; Welch, R.; Guidry, J.T.; Ali, A.; Ohashi, M.; Makielski, K.R.; McChesney, K.; Van Sciver, N.; Lambert, P.F.; Keleș, S.; et al. Epstein-Barr Virus Infection Promotes Epithelial Cell Growth by Attenuating Differentiation-Dependent Exit from the Cell Cycle. MBio 2019, 10. [Google Scholar] [CrossRef]
- Jung, H.; Kim, H.S.; Kim, J.Y.; Sun, J.-M.; Ahn, J.S.; Ahn, M.-J.; Park, K.; Esteller, M.; Lee, S.-H.; Choi, J.K. DNA methylation loss promotes immune evasion of tumours with high mutation and copy number load. Nat. Commun. 2019, 10, 4278. [Google Scholar] [CrossRef] [PubMed]
- Geddert, H.; Zur Hausen, A.; Gabbert, H.E.; Sarbia, M. EBV-infection in cardiac and non-cardiac gastric adenocarcinomas is associated with promoter methylation of p16, p14 and APC, but not hMLH1. Anal. Cell. Pathol. (Amst.) 2010, 33, 143–149. [Google Scholar] [CrossRef]
- Xin Yu, J.; Hubbard-Lucey, V.M.; Tang, J. Immuno-oncology drug development goes global. Nat. Rev. Drug Discov. 2019, 18, 899–900. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, Y.; Wang, H. Use of immunotherapy in the treatment of gastric cancer (Review). Oncol. Lett. 2019, 18, 5681–5690. [Google Scholar] [CrossRef]
- Zhao, L.; Li, J.; Bai, C.; Nie, Y.; Lin, G. Multi-Modality Treatment for Patients With Metastatic Gastric Cancer: A Real-World Study in China. Front. Oncol. 2019, 9, 1155. [Google Scholar] [CrossRef]
- Ebinger, S.M.; Warschkow, R.; Tarantino, I.; Schmied, B.M.; Güller, U.; Schiesser, M. Modest overall survival improvements from 1998 to 2009 in metastatic gastric cancer patients: A population-based SEER analysis. Gastric Cancer 2016, 19, 723–734. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, T.N.; Scheper, W.; Kvistborg, P. Cancer Neoantigens. Annu. Rev. Immunol. 2019, 37, 173–200. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, W.; Simeone, I.; Anjum, S.; Mokrab, Y.; Bertucci, F.; Finetti, P.; Curigliano, G.; Seliger, B.; Cerulo, L.; Tomei, S.; et al. Identification of genetic determinants of breast cancer immune phenotypes by integrative genome-scale analysis. Oncoimmunology 2017, 6, e1253654. [Google Scholar] [CrossRef]
- Wagner, J.; Wickman, E.; DeRenzo, C.; Gottschalk, S. CAR T Cell Therapy for Solid Tumors: Bright Future or Dark Reality? Mol. Ther. 2020, 28, 2320–2339. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Rong, L.; Wang, E.; Fang, Y. Pseudoprogression of extramedullary disease in relapsed acute lymphoblastic leukemia after CAR T-cell therapy. Immunotherapy 2021, 13, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Ou, Z. PCN198 Global regulatory challenges of CAR T-Cell therapies: Approval, pricing, and access. Value Health 2019, 22, S93. [Google Scholar] [CrossRef]
- Lin, E.M.; Gong, J.; Klempner, S.J.; Chao, J. Advances in immuno-oncology biomarkers for gastroesophageal cancer: Programmed death ligand 1, microsatellite instability, and beyond. World J. Gastroenterol. 2018, 24, 2686–2697. [Google Scholar] [CrossRef] [PubMed]
- Ratti, M.; Lampis, A.; Hahne, J.C.; Passalacqua, R.; Valeri, N. Microsatellite instability in gastric cancer: Molecular bases, clinical perspectives, and new treatment approaches. Cell. Mol. Life Sci. 2018, 75, 4151–4162. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-Y.; Shin, N.R.; Kim, A.; Lee, H.-J.; Park, W.; Kim, J.-Y.; Lee, C.-H.; Huh, G.-Y.; Park, D.Y. Microsatellite Instability Status in Gastric Cancer: A Reappraisal of Its Clinical Significance and Relationship with Mucin Phenotypes. Korean J. Pathol. 2013, 47, 28. [Google Scholar] [CrossRef] [PubMed]
- Trenner, A.; Sartori, A.A. Harnessing DNA Double-Strand Break Repair for Cancer Treatment. Front. Oncol. 2019, 9, 1388. [Google Scholar] [CrossRef] [PubMed]
- Motegi, A.; Masutani, M.; Yoshioka, K.; Bessho, T. Aberrations in DNA repair pathways in cancer and therapeutic significances. Semin. Cancer Biol. 2019, 58, 29–46. [Google Scholar] [CrossRef] [PubMed]
- Corti, C.; Sajjadi, E.; Fusco, N. Determination of Mismatch Repair Status in Human Cancer and Its Clinical Significance. Adv. Anat. Pathol. 2019, 26, 270–279. [Google Scholar] [CrossRef] [PubMed]
- Lopez, G.; Venetis, K.; Sajjadi, E.; Fusco, N. Mismatch Repair System Genomic Scars in Gastroesophageal Cancers: Biology and Clinical Testing. Gastrointest. Disord. 2020, 2, 341–352. [Google Scholar] [CrossRef]
- Matsuoka, T.; Yashiro, M. Biomarkers of gastric cancer: Current topics and future perspective. World J. Gastroenterol. 2018, 24, 2818–2832. [Google Scholar] [CrossRef] [PubMed]
- Dhakras, P.; Uboha, N.; Horner, V.; Reinig, E.; Matkowskyj, K.A. Gastrointestinal cancers: Current biomarkers in esophageal and gastric adenocarcinoma. Transl. Gastroenterol. Hepatol. 2020, 5, 55. [Google Scholar] [CrossRef] [PubMed]
- Pagni, F.; Guerini-Rocco, E.; Schultheis, A.M.; Grazia, G.; Rijavec, E.; Ghidini, M.; Lopez, G.; Venetis, K.; Croci, G.A.; Malapelle, U.; et al. Targeting Immune-Related Biological Processes in Solid Tumors: We do Need Biomarkers. Int. J. Mol. Sci. 2019, 20, 5452. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, E.; Venetis, K.; Scatena, C.; Fusco, N. Biomarkers for precision immunotherapy in the metastatic setting: Hope or reality? Ecancermedicalscience 2020, 14. [Google Scholar] [CrossRef]
- Polom, K.; Marano, L.; Marrelli, D.; De Luca, R.; Roviello, G.; Savelli, V.; Tan, P.; Roviello, F. Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer. Br. J. Surg. 2018, 105, 159–167. [Google Scholar] [CrossRef]
- Giampieri, R.; Maccaroni, E.; Mandolesi, A.; Del Prete, M.; Andrikou, K.; Faloppi, L.; Bittoni, A.; Bianconi, M.; Scarpelli, M.; Bracci, R.; et al. Mismatch repair deficiency may affect clinical outcome through immune response activation in metastatic gastric cancer patients receiving first-line chemotherapy. Gastric Cancer 2017, 20, 156–163. [Google Scholar] [CrossRef]
- Yuza, K.; Nagahashi, M.; Watanabe, S.; Takabe, K.; Wakai, T. Hypermutation and microsatellite instability in gastrointestinal cancers. Oncotarget 2017, 8, 112103–112115. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Ma, Z.; Han, H.; Zhang, X.; Niu, S.; Du, L.; Zheng, Y.; Liu, H. Expression of programmed death ligand 1 (PD-L1) is associated with metastasis and differentiation in gastric cancer. Life Sci. 2020, 242, 117247. [Google Scholar] [CrossRef]
- Liu, X.; Choi, M.G.; Kim, K.; Kim, K.-M.; Kim, S.T.; Park, S.H.; Cristescu, R.; Peter, S.; Lee, J. High PD-L1 expression in gastric cancer (GC) patients and correlation with molecular features. Pathol. Res. Pract. 2020, 216, 152881. [Google Scholar] [CrossRef] [PubMed]
- Ju, X.; Shen, R.; Huang, P.; Zhai, J.; Qian, X.; Wang, Q.; Chen, M. Predictive relevance of PD-L1 expression with pre-existing TILs in gastric cancer. Oncotarget 2017, 8, 99372–99381. [Google Scholar] [CrossRef]
- Fassan, M.; Brignola, S.; Pennelli, G.; Alberti, G.; Angerilli, V.; Bressan, A.; Pellino, A.; Lanza, C.; Salmaso, R.; Lonardi, S.; et al. PD-L1 expression in gastroesophageal dysplastic lesions. Virchows Arch. 2020, 477, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Patel, K.; Singhi, A.D.; Ren, B.; Zhu, B.; Shaikh, F.; Sun, W. Programmed Death-Ligand 1 Expression Is Common in Gastric Cancer Associated With Epstein-Barr Virus or Microsatellite Instability. Am. J. Surg. Pathol. 2016, 40, 1496–1506. [Google Scholar] [CrossRef]
- Ye, D.; Xu, G.; Ma, W.; Li, Y.; Luo, W.; Xiao, Y.; Liu, Y.; Zhang, Z. Significant function and research progress of biomarkers in gastric cancer (Review). Oncol. Lett. 2019, 19, 17–29. [Google Scholar] [CrossRef]
- Zhang, N.; Cao, M.; Duan, Y.; Bai, H.; Li, X.; Wang, Y. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: A meta-analysis and experimental validation. Arch. Med. Sci. 2020, 16, 1092–1103. [Google Scholar] [CrossRef]
- Lee, J.S.; Won, H.S.; Sun, D.S.; Hong, J.H.; Ko, Y.H. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer. Medicine (Baltimore) 2018, 97, e11769. [Google Scholar] [CrossRef]
- Hendry, S.; Salgado, R.; Gevaert, T.; Russell, P.A.; John, T.; Thapa, B.; Christie, M.; van de Vijver, K.; Estrada, M.V.; Gonzalez-Ericsson, P.I.; et al. Assessing Tumor-Infiltrating Lymphocytes in Solid Tumors. Adv. Anat. Pathol. 2017, 24, 311–335. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Y.; Yu, M.; Liu, Z.; Xu, Y.; Ma, G.; Zhou, W.; Kong, P.; Ling, L.; Wang, S.; et al. Increased Stromal Infiltrating Lymphocytes are Associated with Circulating Tumor Cells and Metastatic Relapse in Breast Cancer Patients After Neoadjuvant Chemotherapy. Cancer Manag. Res. 2019, 11, 10791–10800. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Doi, T.; Jang, R.W.; Muro, K.; Satoh, T.; Machado, M.; Sun, W.; Jalal, S.I.; Shah, M.A.; Metges, J.-P.; et al. Safety and Efficacy of Pembrolizumab Monotherapy in Patients With Previously Treated Advanced Gastric and Gastroesophageal Junction Cancer. JAMA Oncol. 2018, 4, e180013. [Google Scholar] [CrossRef]
- Sholl, L.M.; Hirsch, F.R.; Hwang, D.; Botling, J.; Lopez-Rios, F.; Bubendorf, L.; Mino-Kenudson, M.; Roden, A.C.; Beasley, M.B.; Borczuk, A.; et al. The Promises and Challenges of Tumor Mutation Burden as an Immunotherapy Biomarker: A Perspective from the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020, 15, 1409–1424. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration. FDA Approves Pembrolizumab for Adults and Children with TMB-H Solid Tumors. Available online: https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-pembrolizumab-adults-and-children-tmb-h-solid-tumors (accessed on 19 December 2020).
- Ku, G.Y.; Sanchez-Vega, F.; Chatila, W.; Margolis, M.; Fein, C.; Ilson, D.H.; Hechtman, J.F.; Tuvy, Y.; Bouvier, N.; Kundra, R.; et al. Correlation of benefit from immune checkpoint inhibitors with next gen sequencing (NGS) profiles in esophagogastric cancer (EGC) patients. J. Clin. Oncol. 2017, 35, 4025. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Sanchez-Vega, F.; Jonsson, P.; Chatila, W.K.; Hechtman, J.F.; Ku, G.Y.; Riches, J.C.; Tuvy, Y.; Kundra, R.; Bouvier, N.; et al. Genetic Predictors of Response to Systemic Therapy in Esophagogastric Cancer. Cancer Discov. 2018, 8, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Sundar, R.; Smyth, E.C.; Peng, S.; Yeong, J.P.S.; Tan, P. Predictive Biomarkers of Immune Checkpoint Inhibition in Gastroesophageal Cancers. Front. Oncol. 2020, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, L.; Jiang, X.; Li, Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J. Hematol. Oncol. 2019, 12, 54. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Keegan, P.; Pazdur, R. FDA Approval Summary: Pembrolizumab for the Treatment of Microsatellite Instability-High Solid Tumors. Clin. Cancer Res. 2019, 25, 3753–3758. [Google Scholar] [CrossRef]
- Shitara, K.; Van Cutsem, E.; Bang, Y.-J.; Fuchs, C.; Wyrwicz, L.; Lee, K.-W.; Kudaba, I.; Garrido, M.; Chung, H.C.; Lee, J.; et al. Efficacy and Safety of Pembrolizumab or Pembrolizumab Plus Chemotherapy vs Chemotherapy Alone for Patients With First-line, Advanced Gastric Cancer. JAMA Oncol. 2020, 6, 1571. [Google Scholar] [CrossRef]
- Cho, J.; Kang, S.Y.; Kim, K.-M. MMR protein immunohistochemistry and microsatellite instability in gastric cancers. Pathology 2019, 51, 110–113. [Google Scholar] [CrossRef]
- Luchini, C.; Bibeau, F.; Ligtenberg, M.J.L.; Singh, N.; Nottegar, A.; Bosse, T.; Miller, R.; Riaz, N.; Douillard, J.-Y.; Andre, F.; et al. ESMO recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: A systematic review-based approach. Ann. Oncol. 2019, 30, 1232–1243. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.T.; Cristescu, R.; Bass, A.J.; Kim, K.-M.; Odegaard, J.I.; Kim, K.; Liu, X.Q.; Sher, X.; Jung, H.; Lee, M.; et al. Comprehensive molecular characterization of clinical responses to PD-1 inhibition in metastatic gastric cancer. Nat. Med. 2018, 24, 1449–1458. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, Q.; Shi, B.; Xu, P.; Hu, Z.; Bai, L.; Zhang, X. Development of a sandwich ELISA for evaluating soluble PD-L1 (CD274) in human sera of different ages as well as supernatants of PD-L1+ cell lines. Cytokine 2011, 56, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Duraker, N.; Naci Çelik, A.; Gençler, N. The prognostic significance of gastric juice CA 19–9 and CEA levels in gastric carcinoma patients. Eur. J. Surg. Oncol. 2002, 28, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Morita, M.; Sasajima, M.; Hoshina, R.; Kanda, E.; Urita, Y. Usefulness of gastric cancer screening using the serum pepsinogen test method. Am. J. Gastroenterol. 2003, 98, 735–739. [Google Scholar] [CrossRef]
- Miki, K. Gastric cancer screening using the serum pepsinogen test method. Gastric Cancer 2006, 9, 245–253. [Google Scholar] [CrossRef]
- Zheng, Z.; Bu, Z.; Liu, X.; Zhang, L.; Li, Z.; Wu, A.; Wu, X.; Cheng, X.; Xing, X.; Du, H.; et al. Level of circulating PD-L1 expression in patients with advanced gastric cancer and its clinical implications. Chin. J. Cancer Res. 2014, 26, 104–111. [Google Scholar]
- Shigemori, T.; Toiyama, Y.; Okugawa, Y.; Yamamoto, A.; Yin, C.; Narumi, A.; Ichikawa, T.; Ide, S.; Shimura, T.; Fujikawa, H.; et al. Soluble PD-L1 Expression in Circulation as a Predictive Marker for Recurrence and Prognosis in Gastric Cancer: Direct Comparison of the Clinical Burden Between Tissue and Serum PD-L1 Expression. Ann. Surg. Oncol. 2019, 26, 876–883. [Google Scholar] [CrossRef]
- Gershtein, E.S.; Ognerubov, N.A.; Chang, V.L.; Delektorskaya, V.V.; Korotkova, E.A.; Sokolov, N.Y.; Polikarpova, S.B.; Stilidi, I.S.; Kushlinskii, N.E. [The content of the soluble forms PD-1 and PD-L1 in blood serum of patients with gastric cancer and their relationship with clinical and morphological characteristics of the disease.]. Klin. Lab. Diagn. 2020, 65, 347–352. [Google Scholar] [CrossRef]
- Shapiro, M.; Herishanu, Y.; Katz, B.-Z.; Dezorella, N.; Sun, C.; Kay, S.; Polliack, A.; Avivi, I.; Wiestner, A.; Perry, C. Lymphocyte activation gene 3: A novel therapeutic target in chronic lymphocytic leukemia. Haematologica 2017, 102, 874–882. [Google Scholar] [CrossRef]
- Okamura, T.; Fujio, K.; Sumitomo, S.; Yamamoto, K. Roles of LAG3 and EGR2 in regulatory T cells. Ann. Rheum. Dis. 2012, 71, i96–i100. [Google Scholar] [CrossRef]
- Li, N.; Jilisihan, B.; Wang, W.; Tang, Y.; Keyoumu, S. Soluble LAG3 acts as a potential prognostic marker of gastric cancer and its positive correlation with CD8+T cell frequency and secretion of IL-12 and INF-γ in peripheral blood. Cancer Biomarkers 2018, 23, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Botticelli, A.; Mezi, S.; Pomati, G.; Cerbelli, B.; Di Rocco, C.; Amirhassankhani, S.; Sirgiovanni, G.; Occhipinti, M.; Napoli, V.; Emiliani, A.; et al. The 5-Ws of immunotherapy in head and neck cancer. Crit. Rev. Oncol. Hematol. 2020, 153, 103041. [Google Scholar] [CrossRef] [PubMed]
- Ando, K.; Hamada, K.; Watanabe, M.; Ohkuma, R.; Shida, M.; Onoue, R.; Kubota, Y.; Matsui, H.; Ishiguro, T.; Hirasawa, Y.; et al. Plasma Levels of Soluble PD-L1 Correlate With Tumor Regression in Patients With Lung and Gastric Cancer Treated With Immune Checkpoint Inhibitors. Anticancer Res. 2019, 39, 5195–5201. [Google Scholar] [CrossRef] [PubMed]
- Park, W.; Bang, J.-H.; Nam, A.-R.; Jin, M.H.; Seo, H.; Kim, J.-M.; Oh, K.S.; Kim, T.-Y.; Oh, D.-Y. Prognostic Value of Serum Soluble Programmed Death-Ligand 1 and Dynamics During Chemotherapy in Advanced Gastric Cancer Patients. Cancer Res. Treat. 2021, 53, 199–206. [Google Scholar] [CrossRef]
- Takahashi, N.; Iwasa, S.; Sasaki, Y.; Shoji, H.; Honma, Y.; Takashima, A.; Okita, N.T.; Kato, K.; Hamaguchi, T.; Yamada, Y. Serum levels of soluble programmed cell death ligand 1 as a prognostic factor on the first-line treatment of metastatic or recurrent gastric cancer. J. Cancer Res. Clin. Oncol. 2016, 142, 1727–1738. [Google Scholar] [CrossRef] [PubMed]
- Ribas, A.; Butterfield, L.H.; Glaspy, J.A.; Economou, J.S. Current developments in cancer vaccines and cellular immunotherapy. J. Clin. Oncol. 2003, 21, 2415–2432. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, A.D.; Watson, S.A. G17DT: An antigastrin immunogen for the treatment of gastrointestinal malignancy. Expert Opin. Biol. Ther. 2007, 7, 397–404. [Google Scholar] [CrossRef]
- Park, D.J.; Thomas, N.J.; Yoon, C.; Yoon, S.S. Vascular Endothelial Growth Factor A Inhibition in Gastric Cancer. Gastric Cancer 2015, 18, 33–42. [Google Scholar] [CrossRef]
- Sundar, R.; Rha, S.Y.; Yamaue, H.; Katsuda, M.; Kono, K.; Kim, H.S.; Kim, C.; Mimura, K.; Kua, L.-F.; Yong, W.P. A phase I/Ib study of OTSGC-A24 combined peptide vaccine in advanced gastric cancer. BMC Cancer 2018, 18, 332. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, A.; Watson, S.; Henwood, M.; McKenzie, A.; Humphreys, J.; Elder, J.; Iftikhar, S.; Welch, N.; Fielding, J.; Broome, P.; et al. A phase II study of G17DT in gastric carcinoma. Eur. J. Surg. Oncol. 2004, 30, 536–543. [Google Scholar] [CrossRef]
- Ajani, J.A.; Randolph Hecht, J.; Ho, L.; Baker, J.; Oortgiesen, M.; Eduljee, A.; Michaeli, D. An open-label, multinational, multicenter study of G17DT vaccination combined with cisplatin and 5-fluorouracil in patients with untreated, advanced gastric or gastroesophageal cancer: The GC4 study. Cancer 2006, 106, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Masuzawa, T.; Yoshiyuki, F.; Okada, K.; Nakamura, A.; Takiguchi, S.; Nakajima, K.; Miyata, H.; Yamasaki, M.; Kurokawa, Y.; Osawa, R.; et al. Phase I/II study of S-1 plus cisplatin combined with peptide vaccines for human vascular endothelial growth factor receptor 1 and 2 in patients with advanced gastric cancer. Int. J. Oncol. 2012, 41, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Stauss, H.J.; Morris, E.C.; Abken, H. Cancer gene therapy with T cell receptors and chimeric antigen receptors. Curr. Opin. Pharmacol. 2015, 24, 113–118. [Google Scholar] [CrossRef]
- Li, J.; Li, W.; Huang, K.; Zhang, Y.; Kupfer, G.; Zhao, Q. Chimeric antigen receptor T cell (CAR-T) immunotherapy for solid tumors: Lessons learned and strategies for moving forward. J. Hematol. Oncol. 2018, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Gill, S.; Maus, M.V.; Porter, D.L. Chimeric antigen receptor T cell therapy: 25years in the making. Blood Rev. 2016, 30, 157–167. [Google Scholar] [CrossRef]
- Whilding, L.M.; Maher, J. ErbB-targeted CAR T-cell immunotherapy of cancer. Immunotherapy 2015, 7, 229–241. [Google Scholar] [CrossRef]
- Wang, L.; Ma, N.; Okamoto, S.; Amaishi, Y.; Sato, E.; Seo, N.; Mineno, J.; Takesako, K.; Kato, T.; Shiku, H. Efficient tumor regression by adoptively transferred CEA-specific CAR-T cells associated with symptoms of mild cytokine release syndrome. Oncoimmunology 2016, 5, e1211218. [Google Scholar] [CrossRef]
- Guest, R.D.; Kirillova, N.; Mowbray, S.; Gornall, H.; Rothwell, D.G.; Cheadle, E.J.; Austin, E.; Smith, K.; Watt, S.M.; Kühlcke, K.; et al. Definition and application of good manufacturing process-compliant production of CEA-specific chimeric antigen receptor expressing T-cells for phase I/II clinical trial. Cancer Immunol. Immunother. 2014, 63, 133–145. [Google Scholar] [CrossRef]
- Warneke, V.S.; Behrens, H.-M.; Haag, J.; Krüger, S.; Simon, E.; Mathiak, M.; Ebert, M.P.A.; Röcken, C. Members of the EpCAM signalling pathway are expressed in gastric cancer tissue and are correlated with patient prognosis. Br. J. Cancer 2013, 109, 2217–2227. [Google Scholar] [CrossRef][Green Version]
- Smyth, E.C.; Verheij, M.; Allum, W.; Cunningham, D.; Cervantes, A.; Arnold, D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v38–v49. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Van Cutsem, E.; Fuchs, C.S.; Ohtsu, A.; Tabernero, J.; Ilson, D.H.; Hyung, W.J.; Strong, V.E.; Goetze, T.O.; Yoshikawa, T.; et al. KEYNOTE-585: Phase III study of perioperative chemotherapy with or without pembrolizumab for gastric cancer. Future Oncol. 2019, 15, 943–952. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.-G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.L.; Motoyama, S.; Lièvre, A.; et al. LBA9_PR Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer (EC/GEJC) following neoadjuvant chemoradiation therapy (CRT): First results of the CheckMate 577 study. Ann. Oncol. 2020, 31, S1193–S1194. [Google Scholar] [CrossRef]
- Moehler, M.; Shitara, K.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; Liu, T.; et al. LBA6_PR Nivolumab (nivo) plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study. Ann. Oncol. 2020, 31, S1191. [Google Scholar] [CrossRef]
- Boku, N.; Ryu, M.H.; Oh, D.-Y.; Oh, S.C.; Chung, H.C.; Lee, K.-W.; Omori, T.; Shitara, K.; Sakuramoto, S.; Chung, I.J.; et al. LBA7_PR Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538–37) study. Ann. Oncol. 2020, 31, S1192. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Maron, S.B.; Chatila, W.K.; Millang, B.; Chavan, S.S.; Alterman, C.; Chou, J.F.; Segal, M.F.; Simmons, M.Z.; Momtaz, P.; et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: An open-label, single-arm, phase 2 trial. Lancet Oncol. 2020, 21, 821–831. [Google Scholar] [CrossRef]
- Moehler, M.; Dvorkin, M.; Boku, N.; Özgüroğlu, M.; Ryu, M.-H.; Muntean, A.S.; Lonardi, S.; Nechaeva, M.; Bragagnoli, A.C.; Coşkun, H.S.; et al. Phase III Trial of Avelumab Maintenance After First-Line Induction Chemotherapy Versus Continuation of Chemotherapy in Patients With Gastric Cancers: Results From JAVELIN Gastric 100. J. Clin. Oncol. 2020, 39, 966–977. [Google Scholar] [CrossRef]
- Shitara, K.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.-H.; Fornaro, L.; Olesiński, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): A randomised, open-label, controlled, phase 3 trial. Lancet 2018, 392, 123–133. [Google Scholar] [CrossRef]
- Kang, Y.-K.; Boku, N.; Satoh, T.; Ryu, M.-H.; Chao, Y.; Kato, K.; Chung, H.C.; Chen, J.-S.; Muro, K.; Kang, W.K.; et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538–12, ATTRACTION-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (Lond. Engl.) 2017, 390, 2461–2471. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Bendell, J.; Calvo, E.; Kim, J.W.; Ascierto, P.A.; Sharma, P.; Ott, P.A.; Peltola, K.; Jaeger, D.; Evans, J.; et al. CheckMate-032 Study: Efficacy and Safety of Nivolumab and Nivolumab Plus Ipilimumab in Patients With Metastatic Esophagogastric Cancer. J. Clin. Oncol. 2018, 36, 2836–2844. [Google Scholar] [CrossRef] [PubMed]
- Bang, Y.-J.; Ruiz, E.Y.; Van Cutsem, E.; Lee, K.-W.; Wyrwicz, L.; Schenker, M.; Alsina, M.; Ryu, M.-H.; Chung, H.-C.; Evesque, L.; et al. Phase III, randomised trial of avelumab versus physician’s choice of chemotherapy as third-line treatment of patients with advanced gastric or gastro-oesophageal junction cancer: Primary analysis of JAVELIN Gastric 300. Ann. Oncol. 2018, 29, 2052–2060. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Bang, Y.; Van Cutsem, E.; Fuchs, C.; Janjigian, Y.; Bhagia, P.; Li, K.; Adelberg, D.; Qin, S. P-38 KEYNOTE-859: A randomized, double-blind, placebo-controlled phase 3 trial of first-line pembrolizumab plus chemotherapy in patients with advanced gastric or gastroesophageal junction adenocarcinoma. Ann. Oncol. 2020, 31, S101–S102. [Google Scholar] [CrossRef]
- Chung, H.C.; Bang, Y.-J.; Fuchs, C.S.; Qin, S.-K.; Satoh, T.; Shitara, K.; Tabernero, J.; van Cutsem, E.; Alsina, M.; Cao, Z.A.; et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Futur. Oncol. 2020, 17, 491–501. [Google Scholar] [CrossRef]
- Loi, S.; Giobbie-Hurder, A.; Gombos, A.; Bachelot, T.; Hui, R.; Curigliano, G.; Campone, M.; Biganzoli, L.; Bonnefoi, H.; Jerusalem, G.; et al. Pembrolizumab plus trastuzumab in trastuzumab-resistant, advanced, HER2-positive breast cancer (PANACEA): A single-arm, multicentre, phase 1b–2 trial. Lancet Oncol. 2019, 20, 371–382. [Google Scholar] [CrossRef]
- Petrillo, A.; Smyth, E.C. Biomarkers for Precision Treatment in Gastric Cancer. Visc. Med. 2020, 36, 364–372. [Google Scholar] [CrossRef]
- Parisi, A.; Porzio, G.; Ficorella, C. Multimodality Treatment in Metastatic Gastric Cancer: From Past to Next Future. Cancers (Basel) 2020, 12, 2598. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.; Muro, K.; Van Cutsem, E.; Oh, S.-C.; Bodoky, G.; Shimada, Y.; Hironaka, S.; Sugimoto, N.; Lipatov, O.; Kim, T.-Y.; et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol. 2014, 15, 1224–1235. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Tomasek, J.; Yong, C.J.; Dumitru, F.; Passalacqua, R.; Goswami, C.; Safran, H.; dos Santos, L.V.; Aprile, G.; Ferry, D.R.; et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet 2014, 383, 31–39. [Google Scholar] [CrossRef]
- Parisi, A.; Cortellini, A.; Roberto, M.; Venditti, O.; Santini, D.; Dell’Aquila, E.; Stellato, M.; Marchetti, P.; Occhipinti, M.A.; Zoratto, F.; et al. Weight loss and body mass index in advanced gastric cancer patients treated with second-line ramucirumab: A real-life multicentre study. J. Cancer Res. Clin. Oncol. 2019, 145, 2365–2373. [Google Scholar] [CrossRef]
- Di Bartolomeo, M.; Niger, M.; Tirino, G.; Petrillo, A.; Berenato, R.; Laterza, M.M.; Pietrantonio, F.; Morano, F.; Antista, M.; Lonardi, S.; et al. Ramucirumab as Second-Line Therapy in Metastatic Gastric Cancer: Real-World Data from the RAMoss Study. Target. Oncol. 2018, 13, 227–234. [Google Scholar] [CrossRef]
- Paulson, A.S.; Hess, L.M.; Liepa, A.M.; Cui, Z.L.; Aguilar, K.M.; Clark, J.; Schelman, W. Ramucirumab for the treatment of patients with gastric or gastroesophageal junction cancer in community oncology practices. Gastric Cancer 2018, 21, 831–844. [Google Scholar] [CrossRef]
- Shitara, K.; Doi, T.; Dvorkin, M.; Mansoor, W.; Arkenau, H.-T.; Prokharau, A.; Alsina, M.; Ghidini, M.; Faustino, C.; Gorbunova, V.; et al. Trifluridine/tipiracil versus placebo in patients with heavily pretreated metastatic gastric cancer (TAGS): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. Oncol. 2018, 19, 1437–1448. [Google Scholar] [CrossRef]
- Petrillo, A.; Tirino, G.; Zito Marino, F.; Pompella, L.; Sabetta, R.; Panarese, I.; Pappalardo, A.; Caterino, M.; Ventriglia, A.; Laterza, M.M.; et al. Nivolumab in Heavily Pretreated Metastatic Gastric Cancer Patients: Real-Life Data from a Western Population. Onco. Targets. Ther. 2020, 13, 867–876. [Google Scholar] [CrossRef]
- Fuchs, C.S.; Özgüroğlu, M.; Bang, Y.-J.; Di Bartolomeo, M.; Mandalà, M.; Ryu, M.; Fornaro, L.; Olesinski, T.; Caglevic, C.; Chung, H.C.; et al. Pembrolizumab versus paclitaxel for previously treated patients with PD-L1–positive advanced gastric or gastroesophageal junction cancer (GC): Update from the phase III KEYNOTE-061 trial. J. Clin. Oncol. 2020, 38, 4503. [Google Scholar] [CrossRef]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 Pathways. Am. J. Clin. Oncol. 2016, 39, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Pitt, J.M.; Marabelle, A.; Eggermont, A.; Soria, J.-C.; Kroemer, G.; Zitvogel, L. Targeting the tumor microenvironment: Removing obstruction to anticancer immune responses and immunotherapy. Ann. Oncol. 2016, 27, 1482–1492. [Google Scholar] [CrossRef]
- Gambardella, V.; Castillo, J.; Tarazona, N.; Gimeno-Valiente, F.; Martínez-Ciarpaglini, C.; Cabeza-Segura, M.; Roselló, S.; Roda, D.; Huerta, M.; Cervantes, A.; et al. The role of tumor-associated macrophages in gastric cancer development and their potential as a therapeutic target. Cancer Treat. Rev. 2020, 86, 102015. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Marchesi, F.; Malesci, A.; Laghi, L.; Allavena, P. Tumour-associated macrophages as treatment targets in oncology. Nat. Rev. Clin. Oncol. 2017, 14, 399–416. [Google Scholar] [CrossRef]
- Arai, H.; Battaglin, F.; Wang, J.; Lo, J.H.; Soni, S.; Zhang, W.; Lenz, H.-J. Molecular insight of regorafenib treatment for colorectal cancer. Cancer Treat. Rev. 2019, 81, 101912. [Google Scholar] [CrossRef]
- Fukuoka, S.; Hara, H.; Takahashi, N.; Kojima, T.; Kawazoe, A.; Asayama, M.; Yoshii, T.; Kotani, D.; Tamura, H.; Mikamoto, Y.; et al. Regorafenib Plus Nivolumab in Patients With Advanced Gastric or Colorectal Cancer: An Open-Label, Dose-Escalation, and Dose-Expansion Phase Ib Trial (REGONIVO, EPOC1603). J. Clin. Oncol. 2020, 38, 2053–2061. [Google Scholar] [CrossRef]
- Kawazoe, A.; Fukuoka, S.; Nakamura, Y.; Kuboki, Y.; Mikamoto, Y.; Shima, H.; Fujishiro, N.; Higuchi, T.; Wakabayashi, M.; Nomura, S.; et al. An open-label phase II study of lenvatinib plus pembrolizumab in patients with advanced gastric cancer (EPOC1706). J. Clin. Oncol. 2020, 38, 374. [Google Scholar] [CrossRef]
- Peyraud, F.; Italiano, A. Combined PARP Inhibition and Immune Checkpoint Therapy in Solid Tumors. Cancers (Basel) 2020, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Zitvogel, L.; Ma, Y.; Raoult, D.; Kroemer, G.; Gajewski, T.F. The microbiome in cancer immunotherapy: Diagnostic tools and therapeutic strategies. Science 2018, 359, 1366–1370. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jiang, Z.; Mortenson, E.D.; Deng, L.; Radkevich-Brown, O.; Yang, X.; Sattar, H.; Wang, Y.; Brown, N.K.; Greene, M.; et al. The Therapeutic Effect of Anti-HER2/neu Antibody Depends on Both Innate and Adaptive Immunity. Cancer Cell 2010, 18, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Catenacci, D.V.T.; Kang, Y.-K.; Park, H.; Uronis, H.E.; Lee, K.-W.; Ng, M.C.H.; Enzinger, P.C.; Park, S.H.; Gold, P.J.; Lacy, J.; et al. Margetuximab plus pembrolizumab in patients with previously treated, HER2-positive gastro-oesophageal adenocarcinoma (CP-MGAH22–05): A single-arm, phase 1b–2 trial. Lancet Oncol. 2020, 21, 1066–1076. [Google Scholar] [CrossRef]
- Li, X.-F.; Ren, P.; Shen, W.-Z.; Jin, X.; Zhang, J. The expression, modulation and use of cancer-testis antigens as potential biomarkers for cancer immunotherapy. Am. J. Transl. Res. 2020, 12, 7002–7019. [Google Scholar] [PubMed]
- Nasr, R.; Shamseddine, A.; Mukherji, D.; Nassar, F.; Temraz, S. The Crosstalk between Microbiome and Immune Response in Gastric Cancer. Int. J. Mol. Sci. 2020, 21, 6586. [Google Scholar] [CrossRef] [PubMed]
- Pietrantonio, F.; Randon, G.; Di Bartolomeo, M.; Luciani, A.; Chao, J.; Smyth, E.C.; Petrelli, F. Predictive role of microsatellite instability for of PD-1 blockade in patients with advanced gastric cancer: A meta-analysis of randomized clinical trials. ESMO Open 2021, 6, 100036. [Google Scholar] [CrossRef]
- Murugaesu, N.; Wilson, G.A.; Birkbak, N.J.; Watkins, T.B.K.; McGranahan, N.; Kumar, S.; Abbassi-Ghadi, N.; Salm, M.; Mitter, R.; Horswell, S.; et al. Tracking the Genomic Evolution of Esophageal Adenocarcinoma through Neoadjuvant Chemotherapy. Cancer Discov. 2015, 5, 821–831. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Huang, Y.; Kim, B.Y.S.; Shan, H.; Wu, D.; Jiang, W. Tumor Vasculatures: A New Target for Cancer Immunotherapy. Trends Pharmacol. Sci. 2019, 40, 613–623. [Google Scholar] [CrossRef]
- Hara, H.; Shoji, H.; Takahari, D.; Esaki, T.; Machida, N.; Nagashima, K.; Aoki, K.; Honda, K.; Miyamoto, T.; Boku, N.; et al. Phase I/II study of ramucirumab plus nivolumab in patients in second-line treatment for advanced gastric adenocarcinoma (NivoRam study). J. Clin. Oncol. 2019, 37, 129. [Google Scholar] [CrossRef]
| Biomarker | Method and Interpretation | Clinical Value | Clinical Setting | Strengths | Limitations |
|---|---|---|---|---|---|
| PD-L1 | IHC 22C3 (CDx): positive if CPS ≥ 1 | Predictive (pembrolizumab); prognostic (poor OS) | FDA: advanced or metastatic GC/GEJC treated with ≥2 lines of therapy | Standardized (CDx), reliable | Relatively expensive if CDx, poor inter-observer reproducibility, high intra-tumor heterogeneity |
| MMR | IHC for MLH1, MSH2, MSH6, and PMS2: deficient if lack of expression in ≥1 biomarker | Predictive (pembrolizumab); prognostic (improved OS) | Tissue/site-agnostic: unresectable or metastatic dMMR GC/GEJC progressed following prior treatment | Reliable, cost-effective, short turn-around times | No CDx and interpretation guidelines, no data on intra-tumor heterogeneity |
| MSI | FoundationOne (CDx); MSI-H by PCR or NGS: hyper-variability ≥2 Bethesda (BAT-25, BAT-26, D2S123, D5S346 and D17S250) or Promega (BAT-25, BAT-26, MONO-27, NR-21 and NR-24) loci | Predictive (pembrolizumab and other ICI) prognostic (improved OS); Validated with tumor specific guidelines | Tissue/site-agnostic: unresectable or metastatic MSI-H GC/GEJC progressed following prior treatment | CDx available, cost-effective (PCR or NGS if high volume) | Expensive (CDx or NGS if low volume), externalized analysis (CDx), no tumor-specific guidelines |
| TILs | sTILs on HE-stained sections; modified from International TILs Working Group guidelines for breast carcinoma (% of the tumor stromal area containing infiltrating mononuclear inflammatory cells) | Predictive for immunotherapies (emerging); Prognostic (improved RFS). | Not performed in clinical practice | Cost-effective | Controversial clinical value |
| TMB | FoundationOne (CDx); NGS: TMB-H if >17 mut/MB; SNVs counting by Oncomine Tumor Mutation Load Assay | Predictive for ICB; Prognostic (enhanced ORR and PFS); associated with clinical response to ICI | Not performed in clinical practice | CDx available | Expensive, externalized analysis (CDx), no guidelines, controversial clinical value |
| EBV | cobas EBV (CDx); EBV-encoded RNA ISH | Prognostic (improved OS and decrease of metastases recurrence); associated with amplification and/or overexpression of PD-L1 and PD-L2 in GC; high density of immune cell infiltration; alterations in the PIK3CA gene | Diagnostic/subtyping | Standardized and cost-effective | Not available in all centers |
| Study Name (NCT Number) | Country | Phase | Line | N | Drugs (Target) | Selected Population | Study Intervention I Experimental Arm/Control Arm or II Experimental Arm | Primary Endpoint |
|---|---|---|---|---|---|---|---|---|
| Non-metastatic gastric cancer | ||||||||
| Keynote-585 (NCT03221426) | Global | III | Perioperative | NA | Pembrolizumab (PD-1) | All comers | fluorouracil/capecitabine plus cisplatin or FLOT +/- pembrolizumab | OS EFS pCR |
| IMAGINE (NCT04062656) | Western | rII | Perioperative | NA | Nivolumab (PD-1) Ipilimumab (CTLA-4) Relatlimab (LAG-3) | All comers | FLOT Nivolumab Nivolumab + ipilimumab Nivolumab + relatlimab | pCR |
| NCT04354662 | Asian | II | Perioperative | NA | Toripalimab (PD-1) | All comers | FLOT + toripalimab | DFS |
| ICONIC (NCT03399071) | Western | II | Perioperative | NA | Avelumab (PD-L1) | All comers | FLOT + avelumab | pCR |
| NCT03878472 | Asian | II | Neoadjuvant | NA | SHR1210 (PD-1) | All comers | SHR1210 SHR1210 + Apatinib SHR1210 + Apatinib + S-1 SHR1210 + Apatinib+ S-1 + oxaliplatin | pRR |
| Checkmate-577 (NCT02743494) | Global | III | Adjuvant | 794 | Nivolumab (PD-1) | All comers | Nivolumab versus placebo after neoadjuvant chemoradiotherapy and surgery | DFS |
| EORTC VESTIGE (NCT03443856) | Western | rII | Adjuvant | NA | Nivolumab (PD-1) Ipilimumab (CTLA-4) | All comers | Nivolumab + ipilimumab versus FLOT after neoadjuvant FLOT and surgery | DFS |
| Metastatic gastric cancer | ||||||||
| Keynote-859 (NCT03675737) | Global | III | 1° | NA | Pembrolizumab (PD-1) | HER-2 negative | cisplatin plus 5-fluorouracil/Xelox +/- pembrolizumab | OS PFS |
| Keynote-811 (NCT03615326) | Global | III | 1° | NA | Pembrolizumab (PD-1) | HER-2 positive | cisplatin plus 5-fluorouracil/Xelox/Folfox/S-1 oxaliplatin + trastuzumab +/- pembrolizumab | PFS OS |
| APICAL-GE (NCT04278222) | Asian | II | 1° | NA | Toripalimab (PD-1) | MSS | Anlotinib Plus Toripalimab | ORR |
| NCT04202484 | Asian | II | 1° | NA | Toripalimab (PD-1) | HER-2 negative | Toripalimab combined with oxaliplatin and Tegafur, Gimeracil and Oteracil Porassium Capsules | ORR |
| SHR-1210-III-316 (NCT04342910) | China | III | 2° | 550 | Camrelizumab (PD-1) Apatinib (VEGFR2) | All comers | Camrelizumab + apatinib paclitaxel or irinotecan | OS |
| NCT04435652 | Asia | II-III | 2° | 492 | QL1604 (PD-1) | HER-2 negative | QL1604 + nab-paclitaxel followed by QL1604 maintenance paclitaxel alone | ORR, safety, OS |
| SEQUEL (NCT04069273) | USA | rII | ≥2° | 58 | Pembrolizumab (PD-1) Ramucirumab (VEGFR2) | All comers | Paclitaxel + ramucirumab + pembrolizumab (patient-tailored algorithm) Paclitaxel + ramucirumab + pembrolizumab | ORR |
| DURIGAST (PRODIGE59-FFCD1707) (NCT03959293) | France | rII | 2° | 105 | Durvalumab (PD-L1) Tremelimumab (CTLA-4) | All comers | FOLFIRI + durvalumab + tremelimumab FOLFIRI + durvalumab | PFS |
| ESR-15-11655 (NCT03579784) | Korea | II | 2° | 40 | Durvalumab (PD-1) Olaparib (PARP) | All comers | Paclitaxel + olaparib + durvalumab | DCR |
| NCC2070 (NCT04140318) | China | II | 2° | 60 | Sintilimab (PD-1) | All comers | Nab-paclitaxel + sintilimab | ORR |
| ASGARD (NCT04089657) | China | II | ≥3° | 40 | Sintilimab (PD-1) Apatinib (VEGFR2) | All comers | Apatinib + sintilimab | DCR |
| RiME (NCT03995017) | USA | II | 2°–3° | 61 | Nivolumab (PD-1) Rucaparib (PARP) Ramucirumab (VEGFR2) | All comers | Rucaparib + ramucirumab + nivolumab Rucaparib + ramucirumab | ORR |
| RAP (AIO-STO-0218) (NCT03966118) | Germany | II | 2° | 59 | Avelumab (PD-1) Ramucirumab (VEGFR2) | All comers | Paclitaxel + ramucirumab + avelumab | OS |
| WaKING (NCT04166721) | UK | II | ≥2° | 52 | Atezolizumab (PD-L1) DKN-01 (DKK1) | MSS/pMMR | Atezolizumab + DKN-01 | Safety, ORR |
| NCT03694977 | Korea | II | >2° | 30 | Lacnotuzumab (CSF-1) Spartalizumab (PD-1) | All comers | Lacnotuzumab + Spartalizumab | Biomarker analysis |
| NCT04592211 | Korea | I-II | 2° | 71 | Pembrolizumab (PD-1) Olaparib (PARP) | HRR/MSS | Pembrolizumab + olaparib + paclitaxel | PFS DLT |
| NCT04209686 | Australia, USA | II | 2° | 36 | Pembrolizumab (PD-1) Olaparib (PARP) | All comers | Pembrolizumab + olaparib + paclitaxel | OS |
| da VINci (NCT03784040) | Asia | Ib | >2° | 40 | OTSGC-A24 (cancer vaccine) Nivolumab (PD-1) Ipilimumab (CTLA-4) | All comers | OTSGC-A24 + nivolumab OTSGC-A24 + nivolumab + ipilimumab | Safety, ORR |
| Study Name [Reference] | Agents (Target) | Country | Phase | Line | PD-L1 Status | Treatment Arms | N | Primary Endpoints | OS | PFS | RR (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Keynote-062 [62] | Pembrolizumab (PD-1) | Global | III | 1° | CPS ≥ 1% | cisplatin + 5-fluorouracil/capecitabine (CT) | 250 | OS, PFS | Non-inferiority: 10.6 (I) vs. 11 (CT) Superiority: 12.5 (CT + I) vs. 11.1 (CT) | Superiority: 6.9 (CT + I) vs. 6.4 (CT) | 48.6 (CT + I) 37.2 (CT) |
| cisplatin + 5-fluorouracil /capecitabine + pembrolizumab (CT + I) | 257 | ||||||||||
| pembrolizumab (I) | 256 | ||||||||||
| Checkmate-649 (preliminary results) [98] | Nivolumab (PD-1) Ipilimumab (CTLA-4) | Global | III | 1° | Unselected | nivolumab + ipilimumab | OS, PFS | CPS ≥ 5%: 7.7 6.1 | NR | ||
| Xelox/Folfox | 482 | 14 | |||||||||
| Xelox/Folfox + nivolumab | 473 | 11.3 | |||||||||
| Attraction-4 (preliminary results) [99] | Nivolumab (PD-1) | Asian | III | 1° | Unselected | Nivolumab + S-1 oxaliplatin/Xelox | 362 | PFS, OS | 17.5 | 10.5 | NR |
| S-1 oxaliplatin/Xelox | 362 | 17.2 | 8.3 | ||||||||
| Janjigian et al. [100] | Pembrolizumab (PD-1) | Global | II | 1° | Unselected | Xelox/Folfox/cisplatin plus 5-fluorouracil+ trastuzumab+ pembrolizumab | 37 | PFS at 6 months | 27.3 | 13 | 100 |
| Javelin Gastric 100 [101] | Avelumab (PD-L1) | Global | III | 1°mantainance | Unselected | Avelumab | 249 | OS | 10.4 | 3.2 | 13.3 |
| Folfox/Xelox | 250 | 10.9 | 4.4 | 14.4 | |||||||
| Keynote-061 [102] | Pembrolizumab (PD-1) | Global | III | 2° | CPS ≥ 1% | Pembrolizumab | 196 | PFS, OS | 9.1 | 1.5 | 16 |
| Paclitaxel | 199 | 8.3 | 4.1 | 14 | |||||||
| Keynote-059 (cohort 1) [54] | Pembrolizumab (PD-1) | Global | II | ≥3° | Unselected (57.1% CPS ≥ 1%) | Pembrolizumab | 259 | RR | 5.6 | 2 | 11.6 |
| Attraction-02 (ONO-4538-12) [103] | Nivolumab (PD-1) | Asian | III | ≥3° | Unselected | Nivolumab | 330 | OS | 7.5 | 1.6 | 11 |
| Placebo | 163 | 5.1 | 1.5 | 20 | |||||||
| Checkmate-032 [104] | Nivolumab (PD-1) Ipilimumab (CTLA-4) | Western | I-II | ≥3° | Unselected | Nivolumab | 59 | RR | 6.2 | 1.4 | 12 |
| Nivolumab1/Ipilimumab3 * | 49 | 6.9 | 1.6 | 24 | |||||||
| Nivolumab3/Ipilimumab1 ** | 52 | 4.8 | 1.6 | 8 | |||||||
| Javelin Gastric 300 [105] | Avelumab (PD-L1) | Global | III | 3° | TPS ≥ 1% | Avelumab | 272 | OS | 4.6 | 1.4 | 4.6 |
| Physician’s choice ‡ | 133 | 5 | 2.7 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghidini, M.; Petrillo, A.; Botticelli, A.; Trapani, D.; Parisi, A.; La Salvia, A.; Sajjadi, E.; Piciotti, R.; Fusco, N.; Khakoo, S. How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches. J. Clin. Med. 2021, 10, 1412. https://doi.org/10.3390/jcm10071412
Ghidini M, Petrillo A, Botticelli A, Trapani D, Parisi A, La Salvia A, Sajjadi E, Piciotti R, Fusco N, Khakoo S. How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches. Journal of Clinical Medicine. 2021; 10(7):1412. https://doi.org/10.3390/jcm10071412
Chicago/Turabian StyleGhidini, Michele, Angelica Petrillo, Andrea Botticelli, Dario Trapani, Alessandro Parisi, Anna La Salvia, Elham Sajjadi, Roberto Piciotti, Nicola Fusco, and Shelize Khakoo. 2021. "How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches" Journal of Clinical Medicine 10, no. 7: 1412. https://doi.org/10.3390/jcm10071412
APA StyleGhidini, M., Petrillo, A., Botticelli, A., Trapani, D., Parisi, A., La Salvia, A., Sajjadi, E., Piciotti, R., Fusco, N., & Khakoo, S. (2021). How to Best Exploit Immunotherapeutics in Advanced Gastric Cancer: Between Biomarkers and Novel Cell-Based Approaches. Journal of Clinical Medicine, 10(7), 1412. https://doi.org/10.3390/jcm10071412










