Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives
Abstract
1. Introduction
2. Clinical Presentation
2.1. Autonomic Neuropathy in Neurological Disorders
2.2. Clinical Presentation of Autonomic Neuropathy in General
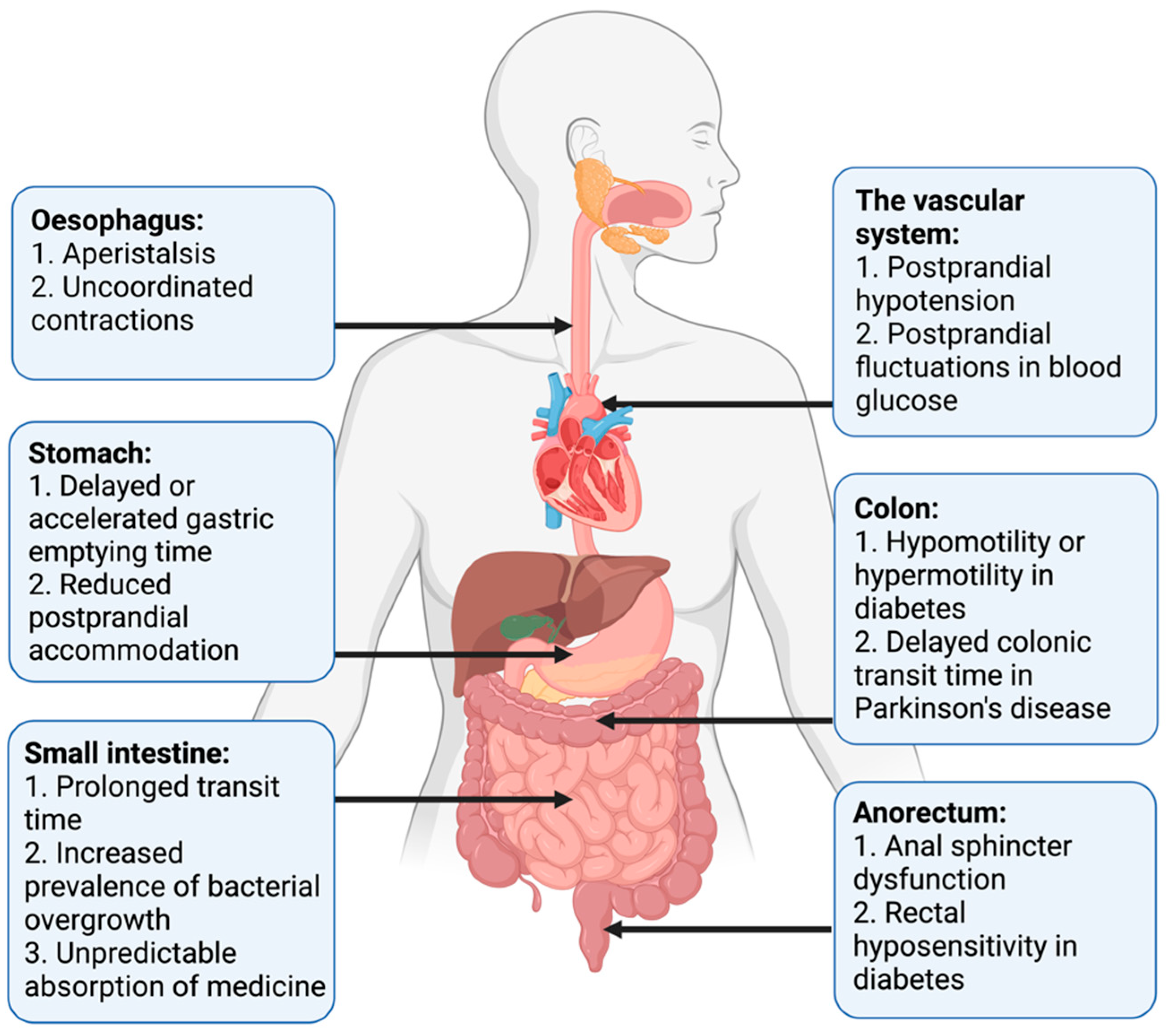
2.3. Clinical Presentation of Gastrointestinal Autonomic Neuropathy
3. Established Methods for Assessment of Gastroenteropathy
3.1. Exclusion of Differential Diagnoses
3.2. Assessment of Symptoms
3.3. Tests of Esophageal Motility
3.4. Gastric Emptying Tests
3.5. Assessment of Gastric and Small Intestinal Motility
3.6. Tests of Small Intestinal and Colonic Transit
3.7. Assessment of Small Intestinal Bacterial Overgrowth
3.8. Tests of Anorectal Motility
3.9. Whole Gut Assessment
4. Emerging Methods for Assessment of Gastroenteropathy
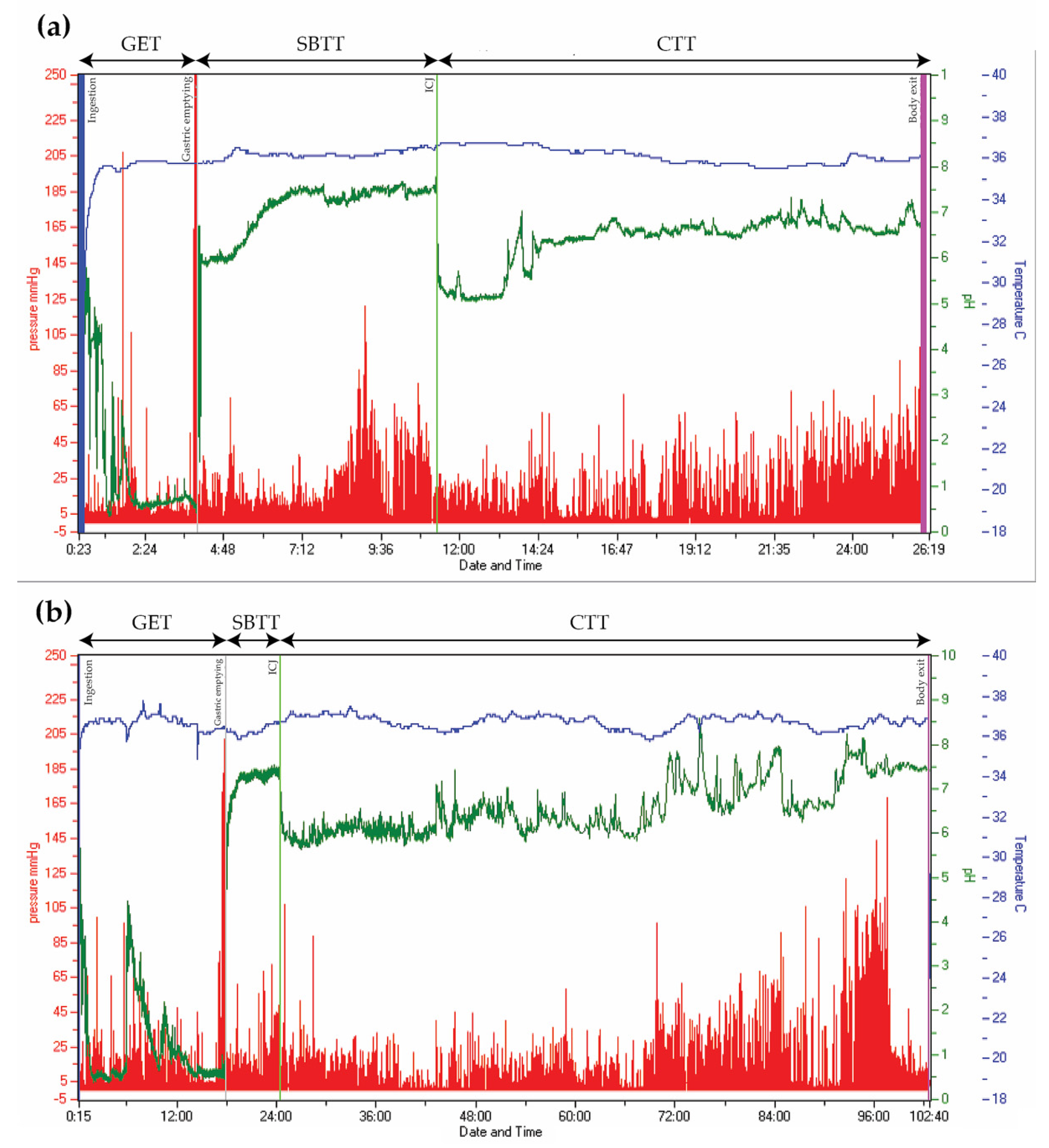
4.1. Whole Gut Assessment
4.2. Tests of Colorectal Contractions
4.3. Imaging
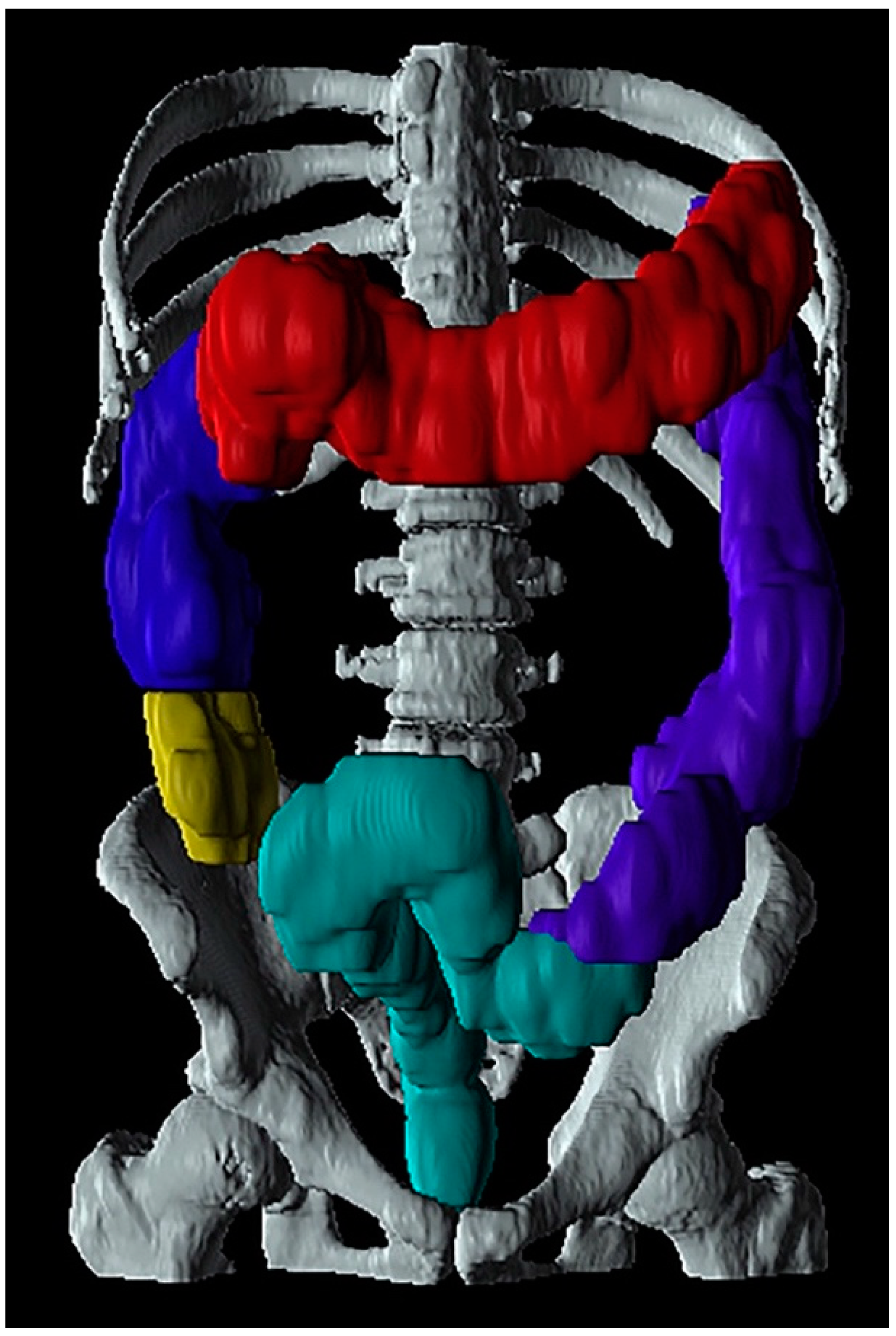
4.3.1. Computed Tomography (CT)
4.3.2. Ultrasound Imaging
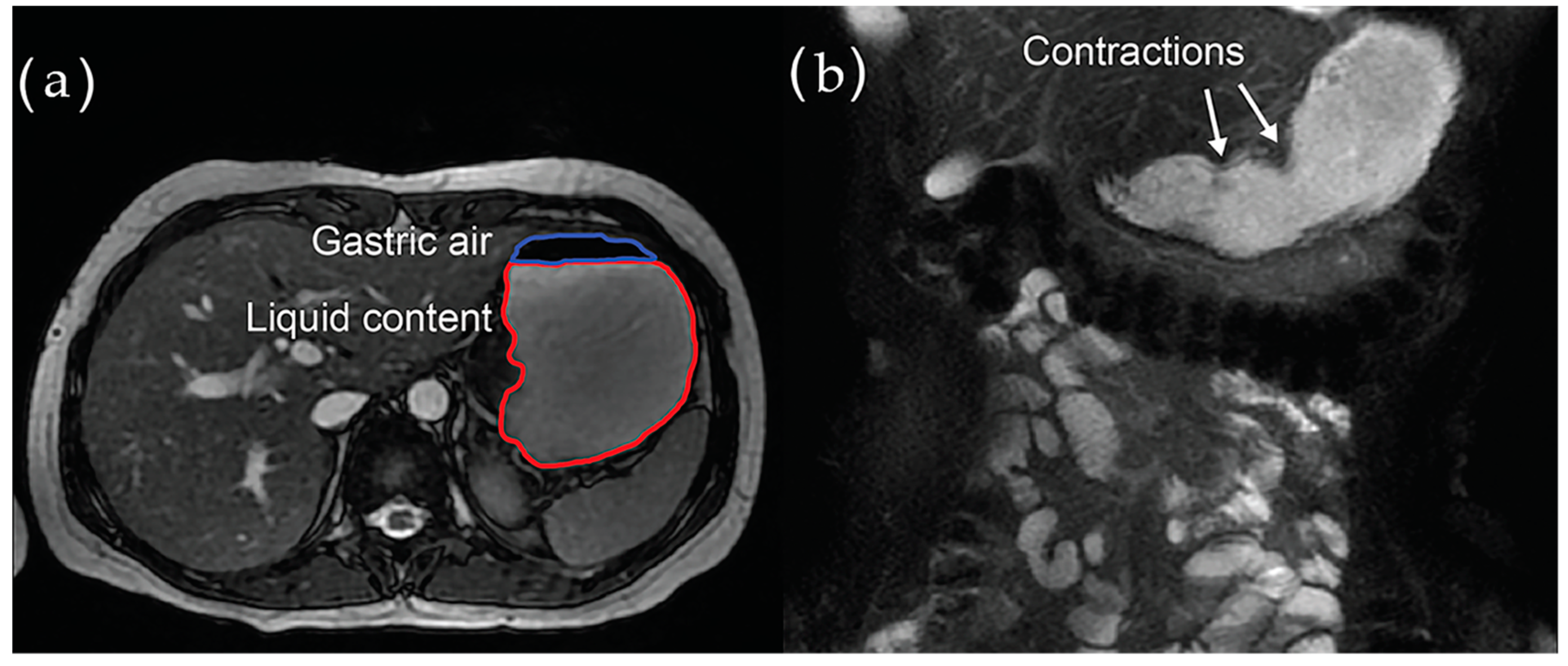
4.3.3. Magnetic Resonance Imaging (MRI)
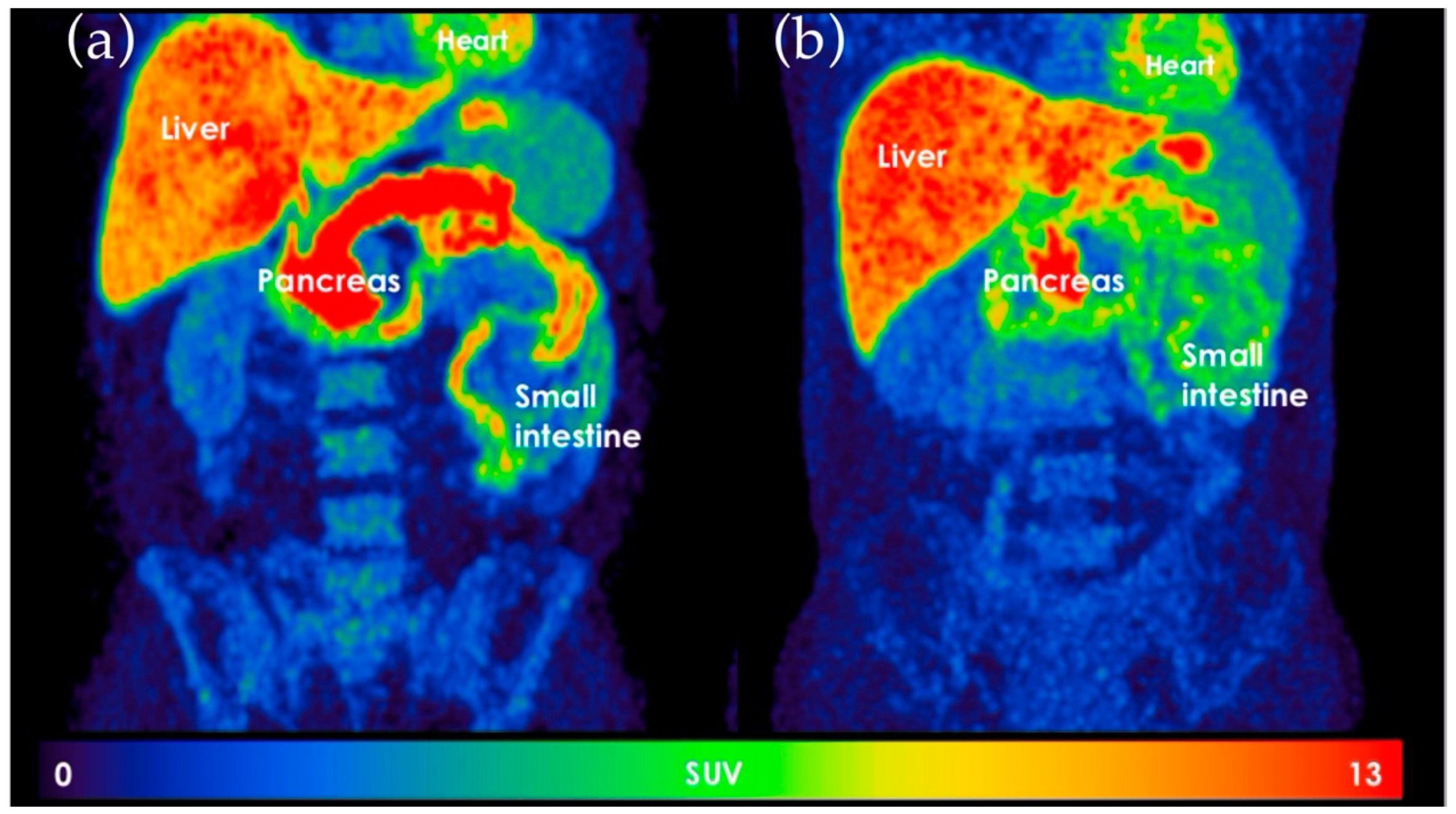
4.3.4. 11C-Donepezil Positron Emission Tomography/Computed Tomography (PET/CT)
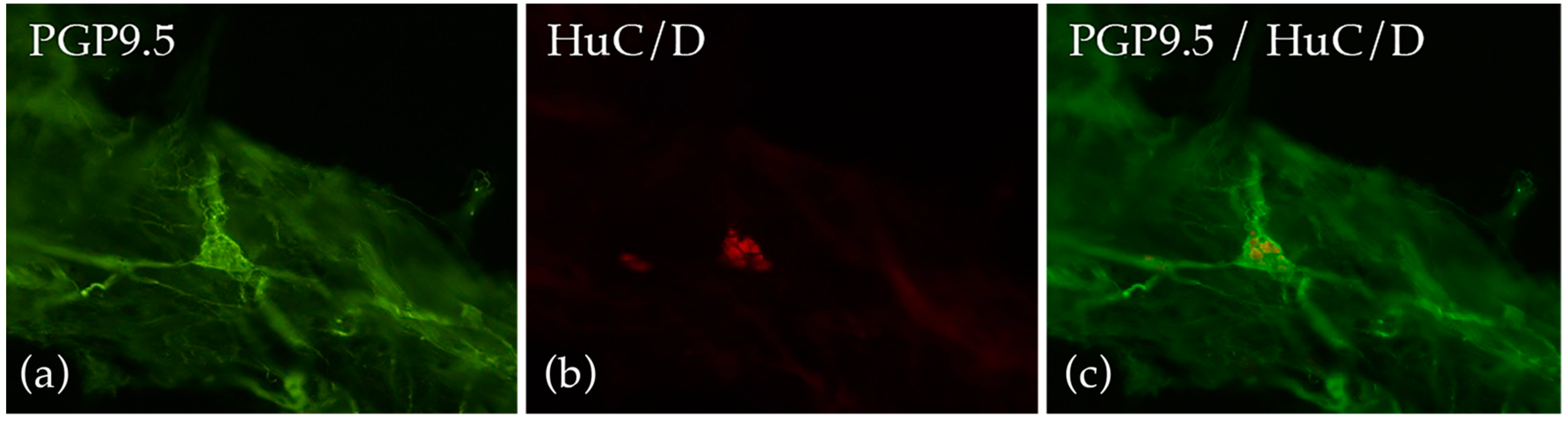
4.4. Gut Biopsies
4.5. Assessment of the Human Gut Microbiota
5. Assessment of Autonomic Neuropathy Outside the Gut
6. Assessment-Guided Treatment
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Benarroch, E.E. Physiology and Pathophysiology of the Autonomic Nervous System. Continuum 2020, 26, 12–24. [Google Scholar] [CrossRef]
- Mathias, C.J.B.R. Autonomic Failure. A Textbook of Clinical Disorders of the Autonomic Nervous System, 5th ed.; University Press: Oxford, UK, 2013. [Google Scholar]
- Chung, K.A.; Pfeiffer, R.F. Gastrointestinal dysfunction in the synucleinopathies. Clin. Auton. Res. 2020. [Google Scholar] [CrossRef]
- Müller, T.; Erdmann, C.; Bremen, D.; Schmidt, W.E.; Muhlack, S.; Woitalla, D.; Goetze, O. Impact of gastric emptying on levodopa pharmacokinetics in Parkinson disease patients. Clin. Neuropharmacol. 2006, 29, 61–67. [Google Scholar] [CrossRef]
- Knudsen, K.; Fedorova, T.D.; Bekker, A.C.; Iversen, P.; Østergaard, K.; Krogh, K.; Borghammer, P. Objective Colonic Dysfunction is Far more Prevalent than Subjective Constipation in Parkinson’s Disease: A Colon Transit and Volume Study. J. Park. Dis. 2017, 7, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, C.; Arora, Z.; Thota, P.N.; Lopez, R.; Santisi, J.; Funk, C.; Cline, M. Role of wireless motility capsule in the assessment and management of gastrointestinal dysmotility in patients with diabetes mellitus. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Gustafsson, R.J.; Littorin, B.; Berntorp, K.; Frid, A.; Thorsson, O.; Olsson, R.; Ekberg, O.; Ohlsson, B. Esophageal dysmotility is more common than gastroparesis in diabetes mellitus and is associated with retinopathy. Rev. Diabet. Stud. 2011, 8, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Bharucha, A.E.; Camilleri, M.; Forstrom, L.A.; Zinsmeister, A.R. Relationship between clinical features and gastric emptying disturbances in diabetes mellitus. Clin. Endocrinol. 2009, 70, 415–420. [Google Scholar] [CrossRef]
- Karemaker, J.M. An introduction into autonomic nervous function. Physiol. Meas. 2017, 38, R89–R118. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Brookes, S.J. The enteric nervous system. Am. J. Gastroenterol. 1994, 89, S129–S137. [Google Scholar] [CrossRef]
- Meldgaard, T.; Olesen, S.S.; Farmer, A.D.; Krogh, K.; Wendel, A.A.; Brock, B.; Drewes, A.M.; Brock, C. Diabetic Enteropathy: From Molecule to Mechanism-Based Treatment. J. Diabetes Res. 2018, 2018, 3827301. [Google Scholar] [CrossRef]
- Gibbons, C.H.; Freeman, R. Treatment-induced neuropathy of diabetes: An acute, iatrogenic complication of diabetes. Brain 2015, 138, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Coon, E.A.; Cutsforth-Gregory, J.K.; Benarroch, E.E. Neuropathology of autonomic dysfunction in synucleinopathies. Mov. Disord. 2018, 33, 349–358. [Google Scholar] [CrossRef]
- Terkelsen, A.J.; Karlsson, P.; Lauria, G.; Freeman, R.; Finnerup, N.B.; Jensen, T.S. The diagnostic challenge of small fibre neuropathy: Clinical presentations, evaluations, and causes. Lancet Neurol. 2017, 16, 934–944. [Google Scholar] [CrossRef]
- Benarroch, E.E. Postural tachycardia syndrome: A heterogeneous and multifactorial disorder. Mayo Clin. Proc. 2012, 87, 1214–1225. [Google Scholar] [CrossRef]
- Weinstock, L.B.; Pace, L.A.; Rezaie, A.; Afrin, L.B.; Molderings, G.J. Mast Cell Activation Syndrome: A Primer for the Gastroenterologist. Dig. Dis. Sci. 2020. [Google Scholar] [CrossRef] [PubMed]
- Beckers, A.B.; Keszthelyi, D.; Fikree, A.; Vork, L.; Masclee, A.; Farmer, A.D.; Aziz, Q. Gastrointestinal disorders in joint hypermobility syndrome/Ehlers-Danlos syndrome hypermobility type: A review for the gastroenterologist. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Benarroch, E.E. The clinical approach to autonomic failure in neurological disorders. Nat. Rev. Neurol. 2014, 10, 396–407. [Google Scholar] [CrossRef] [PubMed]
- Soedamah-Muthu, S.S.; Chaturvedi, N.; Witte, D.R.; Stevens, L.K.; Porta, M.; Fuller, J.H. Relationship between risk factors and mortality in type 1 diabetic patients in Europe: The EURODIAB Prospective Complications Study (PCS). Diabetes Care 2008, 31, 1360–1366. [Google Scholar] [CrossRef]
- Spallone, V. Update on the Impact, Diagnosis and Management of Cardiovascular Autonomic Neuropathy in Diabetes: What Is Defined, What Is New, and What Is Unmet. Diabetes Metab J. 2019, 43, 3–30. [Google Scholar] [CrossRef]
- Bharucha, A.E.; Kudva, Y.C.; Prichard, D.O. Diabetic Gastroparesis. Endocr. Rev. 2019, 40, 1318–1352. [Google Scholar] [CrossRef]
- Bytzer, P.; Talley, N.J.; Leemon, M.; Young, L.J.; Jones, M.P.; Horowitz, M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: A population-based survey of 15,000 adults. Arch. Int. Med. 2001, 161, 1989–1996. [Google Scholar] [CrossRef] [PubMed]
- Talley, N.J.; Young, L.; Bytzer, P.; Hammer, J.; Leemon, M.; Jones, M.; Horowitz, M. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am. J. Gastroenterol. 2001, 96, 71–76. [Google Scholar] [CrossRef]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef]
- Bytzer, P.; Talley, N.J.; Hammer, J.; Young, L.J.; Jones, M.P.; Horowitz, M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am. J. Gastroenterol. 2002, 97, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Feldman, M.; Schiller, L.R. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann. Intern. Med. 1983, 98, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.; Krogh, K.; Østergaard, K.; Borghammer, P. Constipation in parkinson’s disease: Subjective symptoms, objective markers, and new perspectives. Mov. Disord. 2017, 32, 94–105. [Google Scholar] [CrossRef]
- Verbaan, D.; Marinus, J.; Visser, M.; van Rooden, S.M.; Stiggelbout, A.M.; van Hilten, J.J. Patient-reported autonomic symptoms in Parkinson disease. Neurology 2007, 69, 333–341. [Google Scholar] [CrossRef]
- Wang, L.B.; Culbertson, C.J.; Deb, A.; Morgenshtern, K.; Huang, H.; Hohler, A.D. Gastrointestinal dysfunction in postural tachycardia syndrome. J. Neurol. Sci. 2015, 359, 193–196. [Google Scholar] [CrossRef] [PubMed]
- DiBaise, J.K.; Harris, L.A.; Goodman, B. Postural Tachycardia Syndrome (POTS) and the GI Tract: A Primer for the Gastroenterologist. Am. J. Gastroenterol. 2018, 113, 1458–1467. [Google Scholar] [CrossRef]
- Claus, I.; Suttrup, J.; Muhle, P.; Suntrup-Krueger, S.; Siemer, M.L.; Lenze, F.; Dziewas, R.; Warnecke, T. Subtle Esophageal Motility Alterations in Parkinsonian Syndromes: Synucleinopathies vs. Tauopathies. Mov. Disord. Clin. Pract. 2018, 5, 406–412. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.; Borghammer, P. Imaging the Autonomic Nervous System in Parkinson’s Disease. Curr. Neurol. Neurosci. Rep. 2018, 18, 79. [Google Scholar] [CrossRef]
- Brock, C.; Søfteland, E.; Gunterberg, V.; Frøkjær, J.B.; Lelic, D.; Brock, B.; Dimcevski, G.; Gregersen, H.; Simrén, M.; Drewes, A.M. Diabetic autonomic neuropathy affects symptom generation and brain-gut axis. Diabetes Care 2013, 36, 3698–3705. [Google Scholar] [CrossRef]
- Azpiroz, F.; Malagelada, C. Diabetic neuropathy in the gut: Pathogenesis and diagnosis. Diabetologia 2016, 59, 404–408. [Google Scholar] [CrossRef]
- Farmer, A.D.; Pedersen, A.G.; Brock, B.; Jakobsen, P.E.; Karmisholt, J.; Mohammed, S.D.; Scott, S.M.; Drewes, A.M.; Brock, C. Type 1 diabetic patients with peripheral neuropathy have pan-enteric prolongation of gastrointestinal transit times and an altered caecal pH profile. Diabetologia 2017, 60, 709–718. [Google Scholar] [CrossRef] [PubMed]
- Pavelić, A.; Krbot Skorić, M.; Crnošija, L.; Habek, M. Postprandial hypotension in neurological disorders: Systematic review and meta-analysis. Clin. Auton. Res. 2017, 27, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Sletten, D.M.; Suarez, G.A.; Low, P.A.; Mandrekar, J.; Singer, W. COMPASS 31: A refined and abbreviated Composite Autonomic Symptom Score. Mayo Clin. Proc. 2012, 87, 1196–1201. [Google Scholar] [CrossRef]
- Rentz, A.M.; Kahrilas, P.; Stanghellini, V.; Tack, J.; Talley, N.J.; de la Loge, C.; Trudeau, E.; Dubois, D.; Revicki, D.A. Development and psychometric evaluation of the patient assessment of upper gastrointestinal symptom severity index (PAGI-SYM) in patients with upper gastrointestinal disorders. Qual. Life Res. 2004, 13, 1737–1749. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; Rentz, A.M.; Dubois, D.; Kahrilas, P.; Stanghellini, V.; Talley, N.J.; Tack, J. Gastroparesis Cardinal Symptom Index (GCSI): Development and validation of a patient reported assessment of severity of gastroparesis symptoms. Qual. Life Res. Int. J. Qual. Life Asp. Treat. Care Rehabil. 2004, 13, 833–844. [Google Scholar] [CrossRef]
- Nilsson, M.; Poulsen, J.L.; Brock, C.; Sandberg, T.H.; Gram, M.; Frokjaer, J.B.; Krogh, K.; Drewes, A.M. Opioid-induced bowel dysfunction in healthy volunteers assessed with questionnaires and MRI. Eur. J. Gastroenterol. Hepatol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Jones, K.L.; Russo, A.; Stevens, J.E.; Wishart, J.M.; Berry, M.K.; Horowitz, M. Predictors of delayed gastric emptying in diabetes. Diabetes Care 2001, 24, 1264–1269. [Google Scholar] [CrossRef]
- Cassilly, D.W.; Wang, Y.R.; Friedenberg, F.K.; Nelson, D.B.; Maurer, A.H.; Parkman, H.P. Symptoms of gastroparesis: Use of the gastroparesis cardinal symptom index in symptomatic patients referred for gastric emptying scintigraphy. Digestion 2008, 78, 144–151. [Google Scholar] [CrossRef]
- Kulich, K.R.; Madisch, A.; Pacini, F.; Piqué, J.M.; Regula, J.; Van Rensburg, C.J.; Ujszászy, L.; Carlsson, J.; Halling, K.; Wiklund, I.K. Reliability and validity of the Gastrointestinal Symptom Rating Scale (GSRS) and Quality of Life in Reflux and Dyspepsia (QOLRAD) questionnaire in dyspepsia: A six-country study. Health Qual. Life Outcomes 2008, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Revicki, D.A.; Wood, M.; Wiklund, I.; Crawley, J. Reliability and validity of the Gastrointestinal Symptom Rating Scale in patients with gastroesophageal reflux disease. Qual. Life Res. 1998, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Wegeberg, A.L.; Brock, C.; Ejskjaer, N.; Karmisholt, J.S.; Jakobsen, P.E.; Drewes, A.M.; Brock, B.; Farmer, A.D. Gastrointestinal symptoms and cardiac vagal tone in type 1 diabetes correlates with gut transit times and motility index. Neurogastroenterol. Motil. 2020, e13885. [Google Scholar] [CrossRef]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef] [PubMed]
- Simren, M.; Palsson, O.S.; Whitehead, W.E. Update on Rome IV Criteria for Colorectal Disorders: Implications for Clinical Practice. Curr. Gastroenterol. Rep. 2017, 19, 15. [Google Scholar] [CrossRef]
- Quan, C.; Talley, N.J.; Cross, S.; Jones, M.; Hammer, J.; Giles, N.; Horowitz, M. Development and validation of the Diabetes Bowel Symptom Questionnaire. Aliment. Pharmacol. Ther. 2003, 17, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Kahrilas, P.J.; Bredenoord, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.J.; Pandolfino, J.E. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015, 27, 160–174. [Google Scholar] [CrossRef]
- Dhawan, I.; O’Connell, B.; Patel, A.; Schey, R.; Parkman, H.P.; Friedenberg, F. Utility of Esophageal High-Resolution Manometry in Clinical Practice: First, Do HRM. Dig. Dis. Sci. 2018, 63, 3178–3186. [Google Scholar] [CrossRef]
- Yadlapati, R. High-resolution esophageal manometry: Interpretation in clinical practice. Curr. Opin. Gastroenterol. 2017, 33, 301–309. [Google Scholar] [CrossRef]
- George, N.S.; Rangan, V.; Geng, Z.; Khan, F.; Kichler, A.; Gabbard, S.; Ganocy, S.; Fass, R. Distribution of Esophageal Motor Disorders in Diabetic Patients With Dysphagia. J. Clin. Gastroenterol. 2017, 51, 890–895. [Google Scholar] [CrossRef]
- Huang, R.J.; Chun, C.L.; Friday, K.; Triadafilopoulos, G. Manometric abnormalities in the postural orthostatic tachycardia syndrome: A case series. Dig. Dis. Sci. 2013, 58, 3207–3211. [Google Scholar] [CrossRef]
- Fikree, A.; Aziz, Q.; Sifrim, D. Mechanisms underlying reflux symptoms and dysphagia in patients with joint hypermobility syndrome, with and without postural tachycardia syndrome. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Martin-Harris, B.; Canon, C.L.; Bonilha, H.S.; Murray, J.; Davidson, K.; Lefton-Greif, M.A. Best Practices in Modified Barium Swallow Studies. Am. J. Speech Lang. Pathol. 2020, 29, 1078–1093. [Google Scholar] [CrossRef]
- Schiffer, B.L.; Kendall, K. Changes in Timing of Swallow Events in Parkinson’s Disease. Ann. Otol. Rhinol. Laryngol. 2019, 128, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Randall, D.R.; Evangelista, L.M.; Kuhn, M.A.; Belafsky, P.C. Subjective Assessment of Videofluoroscopic Swallow Studies. Otolaryngol. Head Neck Surg. 2017, 156, 901–905. [Google Scholar] [CrossRef] [PubMed]
- Alomari, M.; Hitawala, A.; Chadalavada, P.; Covut, F.; Al Momani, L.; Khazaaleh, S.; Gosai, F.; Al Ashi, S.; Abushahin, A.; Schneider, A. Prevalence and Predictors of Gastrointestinal Dysmotility in Patients with Hypermobile Ehlers-Danlos Syndrome: A Tertiary Care Center Experience. Cureus 2020, 12, e7881. [Google Scholar] [CrossRef] [PubMed]
- Abell, T.L.; Camilleri, M.; Donohoe, K.; Hasler, W.L.; Lin, H.C.; Maurer, A.H.; McCallum, R.W.; Nowak, T.; Nusynowitz, M.L.; Parkman, H.P.; et al. Consensus recommendations for gastric emptying scintigraphy: A joint report of the American Neurogastroenterology and Motility Society and the Society of Nuclear Medicine. Am. J. Gastroenterol. 2008, 103, 753–763. [Google Scholar] [CrossRef]
- Rao, S.S.; Camilleri, M.; Hasler, W.L.; Maurer, A.H.; Parkman, H.P.; Saad, R.; Scott, M.S.; Simren, M.; Soffer, E.; Szarka, L. Evaluation of gastrointestinal transit in clinical practice: Position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol. Motil. 2011, 23, 8–23. [Google Scholar] [CrossRef]
- Madsen, J.L. Scintigraphic assessment of gastrointestinal motility: A brief review of techniques and data interpretation. Clin. Physiol. Funct. Imaging 2014, 34, 243–253. [Google Scholar] [CrossRef]
- Thomaides, T.; Karapanayiotides, T.; Zoukos, Y.; Haeropoulos, C.; Kerezoudi, E.; Demacopoulos, N.; Floodas, G.; Papageorgiou, E.; Armakola, F.; Thomopoulos, Y.; et al. Gastric emptying after semi-solid food in multiple system atrophy and Parkinson disease. J. Neurol. 2005, 252, 1055–1059. [Google Scholar] [CrossRef] [PubMed]
- Loavenbruck, A.; Iturrino, J.; Singer, W.; Sletten, D.M.; Low, P.A.; Zinsmeister, A.R.; Bharucha, A.E. Disturbances of gastrointestinal transit and autonomic functions in postural orthostatic tachycardia syndrome. Neurogastroenterol. Motil. 2015, 27, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Ghoos, Y.F.; Maes, B.D.; Geypens, B.J.; Mys, G.; Hiele, M.I.; Rutgeerts, P.J.; Vantrappen, G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology 1993, 104, 1640–1647. [Google Scholar] [CrossRef]
- Zahn, A.; Langhans, C.D.; Hoffner, S.; Haberkorn, U.; Rating, D.; Haass, M.; Enck, P.; Stremmel, W.; Ruhl, A. Measurement of gastric emptying by 13C-octanoic acid breath test versus scintigraphy in diabetics. Z. Gastroenterol. 2003, 41, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Kato, T.; Nishida, H.; Yamada, M.; Koumura, A.; Sakurai, T.; Hayashi, Y.; Kimura, A.; Hozumi, I.; Araki, H.; et al. Is there delayed gastric emptying in patients with multiple system atrophy? An analysis using the (13)C-acetate breath test. J. Neurol. 2012, 259, 1448–1452. [Google Scholar] [CrossRef]
- Knudsen, K.; Szwebs, M.; Hansen, A.K.; Borghammer, P. Gastric emptying in Parkinson’s disease-A mini-review. Parkinsonism. Relat. Disord. 2018, 55, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, M.; Bharucha, A.E.; di Lorenzo, C.; Hasler, W.L.; Prather, C.M.; Rao, S.S.; Wald, A. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol. Motil. 2008, 20, 1269–1282. [Google Scholar] [CrossRef]
- Samsom, M.; Jebbink, R.J.; Akkermans, L.M.; van Berge-Henegouwen, G.P.; Smout, A.J. Abnormalities of antroduodenal motility in type I diabetes. Diabetes Care 1996, 19, 21–27. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Gonlachanvit, S. Technique of functional and motility test: How to perform antroduodenal manometry. J. Neurogastroenterol. Motil. 2013, 19, 395–404. [Google Scholar] [CrossRef]
- Penning, C.; Gielkens, H.A.; Hemelaar, M.; Lamers, C.B.; Masclee, A.A. Reproducibility of antroduodenal motility during prolonged ambulatory recording. Neurogastroenterol. Motil. 2001, 13, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Bortolotti, M.; Annese, V.; Coccia, G. Twenty-four hour ambulatory antroduodenal manometry in normal subjects (co-operative study). Neurogastroenterol. Motil. 2000, 12, 231–238. [Google Scholar] [CrossRef]
- Desipio, J.; Friedenberg, F.K.; Korimilli, A.; Richter, J.E.; Parkman, H.P.; Fisher, R.S. High-resolution solid-state manometry of the antropyloroduodenal region. Neurogastroenterol. Motil. 2007, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Bonapace, E.S.; Maurer, A.H.; Davidoff, S.; Krevsky, B.; Fisher, R.S.; Parkman, H.P. Whole gut transit scintigraphy in the clinical evaluation of patients with upper and lower gastrointestinal symptoms. Am. J. Gastroenterol. 2000, 95, 2838–2847. [Google Scholar] [CrossRef]
- Maleki, D.; Camilleri, M.; Burton, D.D.; Rath-Harvey, D.M.; Oenning, L.; Pemberton, J.H.; Low, P.A. Pilot study of pathophysiology of constipation among community diabetics. Dig. Dis. Sci. 1998, 43, 2373–2378. [Google Scholar] [CrossRef]
- Camilleri, M.; Thorne, N.K.; Ringel, Y.; Hasler, W.L.; Kuo, B.; Esfandyari, T.; Gupta, A.; Scott, S.M.; McCallum, R.W.; Parkman, H.P.; et al. Wireless pH-motility capsule for colonic transit: Prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol. Motil. 2010, 22, 874–882, e233. [Google Scholar] [CrossRef] [PubMed]
- van der Sijp, J.R.; Kamm, M.A.; Nightingale, J.M.; Britton, K.E.; Mather, S.J.; Morris, G.P.; Akkermans, L.M.; Lennard-Jones, J.E. Radioisotope determination of regional colonic transit in severe constipation: Comparison with radio opaque markers. Gut 1993, 34, 402–408. [Google Scholar] [CrossRef]
- Abrahamsson, H.; Antov, S.; Bosaeus, I. Gastrointestinal and colonic segmental transit time evaluated by a single abdominal X-ray in healthy subjects and constipated patients. Scand. J. Gastroenterol. Suppl. 1988, 152, 72–80. [Google Scholar] [CrossRef]
- Metcalf, A.M.; Phillips, S.F.; Zinsmeister, A.R.; MacCarty, R.L.; Beart, R.W.; Wolff, B.G. Simplified assessment of segmental colonic transit. Gastroenterology 1987, 92, 40–47. [Google Scholar] [CrossRef]
- Sakakibara, R.; Odaka, T.; Uchiyama, T.; Liu, R.; Asahina, M.; Yamaguchi, K.; Yamaguchi, T.; Yamanishi, T.; Hattori, T. Colonic transit time, sphincter EMG, and rectoanal videomanometry in multiple system atrophy. Mov. Disord. 2004, 19, 924–929. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.K.; Kim, D.Y.; Moon, I.H.; Hong, Y.S. Colonic transit time in diabetic patients--comparison with healthy subjects and the effect of autonomic neuropathy. Yonsei Med. J. 2003, 44, 265–272. [Google Scholar] [CrossRef]
- Miller, M.A.; Parkman, H.P.; Urbain, J.L.; Brown, K.L.; Donahue, D.J.; Knight, L.C.; Maurer, A.H.; Fisher, R.S. Comparison of scintigraphy and lactulose breath hydrogen test for assessment of orocecal transit: Lactulose accelerates small bowel transit. Dig. Dis. Sci. 1997, 42, 10–18. [Google Scholar] [CrossRef]
- Simrén, M.; Stotzer, P.O. Use and abuse of hydrogen breath tests. Gut 2006, 55, 297–303. [Google Scholar] [CrossRef]
- Faria, M.; Pavin, E.J.; Parisi, M.C.; Lorena, S.L.; Brunetto, S.Q.; Ramos, C.D.; Pavan, C.R.; Mesquita, M.A. Delayed small intestinal transit in patients with long-standing type 1 diabetes mellitus: Investigation of the relationships with clinical features, gastric emptying, psychological distress, and nutritional parameters. Diabetes Technol. Ther. 2013, 15, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Davies, K.N.; King, D.; Billington, D.; Barrett, J.A. Intestinal permeability and orocaecal transit time in elderly patients with Parkinson’s disease. Postgrad. Med. J. 1996, 72, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Gabrielli, M.; Bonazzi, P.; Scarpellini, E.; Bendia, E.; Lauritano, E.C.; Fasano, A.; Ceravolo, M.G.; Capecci, M.; Rita Bentivoglio, A.; Provinciali, L.; et al. Prevalence of small intestinal bacterial overgrowth in Parkinson’s disease. Mov. Disord. 2011, 26, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, C.; Coss Adame, E.; Attaluri, A.; Valestin, J.; Rao, S.S. Dysmotility and proton pump inhibitor use are independent risk factors for small intestinal bacterial and/or fungal overgrowth. Aliment. Pharmacol. Ther. 2013, 37, 1103–1111. [Google Scholar] [CrossRef]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef]
- Di Stefano, M.; Quigley, E.M.M. The diagnosis of small intestinal bacterial overgrowth: Two steps forward, one step backwards? Neurogastroenterol. Motil. 2018, 30, e13494. [Google Scholar] [CrossRef]
- Yu, D.; Cheeseman, F.; Vanner, S. Combined oro-caecal scintigraphy and lactulose hydrogen breath testing demonstrate that breath testing detects oro-caecal transit, not small intestinal bacterial overgrowth in patients with IBS. Gut 2011, 60, 334–340. [Google Scholar] [CrossRef]
- Scott, S.M.; Carrington, E.V. The London Classification: Improving Characterization and Classification of Anorectal Function with Anorectal Manometry. Curr. Gastroenterol. Rep. 2020, 22, 55. [Google Scholar] [CrossRef]
- Carrington, E.V.; Heinrich, H.; Knowles, C.H.; Fox, M.; Rao, S.; Altomare, D.F.; Bharucha, A.E.; Burgell, R.; Chey, W.D.; Chiarioni, G.; et al. The international anorectal physiology working group (IAPWG) recommendations: Standardized testing protocol and the London classification for disorders of anorectal function. Neurogastroenterol. Motil. 2020, 32, e13679. [Google Scholar] [CrossRef]
- Lee, T.H.; Bharucha, A.E. How to Perform and Interpret a High-resolution Anorectal Manometry Test. J. Neurogastroenterol. Motil. 2016, 22, 46–59. [Google Scholar] [CrossRef]
- Oblizajek, N.R.; Gandhi, S.; Sharma, M.; Chakraborty, S.; Muthyala, A.; Prichard, D.; Feuerhak, K.; Bharucha, A.E. Anorectal pressures measured with high-resolution manometry in healthy people-Normal values and asymptomatic pelvic floor dysfunction. Neurogastroenterol. Motil. 2019, 31, e13597. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yang, X.; Xu, C.; Zhang, Y.; Zhang, X. Normal values and pressure morphology for three-dimensional high-resolution anorectal manometry of asymptomatic adults: A study in 110 subjects. Int. J. Colorectal Dis. 2013, 28, 1161–1168. [Google Scholar] [CrossRef] [PubMed]
- De Pablo-Fernández, E.; Passananti, V.; Zárate-López, N.; Emmanuel, A.; Warner, T. Colonic transit, high-resolution anorectal manometry and MRI defecography study of constipation in Parkinson’s disease. Parkinsonism Relat. Disord. 2019, 66, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Wang, Y.; Wu, G.; Xu, Q.; Tang, Y.; Lin, L. High-resolution Anorectal Manometry in Parkinson Disease With Defecation Disorder: A Comparison With Functional Defecation Disorder. J. Clin. Gastroenterol. 2016, 50, 566–571. [Google Scholar] [CrossRef]
- Sarosiek, I.; Selover, K.H.; Katz, L.A.; Semler, J.R.; Wilding, G.E.; Lackner, J.M.; Sitrin, M.D.; Kuo, B.; Chey, W.D.; Hasler, W.L.; et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment. Pharmacol. Ther. 2010, 31, 313–322. [Google Scholar] [CrossRef]
- Wang, Y.T.; Mohammed, S.D.; Farmer, A.D.; Wang, D.; Zarate, N.; Hobson, A.R.; Hellstrom, P.M.; Semler, J.R.; Kuo, B.; Rao, S.S.; et al. Regional gastrointestinal transit and pH studied in 215 healthy volunteers using the wireless motility capsule: Influence of age, gender, study country and testing protocol. Aliment. Pharmacol. Ther. 2015, 42, 761–772. [Google Scholar] [CrossRef]
- Farmer, A.D.; Wegeberg, A.L.; Brock, B.; Hobson, A.R.; Mohammed, S.D.; Scott, S.M.; Bruckner-Holt, C.E.; Semler, J.R.; Hasler, W.L.; Hellstrom, P.M.; et al. Regional gastrointestinal contractility parameters using the wireless motility capsule: Inter-observer reproducibility and influence of age, gender and study country. Aliment. Pharmacol. Ther. 2018, 47, 391–400. [Google Scholar] [CrossRef]
- Maqbool, S.; Parkman, H.P.; Friedenberg, F.K. Wireless capsule motility: Comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig. Dis. Sci. 2009, 54, 2167–2174. [Google Scholar] [CrossRef]
- Kuo, B.; McCallum, R.W.; Koch, K.L.; Sitrin, M.D.; Wo, J.M.; Chey, W.D.; Hasler, W.L.; Lackner, J.M.; Katz, L.A.; Semler, J.R.; et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment. Pharmacol. Ther. 2008, 27, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.S.; Kuo, B.; McCallum, R.W.; Chey, W.D.; DiBaise, J.K.; Hasler, W.L.; Koch, K.L.; Lackner, J.M.; Miller, C.; Saad, R.; et al. Investigation of colonic and whole-gut transit with wireless motility capsule and radiopaque markers in constipation. Clin. Gastroenterol. Hepatol. 2009, 7, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Fraser, R.J.; Horowitz, M.; Maddox, A.F.; Harding, P.E.; Chatterton, B.E.; Dent, J. Hyperglycaemia slows gastric emptying in type 1 (insulin-dependent) diabetes mellitus. Diabetologia 1990, 33, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Zikos, T.A.; Clarke, J.O.; Nguyen, L.A.; Triadafilopoulos, G.; Neshatian, L. Regional Gastrointestinal Transit and Contractility Patterns Vary in Postural Orthostatic Tachycardia Syndrome (POTS). Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Gandhy, R.; Barlow, C.; Triadafilopoulos, G. Utility of the wireless motility capsule and lactulose breath testing in the evaluation of patients with Parkinson’s disease who present with functional gastrointestinal symptoms. BMJ Open Gastroenterol. 2017, 4, e000132. [Google Scholar] [CrossRef] [PubMed]
- Coleski, R.; Wilding, G.E.; Semler, J.R.; Hasler, W.L. Blunting of Colon Contractions in Diabetics with Gastroparesis Quantified by Wireless Motility Capsule Methods. PLoS ONE 2015, 10, e0141183. [Google Scholar] [CrossRef]
- Mark, E.B.; Poulsen, J.L.; Haase, A.M.; Espersen, M.; Gregersen, T.; Schlageter, V.; Scott, S.M.; Krogh, K.; Drewes, A.M. Ambulatory assessment of colonic motility using the electromagnetic capsule tracking system. Neurogastroenterol. Motil. 2019, 31, e13451. [Google Scholar] [CrossRef]
- Haase, A.M.; Gregersen, T.; Schlageter, V.; Scott, M.S.; Demierre, M.; Kucera, P.; Dahlerup, J.F.; Krogh, K. Pilot study trialling a new ambulatory method for the clinical assessment of regional gastrointestinal transit using multiple electromagnetic capsules. Neurogastroenterol. Motil. 2014, 26, 1783–1791. [Google Scholar] [CrossRef]
- Worsoe, J.; Fynne, L.; Gregersen, T.; Schlageter, V.; Christensen, L.A.; Dahlerup, J.F.; Rijkhoff, N.J.; Laurberg, S.; Krogh, K. Gastric transit and small intestinal transit time and motility assessed by a magnet tracking system. BMC Gastroenterol. 2011, 11, 145. [Google Scholar] [CrossRef]
- Brinck, C.E.; Mark, E.B.; Winther Klinge, M.; Ejerskov, C.; Sutter, N.; Schlageter, V.; Scott, S.M.; Mohr Drewes, A.; Krogh, K. Magnetic tracking of gastrointestinal motility. Physiol. Meas. 2020. [Google Scholar] [CrossRef]
- Sutter, N.; Klinge, M.W.; Mark, E.B.; Nandhra, G.; Haase, A.M.; Poulsen, J.; Knudsen, K.; Borghammer, P.; Schlageter, V.; Birch, M.; et al. Normative values for gastric motility assessed with the 3D-transit electromagnetic tracking system. Neurogastroenterol. Motil. 2020, e13829. [Google Scholar] [CrossRef] [PubMed]
- Nandhra, G.K.; Mark, E.B.; Di Tanna, G.L.; Haase, A.M.; Poulsen, J.; Christodoulides, S.; Kung, V.; Klinge, M.W.; Knudsen, K.; Borghammer, P.; et al. Normative values for region-specific colonic and gastrointestinal transit times in 111 healthy volunteers using the 3D-Transit electromagnet tracking system: Influence of age, gender, and body mass index. Neurogastroenterol. Motil. 2020, 32, e13734. [Google Scholar] [CrossRef] [PubMed]
- Klinge, M.W.; Haase, A.M.; Mark, E.B.; Sutter, N.; Fynne, L.V.; Drewes, A.M.; Schlageter, V.; Lund, S.; Borghammer, P.; Krogh, K. Colonic motility in patients with type 1 diabetes and gastrointestinal symptoms. Neurogastroenterol. Motil. 2020, e13948. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.; Haase, A.M.; Fedorova, T.D.; Bekker, A.C.; Ostergaard, K.; Krogh, K.; Borghammer, P. Gastrointestinal Transit Time in Parkinson’s Disease Using a Magnetic Tracking System. J. Park. Dis. 2017, 7, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Dinning, P.G.; Carrington, E.V.; Scott, S.M. The use of colonic and anorectal high-resolution manometry and its place in clinical work and in research. Neurogastroenterol. Motil. 2015, 27, 1693–1708. [Google Scholar] [CrossRef]
- Dinning, P.G. A new understanding of the physiology and pathophysiology of colonic motility? Neurogastroenterol. Motil. 2018, 30, e13395. [Google Scholar] [CrossRef]
- Corsetti, M.; Costa, M.; Bassotti, G.; Bharucha, A.E.; Borrelli, O.; Dinning, P.; Di Lorenzo, C.; Huizinga, J.D.; Jimenez, M.; Rao, S.; et al. First translational consensus on terminology and definitions of colonic motility in animals and humans studied by manometric and other techniques. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 559–579. [Google Scholar] [CrossRef]
- Campbell-Thompson, M.L.; Kaddis, J.S.; Wasserfall, C.; Haller, M.J.; Pugliese, A.; Schatz, D.A.; Shuster, J.J.; Atkinson, M.A. The influence of type 1 diabetes on pancreatic weight. Diabetologia 2016, 59, 217–221. [Google Scholar] [CrossRef]
- Zhao, J.; Yang, J.; Gregersen, H. Biomechanical and morphometric intestinal remodelling during experimental diabetes in rats. Diabetologia 2003, 46, 1688–1697. [Google Scholar] [CrossRef] [PubMed]
- Klinge, M.W.; Borghammer, P.; Lund, S.; Fedorova, T.; Knudsen, K.; Haase, A.M.; Christiansen, J.J.; Krogh, K. Enteric cholinergic neuropathy in patients with diabetes: Non-invasive assessment with positron emission tomography. Neurogastroenterol. Motil. 2020, 32, e13731. [Google Scholar] [CrossRef]
- Masselli, G.; Gualdi, G. MR imaging of the small bowel. Radiology 2012, 264, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Frøkjaer, J.B.; Brock, C.; Brun, J.; Simren, M.; Dimcevski, G.; Funch-Jensen, P.; Drewes, A.M.; Gregersen, H. Esophageal distension parameters as potential biomarkers of impaired gastrointestinal function in diabetes patients. Neurogastroenterol. Motil. 2012, 24, e1016–e1544. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Pal, A.; Fox, M. Volume and position change of the stomach during gastric accommodation and emptying: A detailed three-dimensional morphological analysis based on MRI. Neurogastroenterol. Motil. 2020, 32, e13865. [Google Scholar] [CrossRef]
- Menys, A.; Keszthelyi, D.; Fitzke, H.; Fikree, A.; Atkinson, D.; Aziz, Q.; Taylor, S.A. A magnetic resonance imaging study of gastric motor function in patients with dyspepsia associated with Ehlers-Danlos Syndrome-Hypermobility Type: A feasibility study. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Lehmann, R.; Borovicka, J.; Kunz, P.; Crelier, G.; Boesiger, P.; Fried, M.; Schwizer, W.; Spinas, G.A. Evaluation of delayed gastric emptying in diabetic patients with autonomic neuropathy by a new magnetic resonance imaging technique and radio-opaque markers. Diabetes Care 1996, 19, 1075–1082. [Google Scholar] [CrossRef] [PubMed]
- Carbone, S.F.; Tanganelli, I.; Capodivento, S.; Ricci, V.; Volterrani, L. Magnetic resonance imaging in the evaluation of the gastric emptying and antral motion: Feasibility and reproducibility of a fast not invasive technique. Eur. J. Radiol. 2010, 75, 212–214. [Google Scholar] [CrossRef]
- Cho, J.; Lee, Y.J.; Kim, Y.H.; Shin, C.M.; Kim, J.M.; Chang, W.; Park, J.H. Quantitative MRI evaluation of gastric motility in patients with Parkinson’s disease: Correlation of dyspeptic symptoms with volumetry and motility indices. PLoS ONE 2019, 14, e0216396. [Google Scholar] [CrossRef]
- Unger, M.M.; Hattemer, K.; Möller, J.C.; Schmittinger, K.; Mankel, K.; Eggert, K.; Strauch, K.; Tebbe, J.J.; Keil, B.; Oertel, W.H.; et al. Real-time visualization of altered gastric motility by magnetic resonance imaging in patients with Parkinson’s disease. Mov. Disord. 2010, 25, 623–628. [Google Scholar] [CrossRef]
- Froehlich, J.M.; Patak, M.A.; von Weymarn, C.; Juli, C.F.; Zollikofer, C.L.; Wentz, K.U. Small bowel motility assessment with magnetic resonance imaging. J. Magn Reson. Imaging 2005, 21, 370–375. [Google Scholar] [CrossRef]
- Ajaj, W.; Goehde, S.C.; Papanikolaou, N.; Holtmann, G.; Ruehm, S.G.; Debatin, J.F.; Lauenstein, T.C. Real time high resolution magnetic resonance imaging for the assessment of gastric motility disorders. Gut 2004, 53, 1256–1261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fedorova, T.D.; Knudsen, K.; Hartmann, B.; Holst, J.J.; Viborg Mortensen, F.; Krogh, K.; Borghammer, P. In vivo positron emission tomography imaging of decreased parasympathetic innervation in the gut of vagotomized patients. Neurogastroenterol. Motil. 2020, 32, e13759. [Google Scholar] [CrossRef]
- Fedorova, T.D.; Seidelin, L.B.; Knudsen, K.; Schacht, A.C.; Geday, J.; Pavese, N.; Brooks, D.J.; Borghammer, P. Decreased intestinal acetylcholinesterase in early Parkinson disease: An (11)C-donepezil PET study. Neurology 2017, 88, 775–781. [Google Scholar] [CrossRef] [PubMed]
- Gjerløff, T.; Fedorova, T.; Knudsen, K.; Munk, O.L.; Nahimi, A.; Jacobsen, S.; Danielsen, E.H.; Terkelsen, A.J.; Hansen, J.; Pavese, N.; et al. Imaging acetylcholinesterase density in peripheral organs in Parkinson’s disease with 11C-donepezil PET. Brain 2015, 138, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Knowles, C.H.; Lindberg, G.; Panza, E.; De Giorgio, R. New perspectives in the diagnosis and management of enteric neuropathies. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Kapur, R.P. Counting myenteric ganglion cells in histologic sections: An empirical approach. Hum. Pathol. 2010, 41, 1097–1108. [Google Scholar] [CrossRef]
- Ippolito, C.; Segnani, C.; De Giorgio, R.; Blandizzi, C.; Mattii, L.; Castagna, M.; Moscato, S.; Dolfi, A.; Bernardini, N. Quantitative evaluation of myenteric ganglion cells in normal human left colon: Implications for histopathological analysis. Cell Tissue Res. 2009, 336, 191–201. [Google Scholar] [CrossRef]
- Coron, E.; Auksorius, E.; Pieretti, A.; Mahé, M.M.; Liu, L.; Steiger, C.; Bromberg, Y.; Bouma, B.; Tearney, G.; Neunlist, M.; et al. Full-field optical coherence microscopy is a novel technique for imaging enteric ganglia in the gastrointestinal tract. Neurogastroenterol. Motil. 2012, 24, e611–e621. [Google Scholar] [CrossRef]
- Boschetti, E.; Malagelada, C.; Accarino, A.; Malagelada, J.R.; Cogliandro, R.F.; Gori, A.; Bonora, E.; Giancola, F.; Bianco, F.; Tugnoli, V.; et al. Enteric neuron density correlates with clinical features of severe gut dysmotility. Am. J. Physiol. Gastrointest. Liver. Physiol. 2019, 317, G793–G801. [Google Scholar] [CrossRef]
- Selim, M.M.; Wendelschafer-Crabb, G.; Redmon, J.B.; Khoruts, A.; Hodges, J.S.; Koch, K.; Walk, D.; Kennedy, W.R. Gastric mucosal nerve density: A biomarker for diabetic autonomic neuropathy? Neurology 2010, 75, 973–981. [Google Scholar] [CrossRef]
- Sprenger, F.S.; Stefanova, N.; Gelpi, E.; Seppi, K.; Navarro-Otano, J.; Offner, F.; Vilas, D.; Valldeoriola, F.; Pont-Sunyer, C.; Aldecoa, I.; et al. Enteric nervous system α-synuclein immunoreactivity in idiopathic REM sleep behavior disorder. Neurology 2015, 85, 1761–1768. [Google Scholar] [CrossRef]
- Lebouvier, T.; Neunlist, M.; Bruley des Varannes, S.; Coron, E.; Drouard, A.; N’Guyen, J.M.; Chaumette, T.; Tasselli, M.; Paillusson, S.; Flamand, M.; et al. Colonic biopsies to assess the neuropathology of Parkinson’s disease and its relationship with symptoms. PLoS ONE 2010, 5, e12728. [Google Scholar] [CrossRef]
- Giancola, F.; Fracassi, F.; Gallucci, A.; Sadeghinezhad, J.; Polidoro, G.; Zini, E.; Asti, M.; Chiocchetti, R. Quantification of nitrergic neurons in the myenteric plexus of gastric antrum and ileum of healthy and diabetic dogs. Auton. Neurosci. 2016, 197, 25–33. [Google Scholar] [CrossRef]
- Blum, H.E. The human microbiome. Adv. Med. Sci. 2017, 62, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Fraher, M.H.; O’Toole, P.W.; Quigley, E.M. Techniques used to characterize the gut microbiota: A guide for the clinician. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Adams, D.H.; Fava, F.; Hermes, G.D.; Hirschfield, G.M.; Hold, G.; Quraishi, M.N.; Kinross, J.; Smidt, H.; Tuohy, K.M.; et al. The gut microbiota and host health: A new clinical frontier. Gut 2016, 65, 330–339. [Google Scholar] [CrossRef]
- Hasegawa, S.; Goto, S.; Tsuji, H.; Okuno, T.; Asahara, T.; Nomoto, K.; Shibata, A.; Fujisawa, Y.; Minato, T.; Okamoto, A.; et al. Intestinal Dysbiosis and Lowered Serum Lipopolysaccharide-Binding Protein in Parkinson’s Disease. PLoS ONE 2015, 10, e0142164. [Google Scholar] [CrossRef]
- Keshavarzian, A.; Green, S.J.; Engen, P.A.; Voigt, R.M.; Naqib, A.; Forsyth, C.B.; Mutlu, E.; Shannon, K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015, 30, 1351–1360. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed]
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Bürmann, J.; Faßbender, K.; Schwiertz, A.; Schäfer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72. [Google Scholar] [CrossRef]
- Brown, C.T.; Davis-Richardson, A.G.; Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; et al. Gut microbiome metagenomics analysis suggests a functional model for the development of autoimmunity for type 1 diabetes. PLoS ONE 2011, 6, e25792. [Google Scholar] [CrossRef]
- Giongo, A.; Gano, K.A.; Crabb, D.B.; Mukherjee, N.; Novelo, L.L.; Casella, G.; Drew, J.C.; Ilonen, J.; Knip, M.; Hyöty, H.; et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011, 5, 82–91. [Google Scholar] [CrossRef]
- Siljander, H.; Honkanen, J.; Knip, M. Microbiome and type 1 diabetes. EBioMedicine 2019, 46, 512–521. [Google Scholar] [CrossRef]
- Miranda, M.C.G.; Oliveira, R.P.; Torres, L.; Aguiar, S.L.F.; Pinheiro-Rosa, N.; Lemos, L.; Guimarães, M.A.; Reis, D.; Silveira, T.; Ferreira, Ê.; et al. Frontline Science: Abnormalities in the gut mucosa of non-obese diabetic mice precede the onset of type 1 diabetes. J. Leukoc. Biol. 2019, 106, 513–529. [Google Scholar] [CrossRef]
- Vaarala, O.; Atkinson, M.A.; Neu, J. The “perfect storm” for type 1 diabetes: The complex interplay between intestinal microbiota, gut permeability, and mucosal immunity. Diabetes 2008, 57, 2555–2562. [Google Scholar] [CrossRef]
- Cani, P.D. Microbiota and metabolites in metabolic diseases. Nat. Rev. Endocrinol. 2019, 15, 69–70. [Google Scholar] [CrossRef]
- Softeland, E.; Brock, C.; Frokjaer, J.B.; Brogger, J.; Madacsy, L.; Gilja, O.H.; Arendt-Nielsen, L.; Simren, M.; Drewes, A.M.; Dimcevski, G. Association between visceral, cardiac and sensorimotor polyneuropathies in diabetes mellitus. J. Diabetes Complicat. 2014, 28, 370–377. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef] [PubMed]
- Low, P.A.; Denq, J.C.; Opfer-Gehrking, T.L.; Dyck, P.J.; O’Brien, P.C.; Slezak, J.M. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle Nerve 1997, 20, 1561–1568. [Google Scholar] [CrossRef]
- Sandroni, P.; Benarroch, E.E.; Low, P.A. Pharmacological dissection of components of the Valsalva maneuver in adrenergic failure. J. Appl. Physiol. 1991, 71, 1563–1567. [Google Scholar] [CrossRef]
- Benarroch, E.E.; Opfer-Gehrking, T.L.; Low, P.A. Use of the photoplethysmographic technique to analyze the Valsalva maneuver in normal man. Muscle Nerve 1991, 14, 1165–1172. [Google Scholar] [CrossRef]
- Freeman, R.; Chapleau, M.W. Testing the autonomic nervous system. Handb. Clin. Neurol. 2013, 115, 115–136. [Google Scholar] [CrossRef]
- Spallone, V. Blood Pressure Variability and Autonomic Dysfunction. Curr. Diabetes Rep. 2018, 18, 137. [Google Scholar] [CrossRef]
- Freeman, R.; Wieling, W.; Axelrod, F.B.; Benditt, D.G.; Benarroch, E.; Biaggioni, I.; Cheshire, W.P.; Chelimsky, T.; Cortelli, P.; Gibbons, C.H.; et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin. Auton. Res. 2011, 21, 69–72. [Google Scholar] [CrossRef]
- Cheshire, W.P., Jr. Autonomic History, Examination, and Laboratory Evaluation. Continuum 2020, 26, 25–43. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R. Autonomic Peripheral Neuropathy. Continuum 2020, 26, 58–71. [Google Scholar] [CrossRef]
- Spallone, V.; Ziegler, D.; Freeman, R.; Bernardi, L.; Frontoni, S.; Pop-Busui, R.; Stevens, M.; Kempler, P.; Hilsted, J.; Tesfaye, S.; et al. Cardiovascular autonomic neuropathy in diabetes: Clinical impact, assessment, diagnosis, and management. Diabetes Metab Res. Rev. 2011, 27, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Sletten, D.M.; Weigand, S.D.; Low, P.A. Relationship of Q-sweat to quantitative sudomotor axon reflex test (QSART) volumes. Muscle Nerve 2010, 41, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, T.W. Pancreatic polypeptide: A unique model for vagal control of endocrine systems. J. Auton. Nerv. Syst. 1983, 9, 99–111. [Google Scholar] [CrossRef]
- Knudsen, K.; Hartmann, B.; Fedorova, T.D.; Østergaard, K.; Krogh, K.; Møller, N.; Holst, J.J.; Borghammer, P. Pancreatic Polypeptide in Parkinson’s Disease: A Potential Marker of Parasympathetic Denervation. J. Park. Dis. 2017, 7, 645–652. [Google Scholar] [CrossRef]
- Desai, A.; Low, P.A.; Camilleri, M.; Singer, W.; Burton, D.; Chakraborty, S.; Bharucha, A.E. Utility of the plasma pancreatic polypeptide response to modified sham feeding in diabetic gastroenteropathy and non-ulcer dyspepsia. Neurogastroenterol. Motil. 2020, 32, e13744. [Google Scholar] [CrossRef]
- Parkman, H.P.; Yates, K.P.; Hasler, W.L.; Nguyan, L.; Pasricha, P.J.; Snape, W.J.; Farrugia, G.; Calles, J.; Koch, K.L.; Abell, T.L.; et al. Dietary intake and nutritional deficiencies in patients with diabetic or idiopathic gastroparesis. Gastroenterology 2011, 141, 486–498, 498.e481–487. [Google Scholar] [CrossRef]
- Astarloa, R.; Mena, M.A.; Sánchez, V.; de la Vega, L.; de Yébenes, J.G. Clinical and pharmacokinetic effects of a diet rich in insoluble fiber on Parkinson disease. Clin. Neuropharmacol. 1992, 15, 375–380. [Google Scholar] [CrossRef]
- Jalleh, R.; Marathe, C.S.; Rayner, C.K.; Jones, K.L.; Horowitz, M. Diabetic Gastroparesis and Glycaemic Control. Curr. Diabetes Rep. 2019, 19, 153. [Google Scholar] [CrossRef]
- Acosta, A.; Camilleri, M. Prokinetics in gastroparesis. Gastroenterol. Clin. N. Am. 2015, 44, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi, J.R.; Ackerman, M.J.; Camilleri, M. Cardiovascular safety of prokinetic agents: A focus on drug-induced arrhythmias. Neurogastroenterol. Motil. 2018, 30, e13302. [Google Scholar] [CrossRef]
- Kumar, M.; Chapman, A.; Javed, S.; Alam, U.; Malik, R.A.; Azmi, S. The Investigation and Treatment of Diabetic Gastroparesis. Clin. Ther. 2018, 40, 850–861. [Google Scholar] [CrossRef] [PubMed]
- Iodice, V.; Kimpinski, K.; Vernino, S.; Sandroni, P.; Low, P.A. Immunotherapy for autoimmune autonomic ganglionopathy. Auton. Neurosci. 2009, 146, 22–25. [Google Scholar] [CrossRef] [PubMed]
- McCallum, R.W.; Lin, Z.; Forster, J.; Roeser, K.; Hou, Q.; Sarosiek, I. Gastric electrical stimulation improves outcomes of patients with gastroparesis for up to 10 years. Clin. Gastroenterol. Hepatol. 2011, 9, 314–319.e1. [Google Scholar] [CrossRef]
- Klinge, M.W.; Rask, P.; Mortensen, L.S.; Lassen, K.; Ejskjaer, N.; Ehlers, L.H.; Krogh, K. Early Assessment of Cost-effectiveness of Gastric Electrical Stimulation for Diabetic Nausea and Vomiting. J. Neurogastroenterol. Motil. 2017, 23, 541–549. [Google Scholar] [CrossRef]
- Okdahl, T.; Bertoli, D.; Brock, B.; Krogh, K.; Krag Knop, F.; Brock, C.; Drewes, A.M. Study protocol for a multicentre, randomised, parallel group, sham-controlled clinical trial investigating the effect of transcutaneous vagal nerve stimulation on gastrointestinal symptoms in people with diabetes complicated with diabetic autonomic neuropathy: The DAN-VNS Study. BMJ Open 2021, 11, e038677. [Google Scholar] [CrossRef] [PubMed]
- Lauritano, E.C.; Gabrielli, M.; Scarpellini, E.; Lupascu, A.; Novi, M.; Sottili, S.; Vitale, G.; Cesario, V.; Serricchio, M.; Cammarota, G.; et al. Small intestinal bacterial overgrowth recurrence after antibiotic therapy. Am. J. Gastroenterol. 2008, 103, 2031–2035. [Google Scholar] [CrossRef] [PubMed]
- Selby, A.; Reichenbach, Z.W.; Piech, G.; Friedenberg, F.K. Pathophysiology, Differential Diagnosis, and Treatment of Diabetic Diarrhea. Dig. Dis. Sci. 2019, 64, 3385–3393. [Google Scholar] [CrossRef] [PubMed]
| Investigation | Measurement | Primary Dysmotility Parameters | Advantages/Limitations | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Minimally invasive | Radiation free | Standardized | Inexpensive | High Availability | Ambulatory assessment | Simple data analysis | High reliability | |||
| Established Assessment Methods | ||||||||||
| Esophageal manometry | Esophageal contractility patterns | Reduced peristalsis and uncoordinated contractions | No | Yes | Yes | No | No | No | No | Yes |
| Gastric emptying scintigraphy | Gastric emptying time | Delayed gastric emptying time | Yes | No | Yes | No | Yes | No | No | Yes |
| 13C-octanoic acid breath test | Gastric emptying time | Delayed gastric emptying time | Yes | Yes | Yes | Yes | No | No | Yes | No |
| Antropyloroduodenal manometry | Antropyloroduodenal contractility patterns | Postprandial antral hypomotility and duodenal dysmotility in diabetes | No | Yes | No | No | No | No/Yes | No | Yes |
| Intestinal scintigraphy | Small intestinal and colonic transit times | Prolonged intestinal transit times | Yes | No | No | No | No | No | No | Yes |
| Radio-opaque markers | Small intestinal and colonic transit times | Prolonged whole gut and regional transit times | Yes | No | No | Yes | Yes | Yes | Yes | No |
| Hydrogen and methane breath test | Orocecal transit time and detection of small intestinal bacterial overgrowth | Prolonged orocecal transit time and increased frequency of small intestinal bacterial overgrowth | Yes | Yes | Yes | Yes | Yes | No | Yes | No |
| Anorectal manometry | Anorectal contractility patterns | 1. Dystonic external anal sphincter during defecation in Parkinson’s disease 2. Dysfunction of the internal anal sphincter in diabetes 3. Recto-anal dyscoordination | Yes | Yes | No | No | No | No | No | Yes |
| Wireless motility capsule | 1. Whole gut and regional transit times 2. Motility patterns | Delayed whole gut- and regional transit times | Yes | Yes | Yes | No | No | Yes | Yes | Yes |
| Emerging Assessment Methods | ||||||||||
| Colonic Manometry | Colonic contractility patterns | Colonic dysmotility | No | Yes | No | No | No | No | No | Yes |
| 3D-Transit capsule | Whole gut and regional transit times | Delayed whole gut- and regional transit times | Yes | Yes | No | No | No | Yes | No | Yes |
| Computed tomography imaging | Small intestinal and colonic volume | Increased colonic volume | Yes | No | No | Yes | Yes | No | No | Yes |
| Magnetic resonance imaging | 1. Whole gut and regional transit times 2. Whole gut contractility 3. Organ volumes | Delayed gastric emptying and increased intestinal volume | Yes | Yes | No | No | Yes | No | No | Yes |
| 11C-donepezil positron emission tomography/computed tomography imaging | Whole gut cholinergic innervation | Intestinal parasympathetic denervation | Yes | No | No | No | No | No | No | Yes |
| Submucosal biopsies | Quantification of enteric neurons | 1. Reduced number of neurons in diabetes 2. Aggregation of α-synuclein in Parkinson’s disease | No | Yes | No | No | No | No | No | No |
| Microbiota |
Gut microbiota composition | 1. Less stable and diverse in diabetes 2. Altered in Parkinson’s disease | Yes | Yes | No | No | No | Yes | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornum, D.S.; Terkelsen, A.J.; Bertoli, D.; Klinge, M.W.; Høyer, K.L.; Kufaishi, H.H.A.; Borghammer, P.; Drewes, A.M.; Brock, C.; Krogh, K. Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. J. Clin. Med. 2021, 10, 1392. https://doi.org/10.3390/jcm10071392
Kornum DS, Terkelsen AJ, Bertoli D, Klinge MW, Høyer KL, Kufaishi HHA, Borghammer P, Drewes AM, Brock C, Krogh K. Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. Journal of Clinical Medicine. 2021; 10(7):1392. https://doi.org/10.3390/jcm10071392
Chicago/Turabian StyleKornum, Ditte S., Astrid J. Terkelsen, Davide Bertoli, Mette W. Klinge, Katrine L. Høyer, Huda H. A. Kufaishi, Per Borghammer, Asbjørn M. Drewes, Christina Brock, and Klaus Krogh. 2021. "Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives" Journal of Clinical Medicine 10, no. 7: 1392. https://doi.org/10.3390/jcm10071392
APA StyleKornum, D. S., Terkelsen, A. J., Bertoli, D., Klinge, M. W., Høyer, K. L., Kufaishi, H. H. A., Borghammer, P., Drewes, A. M., Brock, C., & Krogh, K. (2021). Assessment of Gastrointestinal Autonomic Dysfunction: Present and Future Perspectives. Journal of Clinical Medicine, 10(7), 1392. https://doi.org/10.3390/jcm10071392






