Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular Age-Related Macular Degeneration
Abstract
1. Introduction
2. Patients and Methods
2.1. Outcome Variables
2.2. Statistical Analyses
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Authorship
References
- AREDS-HOME Study Research, Group; Chew, E.Y.; Clemons, T.; Bressler, S.B.; Elman, M.J.; Danis, R.P.; Domalpally, A.; Garfinkel, R.A. Randomized trial of the ForeseeHome monitoring device for early detection of neovascular age-related macular degeneration. The HOme Monitoring of the Eye (HOME) study design (HOME Study report number 1). Contemp. Clin. Trials. 2014, 37, 294–300. [Google Scholar] [CrossRef]
- Ying, G.S.; Huang, J.; Maguire, M.G.; Jaffe, G.J.; Grunwald, J.E.; Toth, C.; Daniel, E.; Klein, M.; Pieramici, D.; Wells, J.; et al. Baseline predictors for one-year visual outcomes with ranibizumab or bevacizumab for neovascular age-related macular degeneration. Ophthalmology 2013, 120, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.; Lum, F.; Wood, K.; Salman, C.; Burugapalli, B.; Hall, R.; Singh, S.; Parke, D.W., 2nd; Williams, G.A. Real-World Vision in Age-Related Macular Degeneration Patients Treated with Single Anti-VEGF Drug Type for 1 Year in the IRIS Registry. Ophthalmology 2018, 125, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.M.; Kaiser, P.K.; Michels, M.; Soubrane, G.; Heier, J.S.; Kim, R.Y.; Sy, J.P.; Schneider, S.; ANCHOR Study Group. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1432–1444. [Google Scholar] [CrossRef]
- Heier, J.S.; Brown, D.M.; Chong, V.; Korobelnik, J.F.; Kaiser, P.K.; Nguyen, Q.D.; Kirchhof, B.; Ho, A.; Ogura, Y.; Yancopoulos, G.D.; et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012, 119, 2537–2548. [Google Scholar] [CrossRef] [PubMed]
- Rosenfeld, P.J.; Brown, D.M.; Heier, J.S.; Boyer, D.S.; Kaiser, P.K.; Chung, C.Y.; Kim, R.Y.; MARINA Study Group. Ranibizumab for neovascular age-related macular degeneration. N. Engl. J. Med. 2006, 355, 1419–1431. [Google Scholar] [CrossRef]
- Comparison of Age-related Macular Degeneration Treatments Trials Research Group; Maguire, M.G.; Martin, D.F.; Ying, G.S.; Jaffe, G.J.; Daniel, E.; Grunwald, J.E.; Toth, C.A.; Ferris, F.L., 3rd; Fine, S.L. Five-Year Outcomes with Anti-Vascular Endothelial Growth Factor Treatment of Neovascular Age-Related Macular Degeneration: The Comparison of Age-Related Macular Degeneration Treatments Trials. Ophthalmology 2016, 123, 1751–1761. [Google Scholar] [CrossRef]
- Rofagha, S.; Bhisitkul, R.B.; Boyer, D.S.; Sadda, S.R.; Zhang, K.; Group S-US. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: A multicenter cohort study (SEVEN-UP). Ophthalmology 2013, 120, 2292–2299. [Google Scholar] [CrossRef]
- Mehta, H.; Tufail, A.; Daien, V.; Lee, A.Y.; Nguyen, V.; Ozturk, M.; Barthelmes, D.; Gillies, M.C. Real-world outcomes in patients with neovascular age-related macular degeneration treated with intravitreal vascular endothelial growth factor inhibitors. Prog. Retin. Eye Res. 2018, 65, 127–146. [Google Scholar] [CrossRef]
- Evans, R.N.; Reeves, B.C.; Phillips, D.; Muldrew, K.A.; Rogers, C.; Harding, S.P.; Chakravarthy, U.; IVAN Study Group. Long-term Visual Outcomes after Release from Protocol in Patients who participated in the Inhibition of VEGF in Age-related choroidal Neovascularisation (IVAN) Trial. Ophthalmology 2020, 127, 1191–1200. [Google Scholar] [CrossRef]
- Brown, G.C.; Brown, M.M.; Rapuano, S.; Boyer, D. Cost-Utility Analysis of VEGF-Inhibitors for Treating Neovascular Age-Related Macular Degeneration. Am. J. Ophthalmol. 2020, 218, 225–241. [Google Scholar] [CrossRef]
- Ho, A.C.; Albini, T.A.; Brown, D.M.; Boyer, D.S.; Regillo, C.D.; Heier, J.S. The Potential Importance of Detection of Neovascular Age-Related Macular Degeneration When Visual Acuity Is Relatively Good. JAMA Ophthalmol. 2017, 135, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Ho, A.C. Retrospective Analysis of Real-World Disease Detection and Visual Acuity Outcomes in Patients with Dry AMD Converting to Wet AMD Using the AAO IRIS Registry Database. In Proceedings of the 2018 ASCRS ASOA Annual Meeting, Washington, DC, USA, 13–17 April 2018. [Google Scholar]
- Kim, J.E. Real-World Analysis of Injection Frequency Following nAMD Diagnosis According to Baseline Visual Acuity. In Proceedings of the Macula Society, Bonita Springs, FL, USA, 13–16 February 2019. [Google Scholar]
- Fong, D.S.; Custis, P.; Howes, J.; Hsu, J.W. Intravitreal bevacizumab and ranibizumab for age-related macular degeneration a multicenter, retrospective study. Ophthalmology 2010, 117, 298–302. [Google Scholar] [CrossRef]
- Lee, A.Y.; Lee, C.S.; Butt, T.; Xing, W.; Johnston, R.L.; Chakravarthy, U.; Egan, C.; Akerele, T.; McKibbin, M.; Downey, L.; et al. UK AMD EMR USERS GROUP REPORT V: Benefits of initiating ranibizumab therapy for neovascular AMD in eyes with vision better than 6/12. Br. J. Ophthalmol. 2015, 99, 1045–1050. [Google Scholar] [CrossRef]
- Rayess, N.; Houston, S.K.; Gupta, O.P., 3rd; Ho, A.C.; Regillo, C.D. Treatment outcomes after 3 years in neovascular age-related macular degeneration using a treat-and-extend regimen. Am. J. Ophthalmol. 2015, 159, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.D.; Kelly, S.P.; Sallam, A.; Mohamed, Q.; Tufail, A.; Johnston, R.L. Incidence and baseline clinical characteristics of treated neovascular age-related macular degeneration in a well-defined region of the UK. Br. J. Ophthalmol. 2013, 97, 1168–1172. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.Y.; Dubois, L.; Tadayoni, R.; Fajnkuchen, F.; Nghiem-Buffet, S.; Delahaye-Mazza, C.; Guiberteau, B.; Quentel, G. Results of one-year’s treatment with ranibizumab for exudative age-related macular degeneration in a clinical setting. Am. J. Ophthalmol. 2009, 148, 409–413. [Google Scholar] [CrossRef]
- Hirami, Y.; Mandai, M.; Takahashi, M.; Teramukai, S.; Tada, H.; Yoshimura, N. Association of clinical characteristics with disease subtypes, initial visual acuity, and visual prognosis in neovascular age-related macular degeneration. Jpn J. Ophthalmol. 2009, 53, 396–407. [Google Scholar] [CrossRef]
- Zawinka, C.; Ergun, E.; Stur, M. Prevalence of patients presenting with neovascular age-related macular degeneration in an urban population. Retina 2005, 25, 324–331. [Google Scholar] [CrossRef]
- Schmidt-Erfurth, U.; Waldstein, S.M.; Klimscha, S.; Sadeghipour, A.; Hu, X.; Gerendas, B.S.; Osborne, A.; Bogunovic, H. Prediction of Individual Disease Conversion in Early AMD Using Artificial Intelligence. Invest. Ophthalmol. Vis. Sci. 2018, 59, 3199–3208. [Google Scholar] [CrossRef]
- Friberg, T.R.; Bilonick, R.A.; Brennen, P.M. Risk factors for conversion to neovascular age-related macular degeneration based on longitudinal morphologic and visual acuity data. Ophthalmology 2012, 119, 1432–1437. [Google Scholar] [CrossRef]
- McGuinness, M.B.; Finger, R.P.; Wu, Z.; Luu, C.D.; Chen, F.K.; Arnold, J.J.; Chakravarthy, U.; Guymer, R. Association between Patient-Reported Outcomes and Time to Late Age-Related Macular Degeneration in the Laser Intervention in Early Stages of Age-Related Macular Degeneration Study. Ophthalmol. Retin. 2020, 4, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Loewenstein, A.; Malach, R.; Goldstein, M.; Leibovitch, I.; Barak, A.; Baruch, E.; Alster, Y.; Rafaeli, O.; Avni, I.; Yassur, Y. Replacing the Amsler grid: A new method for monitoring patients with age-related macular degeneration. Ophthalmology 2003, 110, 966–970. [Google Scholar] [CrossRef]
- Chamard, C.; Lacombe, S.; Navarre, S.; Rohart, C.; Daures, J.P.; Allieu, S. Is current age related macular degeneration self-monitoring a good tool for detecting exudative recurrence? J. Fr. Ophtalmol. 2019, 42, 1049–1055. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, S.; Allen, D.; Annan-Phan, S.; Bell, K.; Bolliger, I.; Chong, T.; Druckenmiller, H.; Huang, L.Y.; Hultgren, A.; Krasovich, E.; et al. The effect of large-scale anti-contagion policies on the COVID-19 pandemic. Nature 2020, 584, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E. The HOME Study: Lesion characteristics of early choroidal neovascularization. In Proceedings of the American Society of Retina Specialists 2014, San Diego, CA, USA, 9–13 August 2014. [Google Scholar]
- Gregori, N.Z.; Feuer, W.; Rosenfeld, P.J. Novel method for analyzing snellen visual acuity measurements. Retina 2010, 30, 1046–1050. [Google Scholar] [CrossRef]
- Bressler, N.M.; Bressler, S.B.; Congdon, N.G.; Ferris, F.L., 3rd; Friedman, D.S.; Klein, R.; Lindblad, A.S.; Milton, R.C.; Seddon, J.M. Potential public health impact of Age-Related Eye Disease Study results: AREDS report no. 11. Arch. Ophthalmol. 2003, 121, 1621–1624. [Google Scholar]
- Ferris, F.L.; Davis, M.D.; Clemons, T.E.; Lee, L.Y.; Chew, E.Y.; Lindblad, A.S.; Milton, R.C.; Bressler, S.B.; Klein, R. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch. Ophthalmol. 2005, 123, 1570–1574. [Google Scholar] [PubMed]
- Yu, H.J.; Kiernan, D.F.; Eichenbaum, D.; Sheth, V.S.; Wykoff, C.C. Home Monitoring of Age-Related Macular Degeneration: Real-World Utility of the ForeseeHome Device for Detection of Neovascularization. Ophthalmol. Retina 2020.
- Acharya, N.; Lois, N.; Townend, J.; Zaher, S.; Gallagher, M.; Gavin, M. Socio-economic deprivation and visual acuity at presentation in exudative age-related macular degeneration. Br. J. Ophthalmol. 2009, 93, 627–629. [Google Scholar] [CrossRef]
- Olsen, T.W.; Feng, X.; Kasper, T.J.; Rath, P.P.; Steuer, E.R. Fluorescein angiographic lesion type frequency in neovascular age-related macular degeneration. Ophthalmology 2004, 111, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.S.; Mitchell, P.; Seddon, J.M.; Holz, F.G.; Wong, T.Y. Age-Related Macular Degeneration. Lancet 2012, 379, 1728–1738. [Google Scholar] [CrossRef]
- Ferris, F.L., 3rd; Fine, S.L.; Hyman, L. Age-Related Macular Degeneration and Blindness Due to Neovascular Maculopathy. Arch. Ophthalmol. 1984, 102, 1640–1642. [Google Scholar] [CrossRef] [PubMed]
- Zapata, M.A.; Burés, A.; Gallego-Pinazo, R.; Gutiérrez-Sánchez, E.; Oléñik, A.; Pastor, S.; Abraldes, M. Prevalence of age-related macular degeneration among optometric telemedicine users in Spain: A retrospective nationwide population-based study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 1–11. [Google Scholar] [CrossRef]
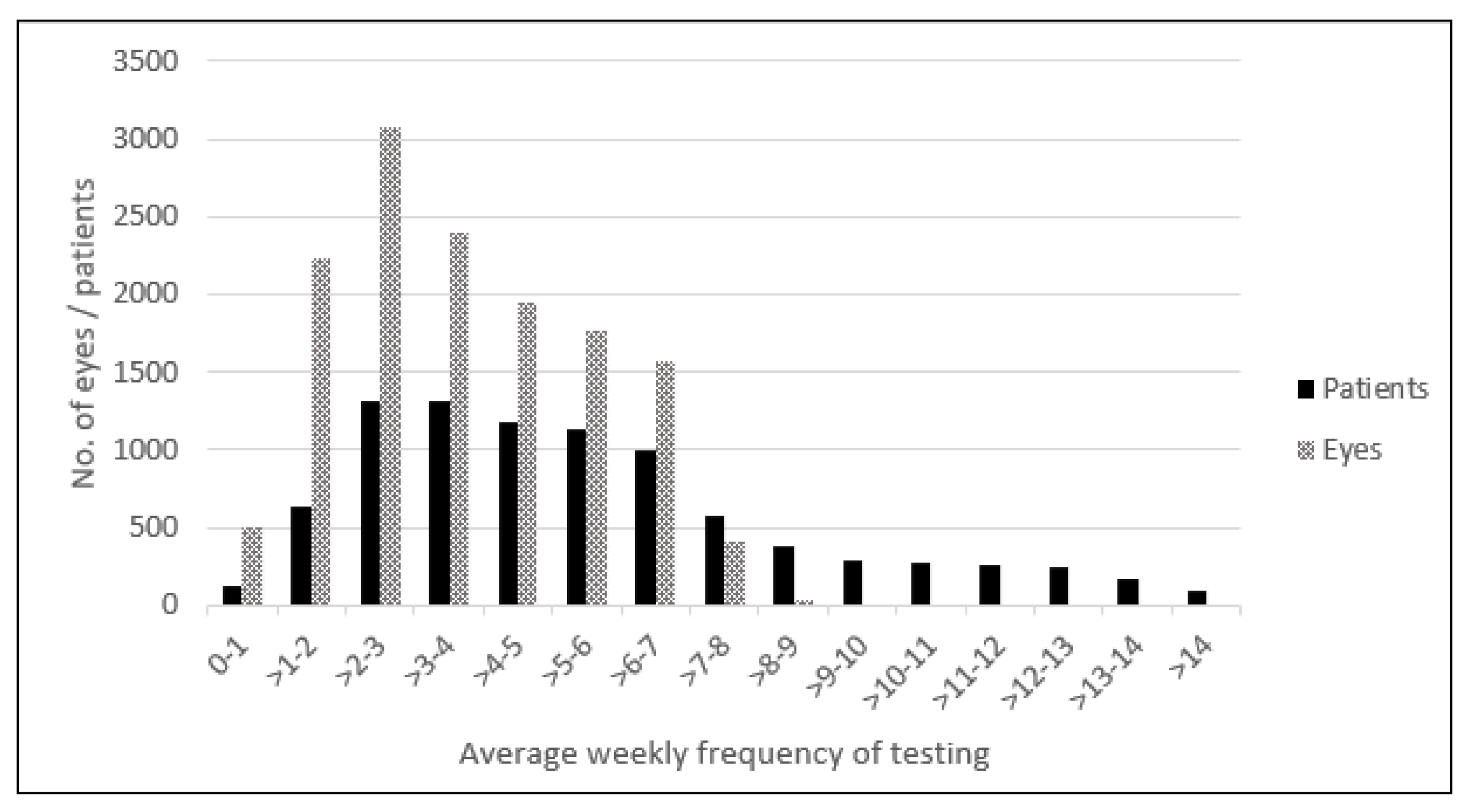
| Modality of Detection, n (%) of Conversions | All Conversions: 306 (100%) | Conversion Detected Following an Alert: 211 (69%) | Conversion Detected during Routine or Symptom-driven Visit to Physician 95 (31%) |
|---|---|---|---|
| Mean age in years (SD) | 75 (7.1) | 76 (6.9) | 73 (7.3) |
| Female sex, n | 199 (65%) | 139 (66%) | 60 (63%) |
| Mean (SD) time from initiation of the at-home device use to conversion, months | 16.8 (16.3) | 15.8 (15.4) | 19.2 (18.0) |
| Median (IQR) time from initiation of the at-home device use to conversion, months | 11.4 (4.3–23.5) | 10.5 (3.9–22.3) | 16.2 (5.4–24.2) |
| VA Outcomes | Baseline VA | VA at Conversion to nAMD | VA at Conversion with Known Baseline VA | VA Change from Baseline to Conversion |
|---|---|---|---|---|
| No. of eyes | 121 | 193 | 121 | |
| Mean VA [SD] letters | 77.7 (7.4) | 72.4 (12.0) | 72.3 (12.7) | −5.46 (10.0) |
| Mean VA Snellen | 20/32 | 20/40 | 20/40 | |
| Median VA [IQR] letters | 79.0 (74.0–84.0) | 75.0 (68.0–81.0) | 74.0 (66.0–81.0) | −3.0 (0.0–(−10.0)) |
| Median VA Snellen | 20/25 | 20/32 | 20/32 | |
| Eyes with nAMD detection triggered by a system alert with known VA | ||||
| No. of eyes | 95 | 151 | 95 | |
| Mean VA [SD] letters | 77.6 (7.1) | 72.7 (11.2) | 73.1 (11.3) | −4.5 (8.6) |
| Mean VA, Snellen | 20/32 | 20/40 | 20/40 | |
| Median VA [IQR] letters | 78.0 (74.0–83.0) | 75.0 (69.0–81.0) | 74.0 (68.5–81.0) | −2.0 (0.0–(−10.0)) |
| Median VA Snellen | 20/32 | 20/32 | 20/32 | |
| Eyes with nAMD detection during a routine or symptom-driven visit to physician with known VA | ||||
| No. of eyes | 26 | 42 | 26 | |
| Mean VA [SD] letters | 78.0 [8.7] | 71.3 [14.7] | 69.4 [16.8] | −8.6 [13.9] |
| Mean VA Snellen | 20/32 | 20/40 | 20/40 | |
| Median VA [IQR] letters | 81.0 [71.75–85.75] | 76.0 [62.25–81.0] | 74.0 [56.25–82.5] | −4.5 [0.0–(−16.5] |
| Median VA Snellen | 20/25 | 20/32 | 20/32 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, A.C.; Heier, J.S.; Holekamp, N.M.; Garfinkel, R.A.; Ladd, B.; Awh, C.C.; Singh, R.P.; Sanborn, G.E.; Jacobs, J.H.; Elman, M.J.; et al. Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular Age-Related Macular Degeneration. J. Clin. Med. 2021, 10, 1355. https://doi.org/10.3390/jcm10071355
Ho AC, Heier JS, Holekamp NM, Garfinkel RA, Ladd B, Awh CC, Singh RP, Sanborn GE, Jacobs JH, Elman MJ, et al. Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine. 2021; 10(7):1355. https://doi.org/10.3390/jcm10071355
Chicago/Turabian StyleHo, Allen C., Jeffrey S. Heier, Nancy M. Holekamp, Richard A. Garfinkel, Byron Ladd, Carl C. Awh, Rishi P. Singh, George E. Sanborn, Jennifer H. Jacobs, Michael J. Elman, and et al. 2021. "Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular Age-Related Macular Degeneration" Journal of Clinical Medicine 10, no. 7: 1355. https://doi.org/10.3390/jcm10071355
APA StyleHo, A. C., Heier, J. S., Holekamp, N. M., Garfinkel, R. A., Ladd, B., Awh, C. C., Singh, R. P., Sanborn, G. E., Jacobs, J. H., Elman, M. J., Loewenstein, A., & Eichenbaum, D. A. (2021). Real-World Performance of a Self-Operated Home Monitoring System for Early Detection of Neovascular Age-Related Macular Degeneration. Journal of Clinical Medicine, 10(7), 1355. https://doi.org/10.3390/jcm10071355






