Resting Heart Rate as a Predictor of Cancer Mortality: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Search and Data Extraction
2.4. Risk of Bias Assessment
2.5. Statistical Analysis
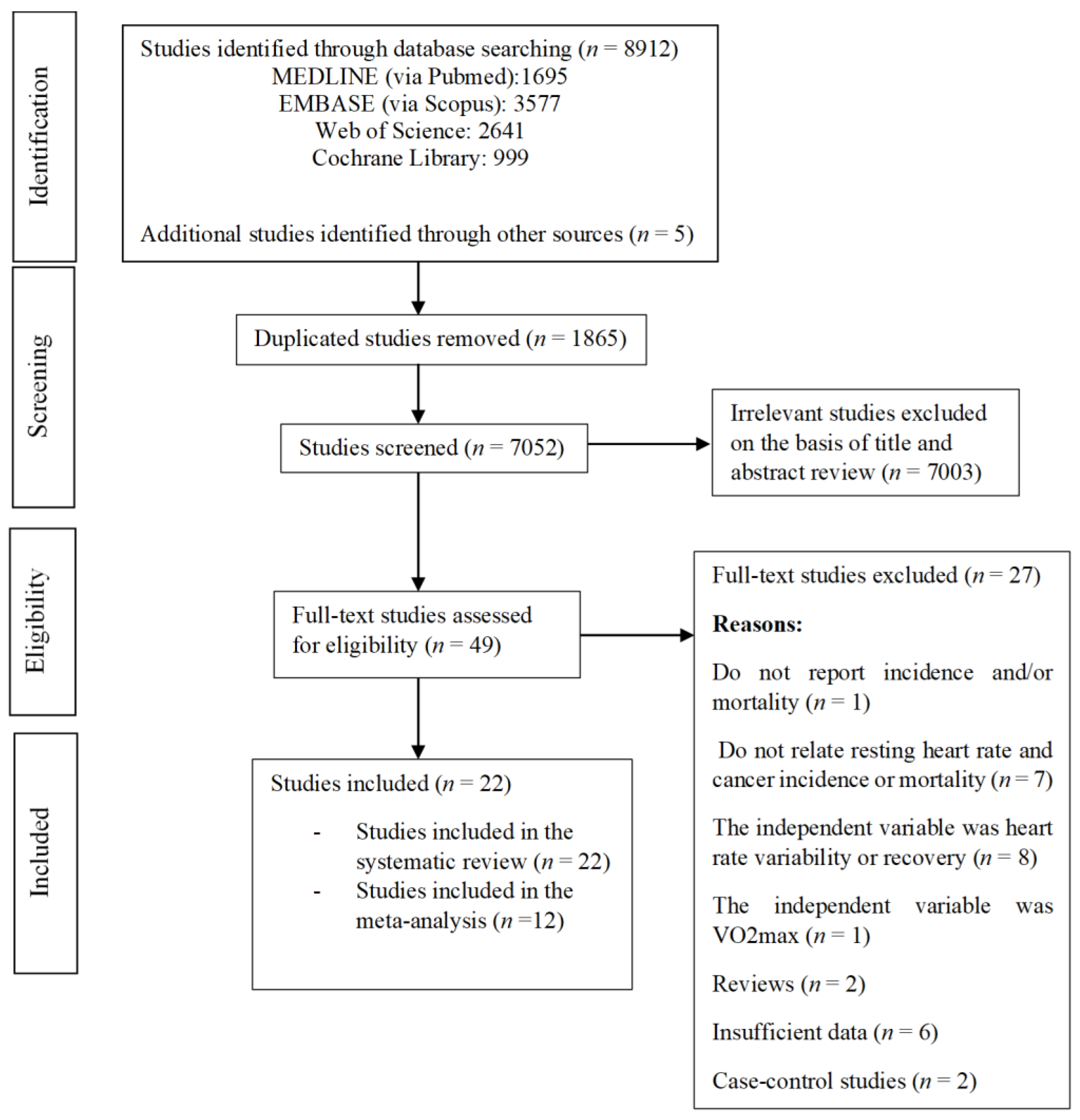
3. Results
3.1. Risk of Bias Assessment
3.2. Pooled Estimates
3.2.1. Cancer Incidence
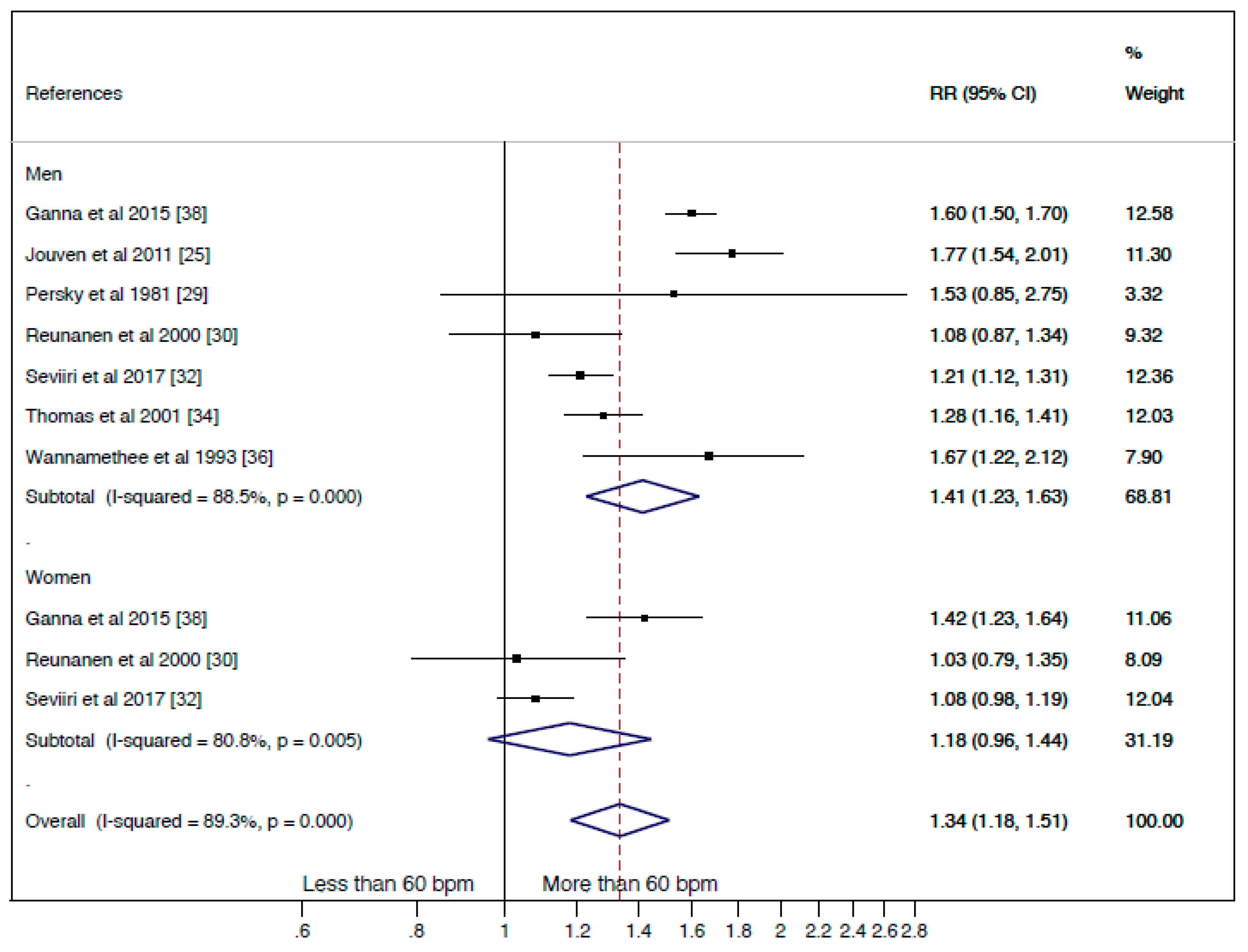
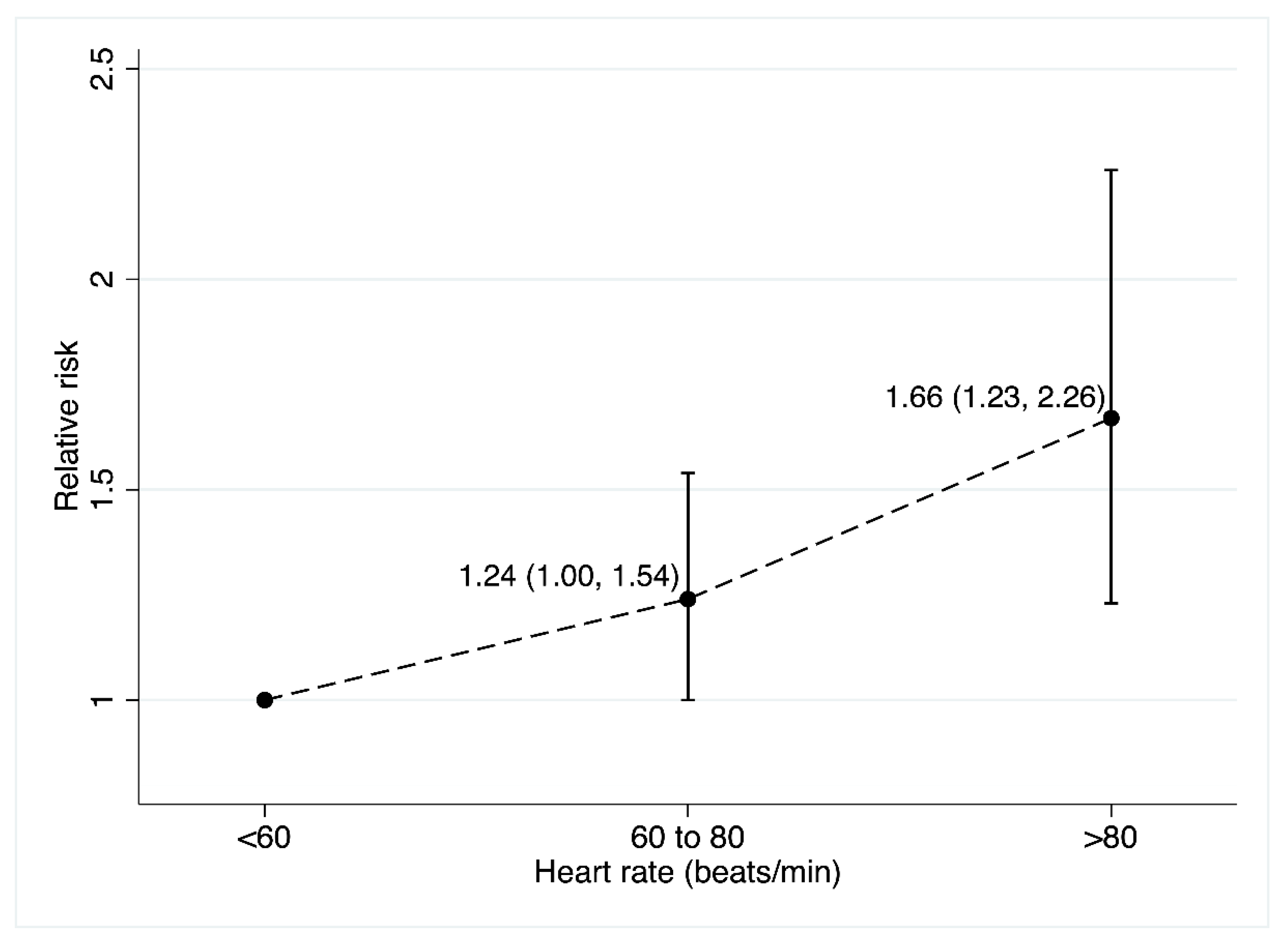
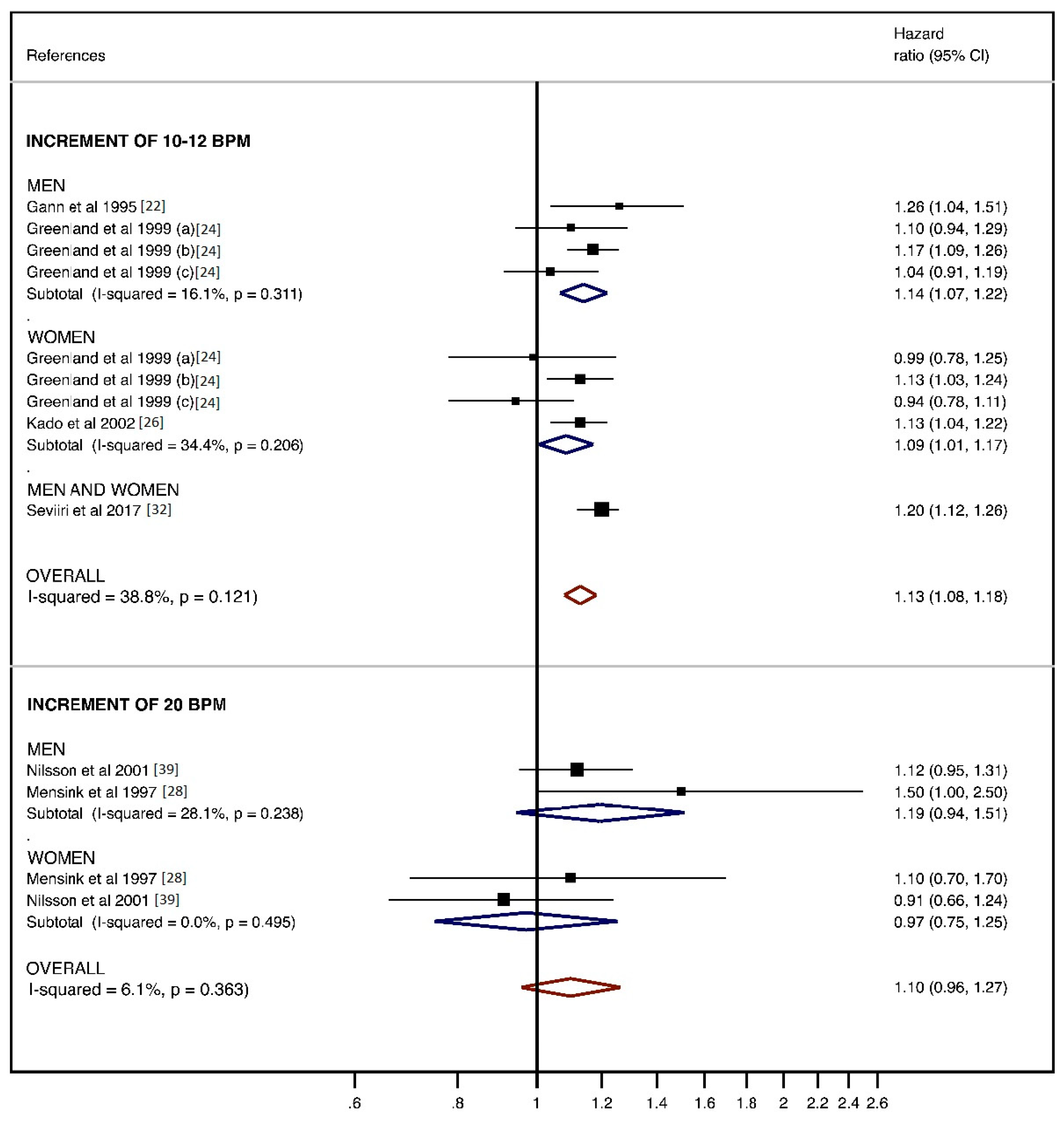
3.2.2. Cancer Mortality
- -
- Cancer mortality comparing the “less than 60 bpm” and “more than 60 bpm” categories
- -
- Analysis for 10–12 and 20 bpm increase and cancer mortality
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jensen, M.T. Resting heart rate and relation to disease and longevity: Past, present and future. Scand. J. Clin. Lab. Investig. 2019, 79, 108–116. [Google Scholar] [CrossRef]
- Kerr, J.; Anderson, C.; Lippman, S.M. Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. Lancet Oncol. 2017, 18, e457–e471. [Google Scholar] [CrossRef]
- Friedenreich, C.M.; Orenstein, M.R. Physical Activity and Cancer Prevention: Etiologic Evidence and Biological Mechanisms. J. Nutr. 2002, 132, 3456S–3464S. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef]
- McTiernan, A. Mechanisms linking physical activity with cancer. Nat. Rev. Cancer 2008, 8, 205–211. [Google Scholar] [CrossRef]
- Taylor, H.L.; Buskirk, E.; Henschel, A. Maximal Oxygen Intake as an Objective Measure of Cardio-Respiratory Performance. J. Appl. Physiol. 1955, 8, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, J.R.; Cavero-Redondo, I.; Ortega, F.B.; Welk, G.J.; Andersen, L.B.; Martinez-Vizcaino, V. Cardiorespiratory fitness cut points to avoid cardiovascular disease risk in children and adolescents; what level of fitness should raise a red flag? A systematic review and meta-analysis. Br. J. Sports Med. 2016, 50, 1451–1458. [Google Scholar] [CrossRef]
- Schmid, D.; Leitzmann, M.F. Cardiorespiratory fitness as predictor of cancer mortality: A systematic review and meta-analysis. Ann. Oncol. 2015, 26, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Pozuelo-Carrascosa, D.; Alvarez-Bueno, C.; Cavero-Redondo, I.; Morais, S.; Lee, I.; Martínez-Vizcaíno, V. Cardiorespiratory fitness and site-specific risk of cancer in men: A systematic review and meta-analysis. Eur. J. Cancer 2019, 113, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Aune, D.; Sen, A.; Ó’Hartaigh, B.; Janszky, I.; Romundstad, P.; Tonstad, S.; Vatten, L. Resting heart rate and the risk of cardiovascular disease, total cancer, and all-cause mortality—A systematic review and dose-response meta-analysis of prospective studies. Nutr. Metab. Cardiovasc. Dis. 2017, 27, 504–517. [Google Scholar] [CrossRef]
- Lee, I.M.; Shiroma, E.J.; Evenson, K.R.; Kamada, M.; LaCroix, A.Z.; Buring, J.E. Accelerometer-Measured Physical Activity and Sedentary Behavior in Relation to All-Cause Mortality: The Women’s Health Study. Circulation 2018, 137, 203–205. [Google Scholar] [CrossRef]
- Kodama, S.; Saito, K.; Tanaka, S.; Maki, M.; Yachi, Y.; Asumi, M.; Sugawara, A.; Totsuka, K.; Shimano, H.; Ohashi, Y.; et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: A meta-analysis. JAMA 2009, 301, 2024–2035. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. J. Clin. Epidemiol. 2009, 62, 1006–1012. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions Version 6.0 (Updated August 2019); Cochrane: Chichester, UK, 2019. [Google Scholar]
- Wells, G.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (accessed on 26 December 2019).
- Zhang, J.; Yu, K.F. What’s the relative risk? A method of correcting the odds ratio in cohort studies of common outcomes. JAMA 1998, 280, 1690–1691. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Egger, M.; Smith, G.D. Systematic reviews in health care: Investigating and dealing with publication and other biases in meta-analysis. BMJ 2001, 323, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Batty, G.D.; Shipley, M.J.; Kivimaki, M.; Marmot, M.; Smith, G.D. Walking Pace, Leisure Time Physical Activity, and Resting Heart Rate in Relation to Disease-Specific Mortality in London: 40 Years Follow-Up of the Original Whitehall Study. An Update of Our Work with Professor Jerry, N. Morris (1910–2009). Ann. Epidemiol. 2010, 20, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Cerhan, J.R.; Pavuk, M.; Wallace, R.B. Positive association between resting pulse and cancer incidence in current and former smokers. Ann Epidemiol. 1999, 9, 34–44. [Google Scholar] [CrossRef]
- Fitzpatrick, A.L.; Daling, J.R.; Furberg, C.D.; Kronmal, R.A.; Weissfeld, J.L. Hypertension, heart rate, use of antihypertensives, and incident prostate cancer. Ann. Epidemiol. 2001, 11, 534–542. [Google Scholar] [CrossRef]
- Gann, P.H.; Daviglus, M.L.; Dyer, A.R.; Stamler, J. Heart rate and prostate cancer mortality: Results of a prospective analysis. Cancer Epidemiol. Biomark. Prev. 1995, 4, 611–616. [Google Scholar]
- Garcia-Palmier, M.R.; Sorlie, P.D.; Costas, R.; Havlik, R.J. An Apparent Inverse Relationship between Serum Cholesterol and Cancer Mortality in Puerto Rico. Am. J. Epidemiol. 1981, 114, 29–40. [Google Scholar] [CrossRef]
- Greenland, P.; Daviglus, M.L.; Dyer, A.R.; Liu, K.; Huang, C.-F.; Goldberger, J.J.; Stamler, J. Resting Heart Rate is a Risk Factor for Cardiovascular and Noncardiovascular Mortality: The Chicago Heart Association Detection Project In Industry. Am. J. Epidemiol. 1999, 149, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Jouven, X.; Escolano, S.; Celermajer, D.; Empana, J.-P.; Bingham, A.; Hermine, O.; Desnos, M.; Perier, M.-C.; Marijon, E.; Ducimetière, P. Heart Rate and Risk of Cancer Death in Healthy Men. PLoS ONE 2011, 6, e21310. [Google Scholar] [CrossRef]
- Kado, D.M.; Lui, L.-Y.L.; Cummings, S.R.; The Study of Osteoporotic Fractures Research Group; Ma, L.-Y.L.L. Rapid Resting Heart Rate: A Simple and Powerful Predictor of Osteoporotic Fractures and Mortality in Older Women. J. Am. Geriatr. Soc. 2002, 50, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kristal-Boneh, E.; Silber, H.; Harari, G.; Froom, P. The association of resting heart rate with cardiovascular, cancer and all-cause mortality. Eight year follow-up of 3527 male Israeli employees (the CORDIS Study). Eur. Heart J. 2000, 21, 116–124. [Google Scholar] [CrossRef]
- Mensink, G.B.M.; Hoffmeister, H. The relationship between resting heart rate and all-cause, cardiovascular and cancer mortality. Eur. Heart J. 1997, 18, 1404–1410. [Google Scholar] [CrossRef]
- Perskly, V.; Dyer, A.R.; Leonas, J.; Stamler, J.; Berkson, D.M.; Lindberg, H.A.; Paul, O.; Shekelle, R.B.; Lepper, M.H.; Schoenberger, J.A. Heart Rate: A Risk Factor for Cancer? Am. J. Epidemiol. 1981, 114, 477–487. [Google Scholar] [CrossRef]
- Reunanen, A.; Karjalainen, J.; Ristola, P.; Heliövaara, M.; Knekt, P.; Aromaa, A. Heart rate and mortality. J. Intern. Med. 2000, 247, 231–239. [Google Scholar] [CrossRef]
- Severson, R.K.; Nomura, A.M.Y.; Grove, J.S.; Stemmermann, G.N. A Prospective Analysis of Physical Activity and Cancer. Am. J. Epidemiol. 1989, 130, 522–529. [Google Scholar] [CrossRef]
- Seviiri, M.; Lynch, B.M.; Hodge, A.M.; Yang, Y.; Liew, D.; English, D.R.; Giles, G.G.; Milne, R.L.; Dugué, P.-A. Resting heart rate, temporal changes in resting heart rate, and overall and cause-specific mortality. Heart 2017, 104, 1076–1085. [Google Scholar] [CrossRef]
- Steenland, K.; Nowlin, S.; Palu, S. Cancer incidence in the National Health and Nutrition Survey, I. Follow-up data: Diabetes, cholesterol, pulse and physical activity. Cancer Epidemiol. Biomark. Prev. 1995, 4, 807–811. [Google Scholar]
- Thomas, F.; Guize, L.; Bean, K.; Benetos, A. Pulse pressure and heart rate: Independent risk factors for cancer? J. Clin. Epidemiol. 2001, 54, 735–740. [Google Scholar] [CrossRef]
- Van Kruijsdijk, R.C.; van der Graaf, Y.; Bemelmans, R.H.; Nathoe, H.M.; Peeters, P.H.; Visseren, F.L. The relation between resting heart rate and cancer incidence, cancer mortality and all-cause mortality in patients with manifest vascular disease. Cancer Epidemiol. 2014, 38, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Wannamethee, G.; Shaper, A.G.; Macfarlane, P.W. Heart Rate, Physical Activity, and Mortality from Cancer and Other Noncardiovascular Diseases. Am. J. Epidemiol. 1993, 137, 735–748. [Google Scholar] [CrossRef] [PubMed]
- Friedman, G.D. Blood pressure and heart rate: No evidence for a positive association with prostate cancer. Ann. Epidemiol. 1997, 7, 486–489. [Google Scholar] [CrossRef]
- Ganna, A.; Ingelsson, E. 5 year mortality predictors in 498 103 UK Biobank participants: A prospective population-based study. Lancet 2015, 386, 533–540. [Google Scholar] [CrossRef]
- Nilsson, P.M.; Nilsson, J.-A.; Hedblad, B.; Berglund, G. Sleep disturbance in association with elevated pulse rate for prediction of mortality—Consequences of mental strain? J. Intern. Med. 2001, 250, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Dekker, J.M.; Crow, R.S.; Folsom, A.R.; Hannan, P.J.; Liao, D.; Swenne, C.A.; Schouten, E.G. Low heart rate variability in a 2-minute rhythm strip predicts risk of coronary heart disease and mortality from several causes: The ARIC Study. Atherosclerosis Risk In Communities. Circulation 2000, 102, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.T.; Marott, J.L.; Allin, K.H.; Nordestgaard, B.G.; Jensen, G.B. Resting heart rate is associated with cardiovascular and all-cause mortality after adjusting for inflammatory markers: The Copenhagen City Heart Study. Eur. J. Prev. Cardiol. 2012, 19, 102–108. [Google Scholar] [CrossRef]
- Cole, S.W.; Sood, A.K. Molecular Pathways: Beta-Adrenergic Signaling in Cancer. Clin. Cancer Res. 2012, 18, 1201–1206. [Google Scholar] [CrossRef]
- Guyton, A.C.; Coleman, T.G.; Granger, H.J. Circulation: Overall Regulation. Annu. Rev. Physiol. 1972, 34, 13–44. [Google Scholar] [CrossRef]
- Eppinga, R.N.; Hagemeijer, Y.; Burgess, S.; Hinds, D.A.; Stefansson, K.; Gudbjartsson, K.S.D.F.; Van Veldhuisen, D.J.; Munroe, P.B.; Verweij, N.; Harst, P. Identification of genomic loci associated with resting heart rate and shared genetic predictors with all-cause mortality. Nat. Genet. 2016, 48, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Azbel, M. Universal biological scaling and mortality. Proc. Natl. Acad. Sci. USA 1994, 91, 12453–12457. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, W.; Li, F. Association between resting heart rate and coronary artery disease, stroke, sudden death and noncardiovascular diseases: A meta-analysis. Can. Med. Assoc. J. 2016, 188, E384–E392. [Google Scholar] [CrossRef] [PubMed]
- Valentini, M.; Parati, G. Variables Influencing Heart Rate. Prog. Cardiovasc. Dis. 2009, 52, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.B.; Banister, E.W.; Blaber, A.P. Effect of Endurance Exercise on Autonomic Control of Heart Rate. Sports Med. 2003, 33, 33–46. [Google Scholar] [CrossRef]
- World Cancer Research Fund/American Institute of Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018. Available online: dietandcanerrepoort.org (accessed on 16 December 2020).
- Díez-Fernández, A.; Sánchez-López, M.; Nieto, J.A.; González-García, A.; Miota-Ibarra, J.; Ortiz-Galeano, I.; Martínez-Vizcaíno, V. Relationship between cardiorespiratory fitness and blood pressure in young adults: A mediation analysis of body composition. Hypertens. Res. 2017, 40, 511–515. [Google Scholar] [CrossRef]
- Rajagopala, S.V.; Vashee, S.; Oldfield, L.M.; Suzuki, Y.; Venter, J.C.; Telenti, A.; Nelson, K.E. The Human Microbiome and Cancer. Cancer Prev. Res. 2017, 10, 226–234. [Google Scholar] [CrossRef]
- Anker, M.S.; Ebner, N.; Hildebrandt, B.; Springer, J.; Sinn, M.; Riess, H.; Anker, S.D.; Landmesser, U.; Haverkamp, W.; Von Haehling, S. Resting heart rate is an independent predictor of death in patients with colorectal, pancreatic, and non-small cell lung cancer: Results of a prospective cardiovascular long-term study. Eur. J. Heart Fail. 2016, 18, 1524–1534. [Google Scholar] [CrossRef]
- Kloter, E.; Barrueto, K.; Klein, S.D.; Scholkmann, F.; Wolf, U. Heart Rate Variability as a Prognostic Factor for Cancer Survival—A Systematic Review. Front. Physiol. 2018, 9, 623. [Google Scholar] [CrossRef]
- Zhang, D.; Shen, X.; Qi, X. Resting heart rate and all-cause and cardiovascular mortality in the general population: A meta-analysis. Can. Med. Assoc. J. 2015, 188, E53–E63. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B., Sr.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2014, 63, 2935–2959. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; De Backer, G.; Gohlke, H.; Graham, I.; Reiner, Z.; Verschuren, W.M.M.; Albus, C.; Benlian, P.; Boysen, G.; Cifkova, R.; et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Eur. J. Prev. Cardiol. 2012, 19, 585–667. [Google Scholar] [CrossRef] [PubMed]
- Dartois, L.; Fagherazzi, G.; Boutron-Ruault, M.-C.; Mesrine, S.; Clavel-Chapelon, F. Association between Five Lifestyle Habits and Cancer Risk: Results from the E3N Cohort. Cancer Prev. Res. 2014, 7, 516–525. [Google Scholar] [CrossRef] [PubMed]
| Reference | Country | Study Name | Period of Data Collection/Follow-Up | Sample Size (Male/Female) Race (White/Black/Asian/Latin/Unknow) Type of Population | Age Distribution | RHR Assessment | Number of Cancer Deaths/Cancer Events | Main Study Exposures | Main Study Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Batty et al. 2010 [19] | UK | Original Whitehall Study | 1967–1970 40 years | 1183M Race NR General population | 40–69 | NR | Mortality: All cancer: 244 | Walking pace, LTPA, and RHR | Disease-specific mortality |
| Cerhan et al. 1999 [20] | USA | Iowa 65+ Rural Health Study | 1982–1993 8.5 years | 2819M&F Race NR General population | 65–102 | Manually | Cancer events: All cancer: 231 | RHR | Cancer incidence |
| Dekker et al. 2000 [40] | USA | ARIC Study | 1987–1989 ~5 years | 15,792 M&F Race NR General population | 45–64 | ECG | Mortality: All cancer:209 | RHR and HRV | Cancer and cardiovascular mortality |
| Fitzpatrick et al. 2001 [21] | USA | The Cardiovascular Health Study (CHS) | 1989–1990 1992–1993 5.6 years | 2442M 318B/2124 Race NR General population | ≥65 | Manually and ECG | Cancer events: Prostate: 209 | BP and RHR | Prostate cancer incidence |
| Friedman et al. 1996 [37] | USA | Kaiser Permanent Medical Care Program (KPMCP) | 1964–1972 ~30 years | 58,704M 46,145W/7535B/2534A/2490U General population | 30–79 | Manually, Air Shield and Godart automated device | Mortality: Prostate: 464 Cancer events: Prostate: 2297 | BP and RHR | Prostate cancer mortality and incidence |
| Gann et al. 1995 [22] | USA | Chicago Heart Association Detection Project | 1967–1973 19.2 years | 22,380M 20,184W/1487B/709U General population | 15–90 | ECG | Mortality: Prostate: 464 | RHR | Prostate cancer mortality |
| Ganna et al. 2015 [38] | UK | UK Biobank | 2007–2010 4.9 years | 498,103 M&F Race NR General population | 37–73 | NR | Mortality: All cancer: 5083 | Several health-related variables. RHR | Cardiovascular, respiratory, digestive, cancer, and other causes mortality |
| Garcia-Palmieri et al. 1981 [23] | Puerto Rico | Puerto Rico Heart Health Program | 1965–1969 ~8 years | 9824M Latin General population | 45–64 | NR | Mortality: All cancer: 179 | Serum cholesterol | Cancer mortality |
| Greenland et al. 1999 [24] | USA | Chicago Heart Association Detection Project | 1967–1973 22 years | 18,787M/12,994F 3357B/28,424 Race NR General population | 18–74 | ECG | Mortality: All cancer: 724 | RHR | Cardiovascular and noncardiovascular mortality |
| Jouven et al. 2011 [25] | France | - | 1967–1972 25 years | 6101M Race NR General population | 42–53 | ECG | Mortality: All cancer: 771 Respiratory cancer: 241 Digestive cancer: 210 | RHR | Cancer mortality |
| Kado et al. 2002 [26] | USA | Study of Osteoporotic Fractures | 1986–1988 8.9 years | 9704F White General population | ≥65 | NR | Mortality: All cancer: 580 | RHR | Osteoporotic fractures and mortality |
| Kristal-Boneh et al. 2000 [27] | Israel | CORDIS Study | 1985–1987 8 years | 3527M White General population | 43 | ECG | Mortality: All cancer: 45 | RHR | Cardiovascular, cancer, and all-cause mortality |
| Mensink et al. 1997 [28] | Germany | Spandau Health Test | 1984–1991 12 years | 1798M/2908F Race NR General population | 40–80 | Manually | Mortality: All cancer: 126 | RHR | Cardiovascular, cancer, and all-cause mortality |
| Nilsson et al. 2001 [39] | Sweden | Malmö Preventive Project | 1974–1992 17 (M)/12 (F) years | 13,466M/9467F Race NR General population | 46.7 | Sphygmomanometer and chronometer | Mortality: All cancer: 841 | Sleep deprivation and RHR | Cardiovascular, cancer, and all-cause mortality |
| Perskly et al. 1981 [29] | USA | Chicago Peoples Gas Company Study Chicago Western Electric Company Study Chicago Heart Association Detection Project | 1958–1976 1957–1974 1967–1979 18 years | 1233M White General population 1899M White General population 5784 White General population | 40–59 40–55 45–64 | ECG | Mortality: All cancer: 99 Mortality: All cancer: 78 Mortality: All cancer: 95 | RHR | Cancer mortality |
| Reunanen et al. 2000 [30] | Finland | - | 1966–1972 23 years | 5598M/5119F Race NR General population | 30–59 | ECG | Mortality: All cancer: 364 | RHR | Cardiovascular, cancer, and all-cause mortality |
| Severson et al. 1989 [31] | USA | - | 1965–1968 20 years | 8006M Asian General population | 46–65 | ECG | Cancer events: Colon: 193 Rectum: 95 Stomach: 171 Lung: 194 Prostate: 206 Bladder: 70 | Physical activity | Cancer incidence |
| Seviiri et al. 2017 [32] | Australia | Melbourne Collaborative Study | 1990–1994 21.9 years | 17,045M/24,469F Race NR General population | 40–69 | Automatic blood pressure monitor | Mortality: All cancer: 3618 Bladder: 69 Brain: 124 Breast: 277 Colorectal: 460 Kidney: 73 Lung: 566 Lymphoid: 415 Prostate: 247 Ovarian: 122 Skin: 122 Upper aerodigestive tract: 118 Other cancer: 1025 | RHR | Cardiovascular, cancer, and all-cause mortality |
| Steenland et al. 1995 [33] | USA | NHANES 1 | 1971–1975 15 years | 13,054M&F 11,096W/1958 Race NR General population | 25–74 | NR | Cancer events: All cancer: 1335 Lung: 210 Colorectal: 176 Breast: 163 Prostate: 156 Bladder: 56 Pancreas: 54 Leukemia: 44 Kidney: 41 Skin: 41 Stomach: 33 Ovary: 27 Liver: 25 Brain: 21 Cervix: Uterus: 43 Esophagus: 17 Larynx: 15 Melanoma: 10 Thyroid: 5 Oral: 21 Other hematopoietic: 63 Other: 94 | Diabetes, cholesterol, RHR, and physical activity | Cancer incidence |
| Thomas et al. 2001 [34] | France | - | 1970–1978 8 years | 125,513M Race NR General population | 20–95 | ECG | Mortality: All cancer: 3618 Respiratory cancer: 416 Digestive cancer: 347 Genito-urinary cancer: 113 | BP and RHR | Cancer mortality |
| van Kruijsdijk et al. 2014 [35] | Netherlands | Second Manifestations of ARTerial disease study (SMART) | 1996–2012 ~ 6 years | 4433M/1574F 5707W/300 Race NR Vascular disease patients | 18–80 | ECG | Mortality: All cancer: 56 Cancer events: All cancer: 126 Lung: 27 Colorectal: 17 Breast: 4 Prostate: 24 | RHR | Cancer mortality and incidence, and all-cause mortality |
| Wannamethee et al. 1993 [36] | UK | British Regional Heart Study | 1978–1980 9.5 years | 7735M Race NR General population | 40–59 | ECG | Mortality: All cancer: 217 | RHR and physical activity | Cancer and other noncardiovascular mortality |
| Reference | Association Measurement | RHR Level Comparison | Mortality | Cancer Events | Adjustment Variables |
|---|---|---|---|---|---|
| ALL CANCER | |||||
| Batty et al. 2010 [19] | HR | ≤64 beats/min 65–74 beats/min ≥75 beats/min | 1.00 1.10 (0.81, 1.49) 1.08 (0.79, 1.49) | NR | Age, employment grade, BMI, smoking and forced expiratory volume in 1 s |
| Cerhan et al. 1999 [20] | HR | <63 beats/min 63–68 beats/min 69–74 beats/min 75–82 beats/min >82 beats/min | NR | 1.00 1.68 (1.06, 2.66) 1.54 (0.95, 2.49) 1.62 (1.03, 2.55) 1.66 (1.03, 2.65) | Age, BMI, smoking, and physical activity |
| Men | |||||
| Women | 1.00 1.06 (0.71,1.57) 0.84 (0.55, 1.30) 1.03 (0.70, 1.50) 1.15 (0.77, 1.73) | ||||
| Dekker et al. 2000 [40] | RR | ≤63 beats/min 64–71 beats/min ≥72 beats/min | 1.00 1.22 (0.79–2.38) 1.04 (0.62–1.76) 1.61 (1.05–2.48) 1.39 (0.86–2.25) | NR | Age, sex, race, field center, current smoking, and cigarette-years. |
| Ganna et al. 2015 [38] Men | RR | 30.5–60 beats/min 60–66 beats/min 66–71.5 beats/min 71.5–79 beats/min 79–174 beats/min | 1.00 1.2 (1.1, 1.4) 1.4 (1.2, 1.6) 1.6 (1.4, 1.8) 2.2 (2.0, 2.5) 0.7 (0.6,0.8) 0.8 (0.7, 0.9) 0.9 (0.8, 1.0) 1.0 1.4 (1.3–1.6) | NR | Age |
| Women | |||||
| Greenland et al. 1999 [24] | HR | Increment of 12 beats/min | NR | Age, education, serum cholesterol, smoking, BMI, major and minor electrocardiographic abnormalities, race, diabetes, and SBP. | |
| Men aged 18–39 years | 1.10 (0.94, 1.29) | ||||
| Women aged 18–39 years | 0.99 (0.78, 1.25) | ||||
| Men aged 40–59 years | 1.17 (1.09, 1.26) | ||||
| Women aged 40–59 years | 1.13 (1.03, 1.24) | ||||
| Men aged 60–74 years | 1.04 (0.91, 1.19) | ||||
| Women aged 60–74 years | 0.94 (0.78, 1.11) | ||||
| Jouven et al. 2011 [25] | RR | <60 beats/min 60–67 beats/min 68–73 beats/min >73 beats/min | 1.00 1.60 (1.20, 2.00) 1.60(1,30, 2.00) 2.40 (1.90, 2.90) | NR | Age and smoking |
| Kado et al. 2002 [26] | HR | Increment of 10 beats/min | 1.13 (1.04, 1.22) | NR | Age, weight, self-reported health, physical activity, hyperthyroidism, hypertension, diabetes, and smoking. |
| <80 beats/min ≥80 beats/min | 1.00 1.20 (0.90, 1.50) | ||||
| Kristal-Boneh et al. 2000 [27] | HR | <70 beats/min 70–79 beats/min 80–89 beats/min ≥90 beats/min | 1.00 0.86 (0.30, 2.00) 1.55 (0.70, 3.50) 1.13 (0.40, 3.00) | NR | Age, smoking, education, sport, and hemoglobin |
| Mensink et al. 1997 [28] | HR | Increment of 20 beats/min | 1.50 (1.00, 2.50) | NR | Age, serum cholesterol, BMI, SBP, smoking and diabetes |
| Men | |||||
| Women | 1.10 (0.70, 1.70) | ||||
| Nilsson et al. 2001 [39] Men | HR | Increment of 20 beats/min | 1.12 (0.95, 1.31) 0.91 (0.66, 1.24) | NR | Age, serum cholesterol, BMI, SBP, smoking, and alcohol problematic drinking habits. |
| Women | |||||
| Perskly et al. 1981 [29] | RR | ≤60 beats/min 61–67 beats/min 68–74 beats/min 75–79 beats/min ≥80 beats/min | 1.00 1.2 (0.55, 2.61) 1.38 (0.69, 2.76) 1.54 (0.79, 3.01) 2.54 (1.34, 4.82) | NR | Age, SBP, serum cholesterol, relative weight, and smoking |
| Reunanen et al. 2000 [30] | RR | ≤60 beats/min 61–83 beats/min ≥84 beats/min | 1.00 0.90 (0.69, 1.18) 0.89 (0.64, 1.24) | NR | Age, smoking, blood pressure, serum cholesterol, diabetes, body mass index, perceived health, job, and leisure time physical activity |
| Seviiri et al. 2017 [32] | HR | Increment of 10 beats/min | 1.10 (1.06, 1.13) | NR | Age, sex, country of birth, level of education, waist circumference, alcohol consumption, smoking, physical activity score, Alternative Healthy Eating Index, total serum cholesterol, sodium/potassium ratio, caffeine, blood pressure, and history of hypertension, angina, asthma, and diabetes |
| <60 beats/min 60–69 beats/min 70–79 beats/min 80–89 beats/min ≥90 beats/min | 1.00 1.08 (0.98, 1.19) 1.15 (1.04, 1.28) 1.41 (1.24, 1.60) 1.40 (1.16, 1.69) | ||||
| Steenland et al. 1995 [33] | OR | <73 beats/min 73–79 beats/min 80–87 beats/min ≥88 beats/min | NR | 1.00 0.97 (0.76, 1.24) 1.09 (0.86, 1.39) 0.97 (0.76, 1.22) | Age, BMI, smoking, alcohol, income, recreational physical activity |
| Thomas et al. 2001 [34] | RR | <60 beats/min 60–80 beats/min >80 beats/min | 1.00 1.18 (0.98, 1.43) 1.33 (1.19, 1.49) | NR | Age, body mass index, gamma-Gt, tobacco, cholesterol, PP, triglycerides, and physical activity |
| van Kruijsdijk et al. 2014 [35] | HR | ≤55 beats/min 56–62 beats/min 63–71 beats/min ≥72 beats/min | 1.00 0.99 (0.68, 1.43) 1.18 (0.83, 1.69) 1.06 (0.73, 1.54) | 1.00 0.90 (0.70, 1.16) 0.94 (0.73, 1.22) 1.03 (0.79, 1.34) | Age, sex, smoking, hemoglobin levels, beta-blockers, calcium channel-blockers, alpha-blockers, diuretics, BMI, diabetes mellitus, physical activity, and high sensitivity C-reactive protein |
| Wannamethee et al. 1993 [36] | RR | <60 beats/min 60–69 beats/min 70–79 beats/min 80–89 beats/min ≥90 beats/min | 1.00 1.50 (0.92, 2.41) 1.67 (1.02, 2.66) 2.08 (1.23, 3.49) 1.65 (0.88, 3.03) | NR | Age, social class, smoking, body mass index, heavy alcohol drinking, systolic blood pressure, blood cholesterol, preexisting ischemic heart disease, physical activity, and forced expiratory volume in 1 s. |
| GENITO-URINARY CANCER | |||||
| Cerhan et al. 1999 [20] | HR | <63 beats/min 63–68 beats/min 69–74 beats/min 75–82 beats/min >82 beats/min | NR | 1.00 3.47 (1.29, 9.31) 1.98 (0.66, 5.94) 1.98 (0.70, 5.62) 3.16 (1.15, 8.71) | Age, BMI, smoking, and physical activity |
| Prostate | |||||
| Fitzpatrick et al. 2001 [21] | HR | <60 beats/min 60–69 beats/min 70–79 beats/min ≥80 beats/min | NR | 1.00 1.20 (0.80, 1.70) 1.10 (0.70, 1.70) 1.60 (1.03, 2.50) | Age, race and BMI |
| Prostate | |||||
| Friedman et al. 1996 [37] | RR | ≤66 beats/min 67–74 beats/min 75–83 beats/min ≥84 beats/min | NR | 1.00 0.96 (0.85, 1.09) 0.90 (0.79, 1.02) 0.89 (0.79, 1.01) | Age |
| Prostate | |||||
| Gann et al. 1995 [22] | RR | Increment of 10 beats/min | 1.26 (1.04, 1.51) | NR | Age, BMI, serum cholesterol, SBP, smoking, postload plasma glucose, and education |
| Prostate | |||||
| <63 beats/min 63–72 beats/min 73–78 beats/min 79–87 beats/min >87 beats/min | 1.00 1.55 (0.69, 3.45) 1.85 (0.84, 4.08) 2.18 (1.01, 4.70) 2.69 (1.28, 5.66) | Age | |||
| Severson et al. 1989 [31] Prostate Bladder | RR | ≤71 beats/min 72–81 beats/min ≥82 beats/min | NR | 1.00 1.12 (0.80, 1.55) 0.97 (0.69, 1.36) 1.00 1.01 (0.55, 1.87) 1.22 (0.69, 2.18) | Age and BMI Age, BMI and smoking |
| Seviiri et al. 2017 [32] | HR | Increment of 10 beats/min | 1.07 (0.94, 1.20) | NR | Age, sex, country of birth, level of education, waist circumference, alcohol consumption, smoking, physical activity score, Alternative Healthy Eating Index, total serum cholesterol, sodium/potassium ratio, caffeine, blood pressure, and history of hypertension, angina, asthma, and diabetes |
| Prostate | |||||
| Bladder | 1.05 (0.83, 1.33) | ||||
| Kidney | 1.27 (1.03, 1.57) | ||||
| Ovarian | 0.87 (0.71, 1.06) | ||||
| Steenland et al. 1995 [33] | OR | <73 beats/min 73–79 beats/min 80–87 beats/min ≥88 beats/min | NR | 1.00 0.93 (0.59, 1.45) 1.20 (0.77, 1.87) 1.28 (0.83, 1.97) | Age, BMI, smoking. alcohol, income, recreational physical activity |
| Thomas et al. 2001 [34] | RR | <60 beats/min 60–80 beats/min >80 beats/min | 1.00 1.23 (0.70, 2.17) 0.86 (0.58, 1.28) | NR | Age, BMI, gamma-Gt, tobacco, cholesterol, PP, triglycerides, and physical activity |
| van Kruijsdijk et al. 2014 [35] | HR | ≤55 beats/min 56–62 beats/min 63–71 beats/min ≥72 beats/min | NR | 1.00 0.85 (0.46, 1.56) 0.45 (0.21, 0.98) 0.87 (0.45, 1.70) | Age, sex, smoking, hemoglobin levels, beta-blockers, calcium channel-blockers, alpha-blockers, diuretics, BMI, diabetes mellitus, physical activity, and high sensitivity C-reactive protein |
| Prostate | |||||
| GASTRO-INTESTINAL CANCER | |||||
| Cerhan et al. 1999 [20] | HR | <63 beats/min 63–68 beats/min 69–74 beats/min 75–82 beats/min >82 beats/min | NR | 1.00 1.49 (0.35, 6.29) 3.94 (1.11, 14.05) 3.10 (0.86, 11.17) 1.70 (0.40, 7.12) | Age, BMI, smoking, and physical activity |
| Colorectal | |||||
| Jouven et al. 2011 [25] | RR | <60 beats/min 60–67 beats/min 68–73 beats/min >73 beats/min | 1.00 1.60 (1.00, 2.50) 1.60 (1,10, 2.50) 2.30 (1.50, 3.30) | NR | Age and smoking |
| Severson et al. 1989 [31] Colon Rectum Stomach | RR | ≤71 beats/min 72–81 beats/min ≥82 beats/min | NR | ||
| 1.00 0.56 (0.39, 0.80) 0.71 (0.51, 0.99) | Age and BMI | ||||
| 1.00 1.31 (0.78, 2.20) 1.41 (0.84, 2.36) | |||||
| 1.00 1.07 (0.73, 1.59) 1.34 (0.92, 1.95) | Age, BMI and smoking | ||||
| Seviiri et al. 2017 [32] Colorectal UADT | HR | Increment of 10 beats/min | 1.18 (1.08, 1.29) 1.16 (0.98, 1.38) | NR | Age, sex, country of birth, level of education, waist circumference, alcohol consumption, smoking, physical activity score, Alternative Healthy Eating Index, total serum cholesterol, sodium/potassium ratio, caffeine, blood pressure, and history of hypertension, angina, asthma, and diabetes |
| Steenland et al. 1995 [33] | OR | <73 beats/min 73–79 beats/min 80–87 beats/min ≥88 beats/min | NR | 1.00 1.34 (0.76, 2.34) 1.08 (0.59, 1.98) 1.33 (0.75, 2.37) | Age, BMI, smoking. alcohol, income, recreational physical activity |
| Colorectal | |||||
| Thomas et al. 2001 [34] | RR | <60 beats/min 60–80 beats/min >80 beats/min | 1.00 1.00 (0.72, 1.38) 1.23 (1.03, 1.57) | NR | Age, BMI, gamma-Gt, tobacco, cholesterol, PP, triglycerides, and physical activity |
| van Kruijsdijk et al. 2014 [35] | HR | ≤55 beats/min 56-62 beats/min 63–71 beats/min ≥72 beats/min | NR | 1.00 0.71 (0.32, 1.53) 0.87 (0.41, 1.87) 1.82 (0.86, 3.84) | Age, sex, smoking, hemoglobin levels, beta-blockers, calcium channel-blockers, alpha-blockers, diuretics, BMI, diabetes mellitus, physical activity, and high sensitivity C-reactive protein |
| Colorectal | |||||
| RESPIRATORY CANCER | |||||
| Jouven et al. 2011 [25] | RR | <60 beats/min 60–67 beats/min 68–73 beats/min >73 beats/min | 1.00 1.80 (1.20, 2.70) 1.50 (1,00, 2.30) 3.00 (2.10, 4.50) | NR | Age and smoking |
| Severson et al. 1989 [31] Lung | RR | ≤71 beats/min 72–81 beats/min ≥82 beats/min | NR | 1.00 1.06 (0.76, 1.48) 0.70 (0.48, 1.01) | Age, BMI, and smoking |
| Seviiri et al. 2017 [32] Lung | HR | Increment of 10 beats/min | 1.19 (1.10, 1.29) | NR | Age, sex, country of birth, level of education, waist circumference, alcohol consumption, smoking, physical activity score, Alternative Healthy Eating Index, total serum cholesterol, sodium/potassium ratio, caffeine, blood pressure, and history of hypertension, angina, asthma, and diabetes |
| Steenland et al. 1995 [33] Lung | OR | <73 beats/min 73–79 beats/min 80–87 beats/min ≥88 beats/min | NR | 1.00 0.67 (0.42, 1.09) 0.91 (0.57, 1.45) 1.21 (0.79, 1.84) | Age. BMI, smoking. alcohol, income, recreational physical activity |
| Thomas et al. 2001 [34] | RR | <60 beats/min 60–80 beats/min >80 beats/min | 1.00 1.25 (0.88, 1.77) 1.52 (1.25, 1.85) | NR | Age, BMI, gamma-Gt, tobacco, cholesterol, PP, triglycerides, and physical activity |
| van Kruijsdijk et al. 2014 [35] Lung | HR | ≤55 beats/min 56–62 beats/min 63–71 beats/min ≥72 beats/min | NR | 1.00 0.80 (0.46, 1.40) 0.87 (0.51, 1.50) 0.86 (0.50, 1.48) | Age, sex, smoking, hemoglobin levels, beta-blockers, calcium channel-blockers, alpha-blockers, diuretics, BMI, diabetes mellitus, physical activity, and high sensitivity C-reactive protein |
| OTHER CANCERS | |||||
| Seviiri et al. 2017 [32] | HR | Increment of 10 beats/min | NR | Age, sex, country of birth, level of education, waist circumference, alcohol consumption, smoking, physical activity score, Alternative Healthy Eating Index, total serum cholesterol, sodium/potassium ratio, caffeine, blood pressure, and history of hypertension, angina, asthma, and diabetes | |
| Breast | 1.15 (1.02, 1.30) | ||||
| Brain | 0.91 (0.75, 1.09) | ||||
| Lymphoid | 1.05 (0.95, 1.15) | ||||
| Steenland et al. 1995 [33] | OR | <73 beats/min 73–79 beats/min 80–87 beats/min ≥88 beats/min | NR | 1.00 0.85 (0.54, 1.34) 0.86 (0.55, 1.35) 0.81 (0.52, 1.26) | Age. BMI, smoking. alcohol, income, recreational physical activity |
| Breast | |||||
| van Kruijsdijk et al. 2014 [35] | HR | ≤55 beats/min 56–62 beats/min 63–71 beats/min ≥72 beats/min | NR | 1.00 1.40 (0.40, 4.83) 0.92 (0.25, 3.40) 0.54 (0.13, 2.21) | Age, sex, smoking, hemoglobin levels, beta-blockers, calcium channel-blockers, alpha-blockers, diuretics, BMI, diabetes mellitus, physical activity, and high sensitivity C-reactive protein |
| Breast | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozuelo-Carrascosa, D.P.; Cavero-Redondo, I.; Lee, I.M.; Álvarez-Bueno, C.; Reina-Gutierrez, S.; Martínez-Vizcaíno, V. Resting Heart Rate as a Predictor of Cancer Mortality: A Systematic Review and Meta-Analysis. J. Clin. Med. 2021, 10, 1354. https://doi.org/10.3390/jcm10071354
Pozuelo-Carrascosa DP, Cavero-Redondo I, Lee IM, Álvarez-Bueno C, Reina-Gutierrez S, Martínez-Vizcaíno V. Resting Heart Rate as a Predictor of Cancer Mortality: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2021; 10(7):1354. https://doi.org/10.3390/jcm10071354
Chicago/Turabian StylePozuelo-Carrascosa, Diana P., Iván Cavero-Redondo, I.M. Lee, Celia Álvarez-Bueno, Sara Reina-Gutierrez, and Vicente Martínez-Vizcaíno. 2021. "Resting Heart Rate as a Predictor of Cancer Mortality: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 10, no. 7: 1354. https://doi.org/10.3390/jcm10071354
APA StylePozuelo-Carrascosa, D. P., Cavero-Redondo, I., Lee, I. M., Álvarez-Bueno, C., Reina-Gutierrez, S., & Martínez-Vizcaíno, V. (2021). Resting Heart Rate as a Predictor of Cancer Mortality: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 10(7), 1354. https://doi.org/10.3390/jcm10071354









