Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender
Abstract
1. Introduction
1.1. Aim
1.2. Objectives
- To use a novel application of linear discriminant analysis including stepwise feature selection using leave-one-out cross validation in rehabilitation research to classify healthy adults accurately into four classes according to age and gender.
- Provide a battery of simple, robust, non- invasive dry biomarkers indicative of MSK health for use in ageing studies.
2. Experimental Section
2.1. Participants
2.2. Physical Performance Assessment
2.2.1. Handgrip Strength
2.2.2. Quadriceps Strength
2.2.3. Peak Flow
2.2.4. Timed Up and GO
2.2.5. Stair Climbing Capacity
2.2.6. Anterior Thigh Thickness (Ultrasound Imaging; USI)
2.2.7. Muscle Mechanical Properties
2.2.8. Self-Reported QoL (SF-36)
2.3. Assessing Reliability for Battery of Tests
2.4. Statistical Analyses
3. Results
3.1. Participant Characteristics and Absolute Values for Battery of Tests
3.2. Classification Using Features from Battery of Test
3.3. Classification Performance Parameters
3.4. Misclassified Cases
3.5. Effect of Body Mass Index on Classification Model
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
A.1. Co-Morbidities
| Medical Conditions Reported | Older Adults (n) | Percent (%) | Valid Percent | Cumulative Percent | |
|---|---|---|---|---|---|
| Valid | 0 | 13 | 17.8 | 18.6 | 18.6 |
| 1 | 40 | 54.8 | 57.1 | 75.7 | |
| 2 | 16 | 21.9 | 22.9 | 98.6 | |
| 3 | 1 | 1.4 | 1.4 | 100.0 | |
| Total | 70 | 95.9 | 100.0 | ||
| Missing | 3 | 4.1 | |||
| Total | 73 | 100.0 | |||
A.2. Information on Use of Prescribed Medication in Older Adults
| Number of Medications | Older Adults (n) | Percent (%) | Valid Percent | Cumulative Percent | |
|---|---|---|---|---|---|
| Valid | 0 | 19 | 26.0 | 27.1 | 27.1 |
| 1 | 22 | 30.1 | 31.4 | 58.6 | |
| 2 | 16 | 21.9 | 22.9 | 81.4 | |
| 3 | 7 | 9.6 | 10.0 | 91.4 | |
| 4 | 3 | 4.1 | 4.3 | 95.7 | |
| 5 | 2 | 2.7 | 2.9 | 98.6 | |
| 6 | 1 | 1.4 | 1.4 | 100.0 | |
| Total | 70 | 95.9 | 100.0 | ||
| Missing | 3 | 4.1 | |||
| Total | 73 | 100.0 | |||
References
- Martin, F.C.; Ortuño, R.R. Longitudinal studies of ageing: From insights to impacts: Commentary to accompany themed collection on longitudinal studies. Age Ageing 2019, 48, 481–485. [Google Scholar] [CrossRef]
- World Health Organisation. What is Healthy Ageing? 2018. Available online: https://www.who.int/ageing/healthy-ageing/en/ (accessed on 24 November 2020).
- Chatterji, S.; Byles, J.; Cutler, D.; Seeman, T.; Verdes, E. Health, functioning, and disability in older adults—present status and future implications. Lancet 2015, 385, 563–575. [Google Scholar] [CrossRef]
- Fox, K.R.; Ku, P.-W.; Hillsdon, M.; Davis, M.G.; Simmonds, B.A.J.; Thompson, J.L.; Stathi, A.; Gray, S.F.; Sharp, D.J.; Coulson, J.C. Objectively assessed physical activity and lower limb function and prospective associations with mortality and newly diagnosed disease in UK older adults: An OPAL four-year follow-up study. Age Ageing 2015, 44, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Guralnik, J.; Brown, J.C.; di Bari, M.; Cesari, M.; Landi, F.; Vaes, B.; Legrand, D.; Verghese, J.; Wang, C.; et al. Short Physical Performance Battery and all-cause mortality: Systematic review and meta-analysis. BMC Med. 2016, 14, 215. [Google Scholar] [CrossRef]
- Simmonds, B.; Fox, K.R.; Davis, M.; Ku, P.-W.; Gray, S.; Hillsdon, M.; Sharp, D.; Sta-thi, A.; Thompson, J.L.; Coulson, J.; et al. Objectively Assessed Physical Activity and Subsequent Health Service Use of UK Adults Aged 70 and Over: A Four to Five Year Follow Up Study. PLoS ONE 2014, 9, e97676. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, C.H.; Bůžková, P.; Robbins, J.A.; Patel, K.V.; Newman, A.B. Predicting late-life disability and death by the rate of decline in physical performance measures. Age Ageing 2012, 41, 155–161. [Google Scholar] [CrossRef]
- Washburn, R.A.; McAuley, E.; Katula, J.; Mihalko, S.L.; Boileau, R.A. The Physical Activity Scale for the Elderly (PASE): Evidence for Validity. J. Clin. Epidemiol. 1999, 52, 643–651. [Google Scholar] [CrossRef]
- Ismail, N.; Hairi, F.; Choo, W.Y.; Hairi, N.N.; Peramalah, D.; Bulgiba, A. The Physical Activity Scale for the Elderly (PASE): Validity and Reliability Among Community-Dwelling Older Adults in Malaysia. Asia Pac. J. Public Health 2015, 27 (Suppl. 8), 62S–72S. [Google Scholar] [CrossRef]
- Samuel, D.; Wilson, K.; Martin, H.J.; Allen, R.; Sayer, A.A.; Stokes, M. Age-associated changes in hand grip and quadriceps muscle strength ratios in healthy adults. Aging Clin. Exp. Res. 2012, 24, 245–250. [Google Scholar] [CrossRef]
- Samuel, D.; Rowe, P.J. Effect of Ageing on Isometric Strength through Joint Range at Knee and Hip Joints in Three Age Groups of Older Adults. Gerontology 2009, 55, 621–629. [Google Scholar] [CrossRef]
- Vaz Fragoso, C.A.; Gahbauer, E.A.; Van Ness, P.H.; Gill, T.M. Reporting peak expiratory flow in older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Almeida, G.J.; Schroeder, C.A.; Gil, A.B.; Fitzgerald, G.K.; Piva, S.R. Interrater Reliability and Validity of the Stair Ascend/Descend Test in Subjects With Total Knee Arthroplasty. Arch. Phys. Med. Rehabil. 2010, 91, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.; Ndanyo, L.S.; Brown, S.; Agyapong-Badu, S.; Warner, M.; Stokes, M.; Samuel, D. Thigh muscle and subcutaneous tissue thickness measured using ultrasound imaging in older females living in extended care: A preliminary study. Aging Clin. Exp. Res. 2018, 30, 463–469. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Narici, M.; Cooper, C.; Stokes, M. Anterior thigh composition measured using ultrasound imaging to quantify relative thickness of muscle and non-contractile tissue: A potential biomarker for musculoskeletal health. Physiol. Meas. 2014, 35, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Gavronski, G.; Veraksits, A.; Vasar, E.; Maaroos, J. Evaluation of viscoelastic parameters of the skeletal muscles in junior triathletes. Physiol. Meas. 2007, 28, 625–637. [Google Scholar] [CrossRef]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Practical considerations for standardized recording of muscle mechanical properties using a myometric device: Recording site, muscle length, state of contraction and prior activity. J. Musculoskelet. Res. 2018, 21, 1850010. [Google Scholar] [CrossRef]
- Haywood, K.L.; Garratt, A.M.; Fitzpatrick, R. Quality of life in older people: A structured review of self-assessed health instruments. Expert Rev. Pharm. Outcomes Res. 2006, 6, 181–194. [Google Scholar] [CrossRef]
- R Core Team (2021). R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Lynch, N.A.; Metter, E.J.; Lindle, R.S.; Fozard, J.L.; Tobin, J.D.; Roy., T.A.; Fleg, J.L.; Hurley, B.F. Muscle quality. I. Age associated differences between arm and leg muscle groups. J. Appl. Physiol. 1999, 86, 188–194. [Google Scholar] [CrossRef]
- Crimmins, E.M.; Shim, H.; Zhang, Y.S.; Kim, J.K. Differences between Men and Women in Mortality and the Health Dimensions of the Morbidity Process. Clin. Chem. 2019, 65, 135–145. [Google Scholar] [CrossRef]
- Mascherini, G.; Castizo-Olier, J.; Irurtia, A.; Petri, C.; Galanti, G. Differences between the sexes in athletes’ body composition and lower limb bioimpedance values. Muscles Ligaments Tendons J. 2017, 7, 573–581. [Google Scholar] [CrossRef] [PubMed]
- Reinders, I.; Murphy, R.A.; Koster, A.; Brouwer, I.A.; Visser, M.; Garcia, M.E.; Harris, T.B. Muscle Quality and Muscle Fat Infiltration in Relation to Incident Mobility Disability and Gait Speed Decline: The Age, Gene/Environment Susceptibility-Reykjavik Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Zamboni, M. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Leng, X.I.; Kritchevsky, S.B. Body Composition and Physical Function in Older Adults with Various Comorbidities. Innov. Aging 2017, 1, igx008. [Google Scholar] [CrossRef] [PubMed]
- Santanasto, A.J.; Goodpaster, B.H.; Kritchevsky, S.B.; Miljkovic, I.; Satterfield, S.; Schwartz, A.V.; Newman, A.B. Body Composition Remodeling and Mortality: The Health Aging and Body Composition Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Trevisan, C.; Rizzuto, D.; Sergi, G.; Maggi, S.; Welmer, A.K.; Vetrano, D.L. Peak expiratory flow, walking speed and survival in older adults: An 18-year longitudinal population-based study. Exp. Gerontol. 2020, 135, 110941. [Google Scholar] [CrossRef]
- Saito, A.; Wakasa, M.; Kimoto, M.; Ishikawa, T.; Tsugaruya, M.; Kume, Y.; Okada, K. Age-related changes in muscle elasticity and thickness of the lower extremities are associated with physical functions among community-dwelling older women. Geriatr. Gerontol. Int. 2019, 19, 61–65. [Google Scholar] [CrossRef]
- Akagi, R.; Yamashita, Y.; Ueyasu, Y. Age-Related Differences in Muscle Shear Moduli in the Lower Extremity. Ultrasound Med. Biol. 2015, 41, 2906–2912. [Google Scholar] [CrossRef]
- Eby, S.F.; Cloud, B.A.; Brandenburg, J.E.; Giambini, H.; Song, P.; Chen, S.; LeBrasseur, N.K.; An, K. Shear wave elastography of passive skeletal muscle stiffness: Influences of sex and age throughout adulthood. Clin. Biomech. 2015, 30, 22–27. [Google Scholar] [CrossRef]
- Herrick, I.; Brown, S.; Agyapong-Badu, S.; Warner, M.; Ewings, S.; Samuel, D.; Stokes, M. Anterior Thigh Tissue Thickness Measured Using Ultrasound Imaging in Older Recreational Female Golfers and Sedentary Controls. Geriatrics 2017, 2, 10. [Google Scholar] [CrossRef]
- Warner, M.B.; Whatling, G.; Worsley, P.R.; Mottram, S.; Chappell, P.H.; Holt, C.A.; Stokes, M.J. Objective classification of scapular kinematics in participants with movement faults of the scapula on clinical assessment. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Kurgansky, M.; Frolov, A.; Ioffe, M.; Karabanov, A.; Chernikova, L.A. Comparative Kinematic Analysis of Cyclical Bending in Patients With Early Stages of Parkinson’s Disease and in Patients With Essential Tremor. Comp. Study 2014, 18, 331. [Google Scholar] [CrossRef]
- André, N.; Ferrand, C.; Albinet, C.; Audiffren, M. Cognitive Strategies and Physical Activity in Older Adults: A Discriminant Analysis. J. Aging Res. 2018, 2018, 8917535. [Google Scholar] [CrossRef]
- Okamoto, K.; Harasawa, Y. Prediction of symptomatic depression by discriminant analysis in Japanese community-dwelling elderly. Arch. Gerontol. Geriatr. 2011, 52, 177–180. [Google Scholar] [CrossRef]
- Argerich, S.; Herrera, S.; Benito, S.; Giraldo, B.F. Evaluation of periodic breathing in respiratory flow signal of elderly patients using SVM and linear discriminant analysis. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4276–4279. [Google Scholar] [CrossRef]
- Kidd, T.; Mold, F.; Jones, C.; Ream, E.; Grosvenor, W.; Sund-Levander, M.; Tingström, P.; Carey, N. What are the most effective interventions to improve physical performance in pre-frail and frail adults? A systematic review of randomised control trials. BMC Geriatr. 2019, 19, 184. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich-Zwahlen, A.K.; Casartelli, N.C.; Item-Glatthorn, J.F.; Maffiuletti, N.A. Validity of resting myotonometric assessment of lower extremity muscles in chronic stroke patients with limited hypertonia: A preliminary study. J. Electromyogr. Kinesiol. 2014, 24, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Agyapong-Badu, S.; Warner, M.; Samuel, D.; Stokes, M. Measurement of ageing effects on muscle tone and mechanical properties of rectus femoris and biceps brachii in healthy males and females using a novel hand-held myometric device. Arch. Gerontol. Geriatr. 2016, 62, 59–67. [Google Scholar] [CrossRef]
- Ditroilo, M.; Cully, L.; Boreham, C.A.G.; De Vito, G. Assessment of musculo-articular and muscle stiffness in young and older men. Muscle Nerve 2012, 46, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Ditroilo, M.; Watsford, M.; Murphy, A.; De Vito, G. Assessing Musculo-Articular Stiffness Using Free Oscillations Theory, Measurement and Analysis. Sports Med. 2011, 41, 1019–1032. [Google Scholar] [CrossRef]
- Merritt, C.E. Comparison of 14 body size variables on adult skeletal age methods: How body mass, BMI, fat and muscle influence age estimations. Ann. Hum. Biol. 2020, 47, 32–41. [Google Scholar] [CrossRef]
- Al-Qahtani, M.; Al-Tayyar, S.; Mirza, E.H.; Al-Musallam, A.; Al-Suwayyid, A.; Javed, R. Body Mass Index and Segmental Mass Correlation With Elastographic Strain Ratios of the Quadriceps Tendon. J. Ultrasound Med. 2019, 38, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Chuang, L.L.; Wu, C.Y.; Lin, K.C. Reliability, validity, and responsiveness of myotonometric measurement of muscle tone, elasticity, and stiffness in patients with stroke. Arch. Phys. Med. Rehabil. 2012, 93, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Marusiak, J.; Jaskólska, A.; Koszewicz, M.; Budrewicz, S.; Jaskólski, A. Myometry revealed medication-induced decrease in resting skeletal muscle stiffness in Parkinson’s disease patients. Clin. Biomech. 2012, 27, 632–635. [Google Scholar] [CrossRef]
- Marusiak, J.; Żeligowska, E.; Mencel, J.; Kisiel-Sajewicz, K.; Majerczak, J.; Zoladz, J.A.; Jaskólski, A.; Jaskólska, A. Interval training-induced alleviation of rigidity and hypertonia in patients with Parkinson’s disease is accompanied by increased basal serum brain-derived neurotrophic factor. J. Rehabil. Med. 2015, 47, 372–375. [Google Scholar] [CrossRef]
- Little, P.; Stuart, B.; Stokes, M.; Nicholls, C.; Roberts, L.; Preece, S.; Cacciatore, T.; Brown, S.; Lewith, G.; Geraghty, A.; et al. Alexander technique and Supervised Physiotherapy Exercises in back paiN (ASPEN): A four-group randomised feasibility trial. Effic. Mech. Eval. 2014, 1, 1–84. [Google Scholar] [CrossRef]
- Kong, P.W.; Chua, Y.H.; Kawabata, M.; Burns, S.F.; Cai, C. Effect of Post-Exercise Massage on Passive Muscle Stiffness Measured Using Myotonometry—A Double-Blind Study. J. Sports Sci. Med. 2018, 17, 599–606. [Google Scholar]
- Berenpas, F.; Weerdesteyn, V.; Geurts, A.C.; van Alfen, N. Long-term use of implanted peroneal functional electrical stimulation for stroke-affected gait: The effects on muscle and motor nerve. J. Neuroeng. Rehabil. 2019, 16, 86. [Google Scholar] [CrossRef] [PubMed]

| KERRYPNX | Young Adults Male (n = 36) Female (n = 27) | Older Adults Male (n = 32) Female (n = 43) | ||
|---|---|---|---|---|
| Age (years) | 24.9 ± 4.8 | 26.8 ± 4.6 | 74.1 ± 5.7 | 75.5 ± 5.9 |
| Body mass index (kg/m2) | 24.1 ± 4.1 | 23.2 ± 3.2 | 26.5 ± 3.5 | 27.1 ± 3.8 * |
| SF-36 | ||||
| Physical function | 97.4 ± 4.1 | 96.7 ± 6.8 | 90.7 ± 11 | 83.8 ± 16.8 * |
| Physical activity scale for the | ||||
| Elderly (PASE) | N/A | N/A | 173 ± 69.7 | 142 ± 52.3 † |
| Grip strength (kg) | 45.3 ± 8.3 | 27.4 ± 5.2 | 35.7 ± 5.4 | 22.1 ± 4.6 *,† |
| Quadriceps strength (N) | 514 ± 138 | 334 ± 101 | 343 ± 66 | 214 ± 54 *,† |
| Peak flow (L/min) | 564 ± 80 | 395 ± 70 | 446 ± 109 | 307 ± 63 *,† |
| Timed up and go (s) | 5.5 ± 0.9 | 5.3 ± 0.8 | 7.3 ± 2.4 | 7.7 ± 1.8 * |
| Stair climbing time (s) | 7.1 ± 1.7 | 8.7 ± 2.8 | 11.8 ± 4.9 | 14.6 ± 5.1 *,† |
| Ultrasound Imaging | ||||
| Non-contractile tissue thickness (mm) | 8 ± 3.4 | 14 ± 4.2 | 9 ± 4.5 | 16 ± 4.7 *,† |
| Muscle thickness (mm) | 39 ± 7.5 | 29 ± 6.1 | 25 ± 4.4 | 20 ± 5.2 *,† |
| % Non-contractile tissue | 17 ± 6 | 32 ± 7 | 26 ± 8 | 44 ± 7 |
| % Muscle | 83 ± 6 | 68 ± 7 | 74 ± 8 | 56 ± 8 |
| Muscle mechanical properties | 285 ± 58 15.7 ± 1.8 1.5 ± 0.4 | 291 ± 52 * 15 ± 2 * 1.6 ± 0.3 * 300 ± 44.4 *,† 14.6 ± 1.9 † 1.6 ± 0.2 * | ||
| Biceps Brachii | ||||
| Stiffness (N/m) | 214 ± 25 | 216 ± 28 | ||
| Tone (Hz) | 14 ± 0.8 | 13.7 ± 1.2 | ||
| Decrement (log) | 1 ± 0.2 | 1.1 ± 0.2 | ||
| Rectus Femoris | ||||
| Stiffness (N/m) | 290 ± 39.5 | 231 ± 5.3 | 324 ± 31.4 | |
| Tone (Hz) | 16.2 ± 1.7 | 13.5 ± 1.3 | 16.5 ± 1.8 | |
| Decrement (log) | 1.3 ± 0.2 | 1.2 ± 0.2 | 1.6 ± 0.3 | |
| Prediction | Reference | |||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
| 1 | 32 | 0 | 1 | 0 |
| 2 | 2 | 24 | 3 | 1 |
| 3 | 0 | 0 | 23 | 1 |
| 4 | 0 | 1 | 1 | 37 |
| Class 1 | Class 2 | Class 3 | Class 4 | |
|---|---|---|---|---|
| Sensitivity | 94 | 96 | 82 | 95 |
| Specificity | 99 | 94 | 99 | 98 |
| Prevalence | 27 | 20 | 22 | 31 |
| PPV | 97 | 80 | 96 | 95 |
| NPV | 98 | 99 | 95 | 98 |
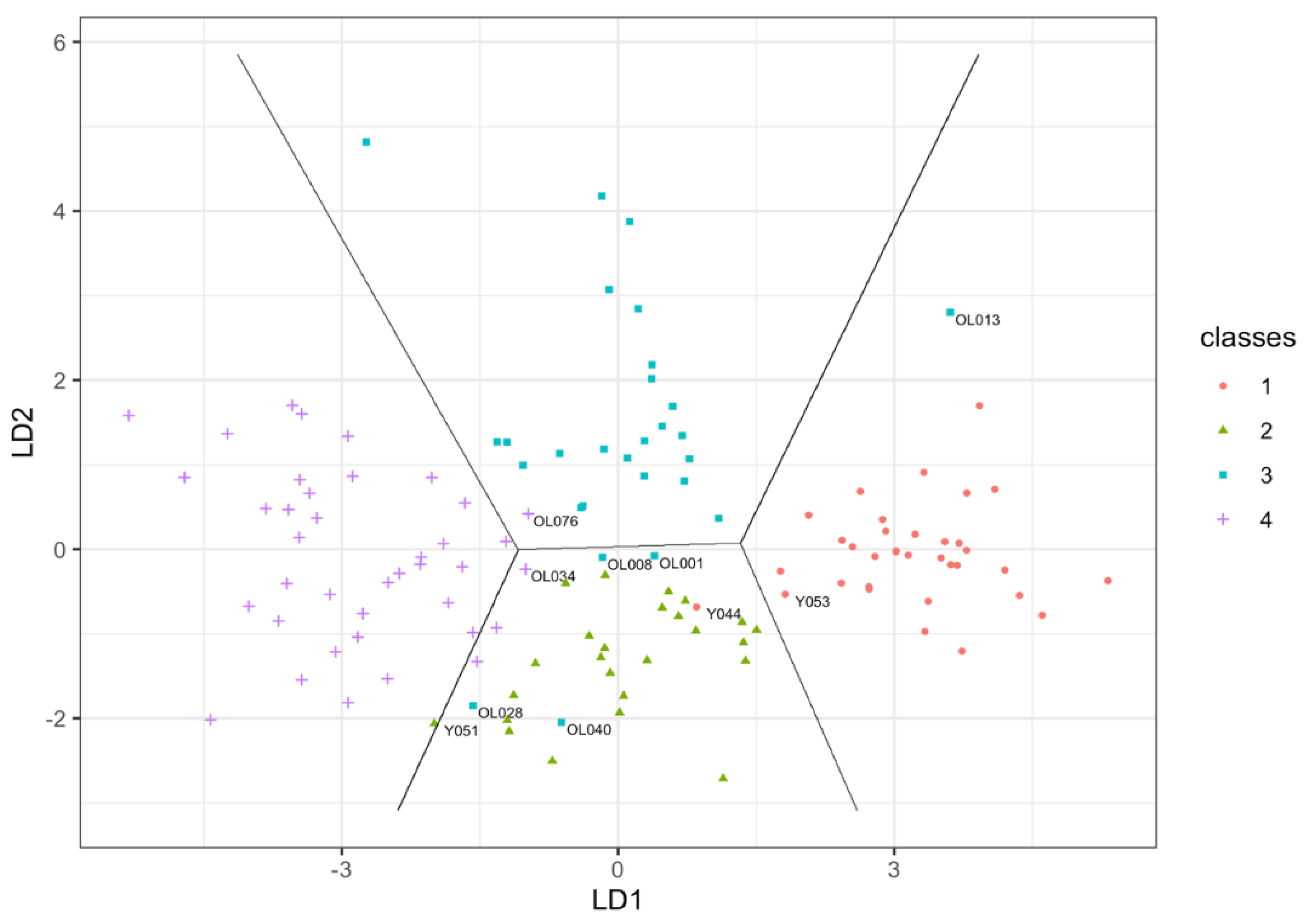
| Participant ID Age/Category | Absolute Values; Peak Flow (L/min), TUG (s); Decrement (Log), Anterior Thigh Muscle Thickness (mm); Percentage Thigh Muscle (%); PASE |
|---|---|
| Y044, 28, young male misclassified as young female | Peak flow = 470; TUG = 6.8; BB Decrement = 1.06; Anterior thigh muscle thickness = 29.9; % thigh muscle = 69 |
| Y051, 25, young female misclassified as older female | Peak flow = 290; TUG = 4.5; BB Decrement = 1.08; Anterior thigh muscle thickness = 19.2; % thigh muscle = 56 |
| Y053, 21, young male misclassified as young female | Peak flow = 450; TUG = 4; BB Decrement = 0.81; Anterior thigh muscle thickness = 25.4; % thigh muscle = 84 |
| OL001, 68, older male misclassified as young female | Peak flow = 510; TUG = 4.9; BB Decrement = 1.25; Anterior thigh muscle thickness = 25.1; % thigh muscle = 68; PASE = 308 |
| OL008, 70, older male misclassified as young female | Peak flow = 300; TUG = 4.9; BB Decrement = 1.22; Anterior thigh muscle thickness = 24.9; % thigh muscle = 81; PASE = 136 |
| OL013, 67, older male misclassified as young male | Peak flow = 810; TUG = 4.4; BB Decrement = 1.47; Anterior thigh muscle thickness = 29.7; % thigh muscle = 85; PASE = 169 |
| OL028, 75, older male misclassified as old female | Peak flow = 370; TUG = 7.1; BB Decrement = 1.13; Anterior thigh muscle thickness = 24.1; % thigh muscle = 52; PASE = 148 |
| OL034, 76, older female misclassified as young female | Peak flow = 400; TUG = 4; BB Decrement = 1.63; Anterior thigh muscle thickness = 28.9; % thigh muscle = 65; PASE = 200 |
| OL040, 73 older male misclassified as young female | Peak flow = 210; TUG = 4.3; BB Decrement = 1.11; Anterior thigh muscle thickness = 32.7; % thigh muscle = 73; PASE = 252 |
| OL076, 73, older female misclassified as old male | Peak flow = 370; TUG = 6.9; BB Decrement = 1.44; Anterior thigh muscle thickness = 22.7; % thigh muscle = 69; PASE = 135 |
| Classification Feature | Correlation |
|---|---|
| SF-36 | −0.22 |
| Physical function | |
| Grip strength (kg) | −0.15 |
| Quadriceps strength (N) | −0.05 |
| Peak flow (L/min) | −0.22 |
| Timed up and go (s) | 0.21 |
| Stair climbing time (s) | 0.36 |
| Ultrasound Imaging | |
| Non-contractile tissue thickness (mm) | 0.01 |
| Muscle thickness (mm) | 0.01 |
| % Non-contractile tissue | 0.41 |
| % Muscle | −0.41 |
| Muscle mechanical properties | |
| Biceps Brachii | |
| Stiffness (N/m) | 0.21 |
| Tone (Hz) | −0.07 |
| Decrement (log) | 0.47 |
| Rectus Femoris | |
| Stiffness (N/m) | 0.01 |
| Tone (Hz) | −0.21 |
| Decrement (log) | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agyapong-Badu, S.; Warner, M.B.; Samuel, D.; Koutra, V.; Stokes, M. Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender. J. Clin. Med. 2021, 10, 1352. https://doi.org/10.3390/jcm10071352
Agyapong-Badu S, Warner MB, Samuel D, Koutra V, Stokes M. Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender. Journal of Clinical Medicine. 2021; 10(7):1352. https://doi.org/10.3390/jcm10071352
Chicago/Turabian StyleAgyapong-Badu, Sandra, Martin B. Warner, Dinesh Samuel, Vasiliki Koutra, and Maria Stokes. 2021. "Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender" Journal of Clinical Medicine 10, no. 7: 1352. https://doi.org/10.3390/jcm10071352
APA StyleAgyapong-Badu, S., Warner, M. B., Samuel, D., Koutra, V., & Stokes, M. (2021). Non-Invasive Biomarkers of Musculoskeletal Health with High Discriminant Ability for Age and Gender. Journal of Clinical Medicine, 10(7), 1352. https://doi.org/10.3390/jcm10071352






